Affiliation:
1Department of Clinical and Experimental Medicine, Endocrinology Section, University of Catania Medical School, 95122 Catania, Italy
2Department of Medical and Surgical Sciences and Advanced Technologies “GF Ingrassia”, University of Catania, 95123 Catania, Italy
ORCID: https://orcid.org/0000-0003-1536-5697
Affiliation:
1Department of Clinical and Experimental Medicine, Endocrinology Section, University of Catania Medical School, 95122 Catania, Italy
ORCID: https://orcid.org/0000-0001-7386-5142
Affiliation:
1Department of Clinical and Experimental Medicine, Endocrinology Section, University of Catania Medical School, 95122 Catania, Italy
Email: laura.sciacca@unict.it
ORCID: https://orcid.org/0000-0002-3258-7200
Explor Med. 2022;3:173–180 DOI: https://doi.org/10.37349/emed.2022.00083
Received: February 02, 2022 Accepted: March 09, 2022 Published: April 19, 2022
Academic Editor: Giovanni Targher, University and Azienda Ospedaliera Universitaria Integrata of Verona, Italy
Semaglutide is a glucagon-like peptide 1 receptor agonist (GLP-1 RA) molecule approved for the treatment of both type 2 diabetes (T2D) and obesity. Semaglutide has a greater impact on glycated haemoglobin (HbA1c) reduction, compared to other GLP-1 RAs, and is the first molecule of this class available in oral formulation for T2D therapy, representing a useful option for subjects and physicians less prone to start an injective drug. Interestingly, due to its remarkable effects on weight reduction, higher than other GLP-1 RAs and very close to bariatric surgery, semaglutide is designated to change the approach to obesity therapy also in the subject not affected by diabetes. In addition to these favorable features, semaglutide, similarly to other GLP-1 RAs, offers beneficial effects on cardio-vascular (CV), renal, and liver protection, making this molecule an advantageous choice in the therapeutic management of “diabesity” (coexistence of both diabetes and obesity) and its co-morbidity.
Type 2 diabetes (T2D) is a complex disease that requires the use of different classes of drugs with various mechanisms to obtain both a good metabolic control and a reduction of chronic complications.
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are first-line drugs in the current multifactorial approach to T2D, focused on both the improvement of glucose control and the mitigation of the dysmetabolic-related burden of target organs. GLP-1 RAs reduce glycemia by modulating the release of insulin and glucagon in a glucose-dependent way with a very low risk of hypoglycemia; moreover, they favor weight loss inducing satiety through central mechanisms [1]. Further advantageous effects are the improvement of lipid panel and blood pressure and the reduction of cardio-vascular (CV), renal, and liver damage [1]. The most common side effects, involving the gastrointestinal system (nausea, vomiting), occur in a small rate of subjects and are, in most cases, well-tolerated and reversible.
Semaglutide is, in chronological order, the newest GLP-1 RA approved for the treatment of T2D. Semaglutide is available for once-weekly subcutaneous injection, and recently also for once-daily oral use, representing the first oral GLP-1 RAs and a well-suited option to improve therapeutic adherence in subjects less prone to injections (Table 1).
Characteristics of GLP-1 RAs available for T2D treatment
| Compound | Approval date | Route | Molecular weight (Da) | Duration of action | Half-life | Dosage | Frequency and timing of administration |
|---|---|---|---|---|---|---|---|
| Daily administration | |||||||
| Exen B.I.D. | 2005 (FDA), 2006 (EMA) | Injective | 4,186.6 | Short | 3–4 h | Starting: 5 μg, maintenance: 10 μg | Twice daily, within 60 min since morning and evening meal |
| Lira | 2009 (EMA), 2010 (FDA) | Injective | 3,751.2 | Long | 12–14 h | Starting: 0.6 mg, maintenance: 1.2 mg or 1.8 mg | Once daily, any time (independent of meals) but preferably at the same time each day |
| Lixi | 2013 (EMA), 2016 (FDA) | Injective | 4,858.5 | Short | 2–3 h | Starting: 10 μg, maintenance: 20 μg | Once daily, within 60 min of any meal (preferably the same meal each day) |
| Sema | 2019 (FDA), 2020 (EMA) | Oral | 4,113.6 | Long | 6–7 days | Starting: 3 mg, maintenance: 7 mg or 14 mg | Once daily, empty stomach, 30 min before eating, drinking, or taking other oral medications |
| Weekly administration | |||||||
| Exen LAR | 2011 (EMA), 2012 (FDA) | Injective | 4,186.6 | Long | 3–4 h | 2 mg | Once weekly, any time of day, with or without meals |
| Dula | 2014 (EMA/FDA) | Injective | 59,670.6 | Long | 5–6 days | 0.75 mg or 1.5 mg | Once weekly, any time of day, with or without meals |
| Sema | 2017 (FDA), 2019 (EMA) | Injective | 4,113.6 | Long | 6–7 days | Starting: 0.25 mg, maintenance: 0.5 mg or 1.0 mg | Once weekly, any time of day, with or without meals |
B.I.D.: bis in die; Da: dalton; Dula: dulaglutide; EMA: European Medicines Agency; Exen: exenatide; FDA: Food and Drugs Administration; LAR: long-acting release; Lira: liraglutide; Lixi: lixisenatide; Sema: semaglutide
GLP-1 RAs are the most effective class of diabetes drugs, excluding the well-known effectiveness of insulin in improving glycated haemoglobin (HbA1c). This effect proves to be greater for long-acting compared to short-acting formulations and is more pronounced, in comparative studies, with injective semaglutide at a dosage of 1 mg once weekly, which reduces HbA1c by 1.8% compared to 1.2–1.4% of other injective long-acting GLP-1 RAs and 1.1% of oral semaglutide (Figure 1A) [1, 2].
