Affiliation:
1Department of Psychology and Neuroscience, Duke University, Durham, NC 27708, USA
2Department of Cell Biology, Duke University School of Medicine, Durham, NC 27710, USA
†These authors contributed equally to this work.
Email: Moawiah.naffaa@duke.edu
ORCID: https://orcid.org/0000-0003-0451-5901
Affiliation:
3Department of Internal Medicine, Ascension Saint Francis Hospital, Evanston, IL 60202, USA
†These authors contributed equally to this work.
Explor Med. 2025;6:1001314 DOI: https://doi.org/10.37349/emed.2025.1001314
Received: January 09, 2025 Accepted: April 17, 2025 Published: April 28, 2025
Academic Editor: Haim Werner, Tel Aviv University, Israel
The interaction between cancer and coronavirus disease 2019 (COVID-19) poses significant challenges, particularly for immunocompromised individuals who are at heightened risk for acute infections and long-term complications. The pandemic has exacerbated existing vulnerabilities in cancer care by disrupting treatment protocols and delaying diagnoses, leading to worsened health outcomes. This article emphasizes the importance of investigating the potential impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on cancer progression and highlights the need for effective strategies to protect this high-risk population. Long-term health consequences, including the emergence of long COVID, further emphasize the need for ongoing surveillance and comprehensive healthcare planning for cancer patients during and after pandemics. A multifaceted approach is essential, incorporating vaccination, timely therapeutic interventions, and sustained support for patients with lingering symptoms. This article also discusses and urges continued research into the oncogenic risks associated with SARS-CoV-2, which is crucial for enhancing our understanding of the broader health implications of COVID-19 and for informing public health strategies aimed at safeguarding cancer patients in future pandemics. Moreover, effective data collection and the development of refined clinical guidelines are vital for improving patient outcomes and preparing healthcare systems to support cancer patients during crises. Additionally, this article discusses the importance of investigating the mechanisms by which SARS-CoV-2 may increase cancer susceptibility, including chronic inflammation, cellular senescence, and immune dysregulation. Understanding these mechanisms is crucial for elucidating the virus’s long-term oncogenic potential, particularly among cancer survivors and individuals with chronic infections. Ensuring continuity and resilience in cancer care during global crises requires strategies to mitigate healthcare disruptions, enhance access to screenings and treatments, and address the specific challenges faced by cancer patients experiencing long COVID.
The COVID-19 pandemic has posed unprecedented challenges to global healthcare systems, disproportionately affecting vulnerable populations, including cancer patients [1, 2]. Studies have consistently shown that individuals with cancer are at a heightened risk of contracting SARS-CoV-2 and experiencing more severe disease outcomes, including increased mortality and complications [3, 4]. This vulnerability is attributed to a combination of factors, including advanced age, preexisting comorbidities, and the immunosuppressive effects of cancer itself and its treatments, such as chemotherapy and radiotherapy [5, 6]. However, data on the precise influence of cancer type and treatment regimens on COVID-19 outcomes remain inconsistent, highlighting the need for further investigation.
Patients suffering from various diseases, particularly those related to cerebrovascular conditions and stroke, have also shown significant impacts from COVID-19 in multiple ways [7, 8]. Studies have already explored these connections, and further research holds promise for clarifying and addressing these links. This could play a crucial role in protecting vulnerable patient groups from severe outcomes associated with COVID-19 infections.
Beyond the immediate clinical risks, the pandemic has significantly disrupted cancer care. Delays in diagnoses, screenings, and treatments have led to an increase in advanced-stage cancer cases, potentially worsening long-term survival rates [9, 10]. Healthcare providers have faced difficult decisions in balancing the urgency of cancer treatment with the risks of SARS-CoV-2 exposure in immunocompromised patients [11, 12]. While vaccines and therapeutic advancements have facilitated a return to standard care, managing cancer in the context of COVID-19 remains a persistent challenge [10, 13].
The relationship between viral infections and cancer is well established, with several viruses, including human papillomavirus (HPV), hepatitis B and C (HBV, HCV), and Epstein-Barr virus (EBV), recognized as oncogenic agents [14–16]. These viruses contribute to tumorigenesis by interfering with tumor suppressor genes, promoting chronic inflammation, or inducing immune evasion. Emerging evidence suggests that coronaviruses, including SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV), may interact with cancer-related pathways. These interactions could potentially influence oncogenesis through mechanisms such as oxidative stress, immune dysregulation, and disruption of cell cycle regulation [14, 15]. SARS-CoV-1, for instance, has been shown to repress the tumor suppressor protein retinoblastoma (pRB) and interfere with cell-cell contact inhibition, raising concerns about whether SARS-CoV-2 might exert similar oncogenic effects [17, 18].