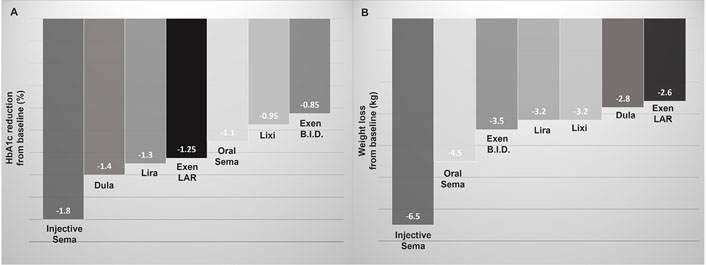
HbA1c and body weight reduction related to the use of GLP-1 RAs in comparative clinical studies. A. Mean HbA1c reduction; B. mean body weight reduction
CV risk reduction related to GLP-1 RAs is in part explained by the improvement of lipid panel and blood pressure. In a randomized controlled crossover trial involving 30 obese subjects not affected by T2D, semaglutide 1 mg significantly reduced, after 12 weeks of treatment, fasting total cholesterol, high-density lipoprotein (HDL), triglycerides and very low density lipoprotein (VLDL), and post-prandial triglycerides, VLDL, and apolipoprotein B-48 (Apob-48) [3]. In a later trial conducted on 15 subjects with T2D treated with oral semaglutide (14 mg/day), Dahl et al. [4] observed a similar improvement of lipid profile. The effect on lipid metabolism, stronger on post-prandial lipid level, could be related to the slow gastric emptying induced by GLP-1 RAs.
Semaglutide, similarly to liraglutide and dulaglutide, reduces blood pressure. In SUSTAIN 6 and PIONEER 6 trials, semaglutide, both in injective and oral formulation, resulted in a mean systolic blood pressure reduction of 2.6 mmHg [5, 6]. This is probably due to the release of smooth arterial muscles and increased natriuresis mediated by atrial natriuretic peptides and independent from weight loss.
Body weight reduction is strongly recommended in all subjects affected by “diabesity”. It is well known that the loss of at least 5% of body weight entails metabolic and CV benefits. GLP-1 RAs induce weight reduction through central mechanisms resulting in greater control of food intake and lower calorie intake.
Semaglutide appears to be the GLP-1 RA with greater effectiveness on weight loss. In comparative studies involving T2D subjects, the administration of injective semaglutide at a dosage of 1 mg once weekly was related to a mean weight reduction of 6.5 kg, while once-daily oral semaglutide determined a mean weight reduction of 4.5 kg (Figure 1B) [1, 7]. The other GLP-1 RAs determine a lower mean weight loss (2.5–3.5 kg), in decreasing order: exenatide short-acting, liraglutide and lixisenatide, dulaglutide, and exenatide long-acting (Figure 1B) [1, 7].
The promising effect on weight loss observed in T2D led to testing semaglutide also in obese subjects not affected by diabetes. In STEP 1 trial, a double-blind study recruiting 1,961 obese subjects, the group treated with injective semaglutide at a dosage of 2.4 mg once weekly adjunct to lifestyle intervention obtained a mean weight reduction from baseline of 15.3 kg (14.9%) after 68 weeks of treatment [8]. In particular, among subjects who received semaglutide, 86% lost 5% or more of baseline weight and half of them obtained a weight reduction of at least 15%. Interestingly, one-third of subjects treated with semaglutide lost 20% or more of baseline weight, a reduction similar to that observed in subjects who underwent bariatric surgery, in particular sleeve gastrectomy [8]. The results of the SELECT study (ClinicalTrials.gov NCT03574597) will clarify whether injective semaglutide could reduce major adverse cardiovascular events (MACE) occurrence in obese subjects not affected by diabetes.
The effect of semaglutide on weight loss observed in the STEP 1 trial was greater than that of liraglutide, the first GLP-1 RA approved for weight management, in obese subjects not affected by diabetes treated with liraglutide 3.0 mg once daily in SCALE trial [9]. Recently, in a similar population recruited in the STEP 8 trial, semaglutide at a dosage of 2.4 mg once weekly demonstrated a significantly greater weight loss compared to liraglutide 3.0 mg once daily after 68 weeks of treatment (–15.8% vs. –6.4%, respectively, P < 0.001) [10]. These results could potentially change the therapeutic approach to obesity and allow the approval of semaglutide for obesity treatment by both FDA and EMA.
Non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) are metabolic liver conditions very common in subjects affected by T2D and/or obesity [11]. These are multifactorial diseases and share their pathogenic mechanisms. The complex, not fully understood interplay between “diabesity” and liver disease, particularly NAFLD and NASH, includes adipose tissue dysfunction, insulin resistance, hyperglycemia, impairment in gut microbiome and cytokines profile, determining a lipotoxic and metabolic liver burden, hepatocytes injury, fibrosis and cirrhosis [12, 13].
To date, there are no approved pharmacological treatments for NAFLD/NASH. Both the improvement of metabolic parameters (blood glucose, insulin-resistance, lipids) and the loss of body weight related to GLP-1 RAs use suggest a role of these drugs in the management of metabolic liver diseases [13–15]. Recently, the effect of once-daily subcutaneous semaglutide (0.1 mg, 0.2 mg, and 0.4 mg) on NASH was tested in a randomized controlled trial involving 320 subjects with or without diabetes [16]. After 72 weeks, the group treated with semaglutide 0.4 mg, compared to placebo, had a significantly higher percentage of subjects achieving NASH resolution (59% vs. 17%, P < 0.001), regardless of diabetes, while no between-group differences were detected on liver fibrosis [17]. In the LEAN trial, involving 52 subjects affected by NASH, those treated with liraglutide 1.8 mg once daily, compared to placebo, were more likely to achieve NASH resolution (39% vs. 9%, P < 0.019) [17].
The SUSTAIN 6 trial evaluated the non-inferiority of injective semaglutide on the incidence of MACE in people with T2D compared to placebo. The 83% of the 3,297 enrolled subjects had established CV disease, chronic kidney disease, or both [5]. Semaglutide significantly reduced both the incidence of MACE [hazard ratio (HR) 0.74, P < 0.001 for non-inferiority, P = 0.02 for superiority], mostly by decreasing the occurrence of non-fatal stroke, and the progression or new onset of nephropathy, mainly by improving albuminuria (HR 0.64, P = 0.005) [5].
Further data on the efficacy and safety of semaglutide in T2D subjects with renal dysfunction were provided by PIONEER 5 trial [18]. This study evaluated oral semaglutide at a dosage of 14 mg once daily vs. placebo in 324 T2D patients with moderate renal impairment. After 26 weeks of treatment, no changes in renal function were observed between groups, despite a significant reduction of both HbA1c and body weight in the semaglutide group [18].
The FLOW clinical trial (ClinicalTrials.gov NCT03819153) is currently evaluating the effect of injective semaglutide on the progression of renal damage in subjects with chronic kidney disease.
The cardio-renal protection could be explained by both the reduction of glycemia, blood pressure, and weight, and through direct protective mechanisms (improved endothelial dysfunction, inflammation, and oxidative stress) of GLP-1 RAs on target organs [19].
In PIONEER 6, oral semaglutide was compared with placebo and it showed non-inferiority (HR 0.79, P < 0.001) but not the superiority (P = 0.17) about the risk of MACE incidence, in subjects at high CV risk [6]. To date, the randomized controlled trials investigating the cardio-renal protection of semaglutide focused mainly on subjects with high CV risk or in secondary prevention. Conversely, in a combined post hoc analysis of the SUSTAIN and PIONEER trials, Husain and colleagues [20] evaluated CV effects of semaglutide across a continuum of baseline CV risk. The authors found a reduced relative and absolute risk of MACE for semaglutide, compared to comparators, across the entire continuum of CV risk [20]. In particular, the relative risk reduction was largest in the low CV risk group, while the largest absolute risk reduction was observed in the intermediate to high CV risk group [20]. Although the mechanisms underlying these findings are unclear, the authors hypothesized a resistance of subjects with more advanced CV disease to the beneficial effects of GLP-1 RAs. The SOUL (ClinicalTrials.gov NCT 03914326) clinical trial is ongoing to evaluate the effect of oral semaglutide on CV risk.