Long-term complications of SARS-CoV-2 infection, commonly referred to as post-acute sequelae of SARS-CoV-2 infection (PASC) or long COVID, have further implications for cancer patients [18]. Chronic inflammation, persistent immune dysfunction, and cellular stress—hallmarks of long COVID—may create a microenvironment conducive to tumorigenesis [19–21]. While direct evidence linking SARS-CoV-2 to cancer remains preliminary, these potential mechanisms warrant further investigation, particularly given the long-term health implications of the virus.
Understanding the interplay between COVID-19 and cancer is essential for guiding future research and healthcare policies. While observational studies have begun to explore potential connections, significant gaps remain due to limitations in data generalizability and comprehensiveness. Determining whether SARS-CoV-2 directly or indirectly influences cancer development and progression requires extensive, in-depth research to clarify the underlying mechanisms.
This study aims to investigate the impact of SARS-CoV-2 infection, including acute COVID-19 and long COVID, on cancer progression, focusing on how immune dysregulation and genetic alterations contribute to oncogenesis. Additionally, it explores the distinct clinical outcomes observed in cancer patients, which result from the complex interplay between their malignancy, ongoing treatments, and viral pathophysiology. To address these issues, this article analyzes the challenges faced by cancer patients with acute and long-term COVID-19, particularly in symptom management and treatment complexities. Additionally, it examines the potential role of SARS-CoV-2 in cancer development and its long-term oncogenic risk. Furthermore, it investigates the genetic and immunological mechanisms underlying viral oncogenesis, with a specific focus on how SARS-CoV-2 may promote cancer progression. The study also assesses the variability in COVID-19 outcomes among cancer patients based on cancer type and treatment regimens. Furthermore, it proposes key strategies for advancing research on cancer care during global health crises, including addressing healthcare disruptions, treatment delays, and the prolonged effects of COVID-19. Ultimately, this work aims to provide a comprehensive understanding of the relationship between COVID-19 and cancer progression, highlighting the need for targeted clinical approaches to mitigate risks in cancer patients. By shaping future research priorities and healthcare policies, this study aims to enhance the resilience of cancer care during pandemics. Additionally, it evaluates the oncogenic potential of SARS-CoV-2 and recommends necessary adaptations in oncology practice to address emerging viral threats.
Given the continued global impact of cancer as a major public health challenge, understanding the interplay between cancer and COVID-19 is essential. Research has already investigated how COVID-19 affects cancer outcomes, associated risk factors, and healthcare disruptions (Table 1); however, further studies are required to deepen this understanding. For instance, a large cohort study analyzed electronic health records (EHRs) from over 500,000 adults, comparing outcomes such as mortality, ICU admissions, mechanical ventilation, and hospitalizations between patients with and without cancer [22]. In fully adjusted models, cancer patients receiving recent treatments exhibited an increased 30-day risk of death, ICU stays, and hospitalization. Conversely, cancer patients without recent treatment had comparable risks of mortality and ICU admission to non-cancer patients, though their risk of mechanical ventilation and hospitalization remained lower [22]. These findings underscore the importance of risk stratification based on recent treatments, highlighting the complexities of managing cancer care during the pandemic.
COVID-19 and cancer: outcomes, risk factors, and healthcare disruptions
| Topic | Details/Findings | References |
|---|---|---|
| Cancer and COVID-19 outcomes | Recent cancer treatment increased mortality, ICU admission, and hospitalization risks, while untreated patients had mortality rates similar to non-cancer individuals. | [22, 23] |
| Risk factors for severe COVID-19 | Older age, comorbidities, male sex, severe obesity, and racial/ethnic background heightened mortality risk. | [4, 24] |
| Impact on cancer care | The pandemic disrupted screenings and treatments; delayed colorectal cancer surgery increased mortality by 13%. | [25] |
| Cancer research disruptions | One-third of clinical trials were delayed or halted, and two-thirds of research labs closed, setting back discoveries by up to 18 months. HPV vaccination declines may raise future cancer risks. | [26] |
| Mortality risk in hospitalized patients | Cancer patients accounted for 10% of hospitalized COVID-19 cases and had higher in-hospital mortality. Younger cancer patients faced disproportionately high mortality. | [27, 28] |
| Vaccine efficacy | Cancer patients, especially those with hematologic malignancies, showed reduced vaccine efficacy. The delta variant widened the mortality gap. | [29, 30] |
| ICU admission trends | Cancer patients were generally less likely to be admitted to the ICU, except for younger patients, whose ICU admission rates matched non-cancer peers despite higher mortality. | [30–32] |
| Long COVID in cancer patients | Nearly 50% of cancer survivors developed long COVID, commonly experiencing fatigue, sleep disturbances, and muscle pain. Women were more affected, and initial infection severity did not predict long COVID risk. | [33–35] |
COVID-19: coronavirus disease 2019; HPV: human papillomavirus
One potential research direction involves conducting longitudinal studies on individuals who have recovered from COVID-19 to assess cancer incidence over time. Given the inflammatory nature of SARS-CoV-2 and its effects on critical tumor suppressor genes [36], monitoring cancer rates in these survivors could help clarify any causal relationship between the virus and cancer development.