The most common semaglutide side effects, similarly to other GLP-1 RAs, involve the digestive system [21]. Injective semaglutide induced nausea (10–20%), vomiting or diarrhea (5–10%) in a higher percentage than comparators, and more frequently in older subjects and in those with renal impairment [5, 21]. A similar rate of gastrointestinal side effects was observed in clinical trials with oral semaglutide [22]. Considering that for both formulations, the gastrointestinal side effects were more frequent for higher doses, a dose-escalation scheme is appropriate and recommended to reduce the discontinuation rate.
An increased risk for cholelithiasis was observed in subjects treated with GLP-1 RAs [22]. Concerning semaglutide, in the SUSTAIN program, 1.4% of subjects treated with injective semaglutide vs. 1.9% in the placebo group, developed a gallbladder adverse event [22].
In the PIONEER studies, the risk of cholecystitis was similar in the oral semaglutide and placebo groups, although the occurrence of cholelithiasis was more frequent in the oral semaglutide group (1.0% vs. 0.6%) [22, 23]. For these reasons, cholelithiasis is included in the summary of product characteristics of both subcutaneous and oral semaglutide.
Caution should be exercised when using semaglutide in patients with proliferative diabetic retinopathy. The increased risk for retinal complications (HR 1.76, P = 0.02) observed in subjects with retinopathy at baseline, probably due to the fast improvement of glucose control, suggests a careful use of semaglutide in these subjects [5]. It could be useful to perform a retinal evaluation before starting semaglutide therapy, in addition, avoid a fast glucose control improvement down titrating other diabetes drugs, in particular the dosage of insulin. The FOCUS trial (ClinicalTrials.gov NCT03811561), designed to further investigate the relationship between the use of semaglutide and the risk of retinopathy, is in progress.
Finally, semaglutide received an official warning for thyroid C-cell cancer in the United States. This alert, shared with other GLP-1 RAs, derives solely from rodent studies [24]. Consequently, semaglutide is contraindicated in the United States in subjects with a personal or family history of medullary thyroid cancer or type 2 multiple endocrine neoplasia.
Current evidence indicates that semaglutide is the GLP-1 RA with a greater impact on HbA1c—despite the low risk of hypoglycemia—and body weight loss. Moreover, the observed cardio-renal and liver protection related to semaglutide enhanced these additional and particularly useful effects of GLP-1 RAs in T2D subjects. These findings, together with their safety and tolerability, make both injective and oral semaglutide an advantageous choice for T2D treatment. Oral semaglutide represents a further option in the treatment of T2D subjects, particularly to increase adherence of subjects reluctant to injective therapies, and this could improve the utilization rate of GLP-1 RAs.
Interestingly, the favorable clinical effects of semaglutide are not limited to diabetes treatment: the relevant weight reduction observed in clinical trials involving obese subjects not affected by diabetes suggests the use of semaglutide in obese subjects, regardless of diabetes, and could potentially remodel the therapeutic approach to obesity. The effect of injective semaglutide on MACE risk reduction of obese subjects without diabetes is currently investigated by SELECT trial. The impact of semaglutide on liver metabolic disease needs to be better explored and the results of further trials are expected to extend the use of this molecule to these conditions.
In conclusion, based on all these findings, not only the broad spectrum of action and clinical application of semaglutide seem related to the “class action” but they also appear to be specific to this molecule. However, further observational and real-life studies are expected to confirm these promising results.
CV: cardio-vascular
EMA: European Medicines Agency
FDA: Food and Drugs Administration
GLP-1 RA: glucagon-like peptide 1 receptor agonist
HbA1c: glycated haemoglobin
HR: hazard ratio
MACE: major adverse cardiovascular events
NAFLD: non-alcoholic fatty liver disease
NASH: non-alcoholic steatohepatitis
T2D: type 2 diabetes
The authors gratefully acknowledge Professor Giuliana Arcidiacono, English and Anglo-American language PhD, University of Catania, for the language revision of the manuscript.
AM and LS conceived the study. AM, LS, and LM researched references, wrote, reviewed/edited the manuscript draft. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work is part of the PhD research program (Clinical and Translational Biomedicine, University of Catania) of Agostino Milluzzo, funded by MIUR—Research and Innovation Plan 2014–2020. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