Cancer patients tend to be older and have more comorbidities than the general population, both of which are associated with adverse COVID-19 outcomes [22, 37]. Additional risk factors identified include male sex, severe obesity, and certain racial and ethnic backgrounds. Broader research has similarly emphasized the role of biological and socioeconomic factors in determining COVID-19 severity in both cancer and non-cancer patients [38]. For example, non-Hispanic Black patients did not exhibit an increased mortality risk after adjusting for comorbidities, highlighting the need for further investigation into how race and ethnicity influence health outcomes [39].
After accounting for factors such as age and comorbidities, mortality rates between cancer and non-cancer patients may be comparable. Studies, such as the Lean European Open Survey on SARS-CoV-2 Infected Patients (LEOSS) registry, reported similar mortality rates between these groups once confounding factors were controlled, emphasizing the complex interplay of factors influencing COVID-19 outcomes [40].
The COVID-19 pandemic has had profound and far-reaching indirect effects on healthcare, particularly in the prevention and treatment of cancer [41]. Research highlights how the pandemic impacted cancer incidence and mortality, primarily through significant disruptions in healthcare services. These disruptions led to delays in cancer screening, diagnosis, and treatment, potentially reversing recent declines in cancer mortality rates [42]. For example, a study found that a one-year disruption, including a 26-week delay in treatment, could result in an additional 1,719 colorectal cancer (CRC) deaths in Australia between 2020 and 2044 [43]. Early detection through screenings is vital, particularly for cancers like colorectal, breast, and melanoma, but many countries postponed screenings during the pandemic [44]. Treatment delays also worsened outcomes; for example, a four-week delay in colon cancer surgery increases mortality risk by 6%, and delayed chemotherapy raises CRC mortality risk by 13% [45]. In Canada, average treatment initiation times for cancer patients increased from 4.5 weeks pre-pandemic to 44 days, significantly elevating death risks for those affected [46].
The pandemic also disrupted cancer research, with a third of clinical trials delayed or stopped, and two-thirds of labs closed, potentially delaying breakthroughs by 18 months [47]. Globally, public health campaigns like HPV vaccination were also interrupted, reducing coverage and increasing future cancer risks. For example, HPV vaccination rates in the UK dropped from 88% to 59%, and projections in the United States suggest up to 6,200 additional oropharyngeal cancer cases by 2100 due to the disruption [48].
The long-term impact of the pandemic on cancer care, emphasizing the need for future public health planning to address these indirect consequences. Understanding how large-scale crises reshape healthcare is essential for preparing for future emergencies and ensuring recovery in cancer care.
Early studies during the COVID-19 pandemic highlighted the heightened vulnerability of cancer patients. In the UK, it was found that 10% of hospitalized COVID-19 patients had a history of cancer, and this group exhibited a significantly higher in-hospital mortality rate compared to non-cancer patients [49]. The hazard ratio (HR) of 1.13 further underscored the increased risk faced by this demographic [50, 51]. This elevated mortality risk emphasizes the severity of COVID-19 outcomes in cancer patients and highlights the unique challenges they face, including delayed cancer treatments due to hospital resource shortages, disruption of ongoing cancer therapies, and lack of timely access to necessary diagnostic services [52]. For instance, during the pandemic, many oncology departments had to postpone elective surgeries, radiation treatments, and even chemotherapy regimens, leading to exacerbated disease progression [53, 54]. In some cases, these delays were further compounded by overwhelmed healthcare systems that struggled to manage both COVID-19 patients and cancer care. Additionally, cancer patients often face difficulty accessing routine imaging and laboratory diagnostics, which are essential for monitoring disease progression and making timely adjustments to treatment plans [55].
Moreover, the shortage of hospital beds, especially during peak COVID-19 surges, resulted in cancer patients being displaced from specialized cancer care units to general wards [1, 56], where the quality of care could be suboptimal for their specific needs. This reallocation of resources hindered the ability to deliver timely and appropriate interventions for cancer patients, worsening prognosis in many cases.
Additionally, many cancer patients experienced the compounding effect of being unable to receive routine care or attend cancer screenings, which resulted in delayed diagnoses and worsened outcomes [57]. The pandemic also led to the cancellation or postponement of cancer screening programs, such as mammograms and colonoscopies, which are critical for early detection of various cancers. This disruption in screening services has likely led to a surge in cancer diagnoses at more advanced stages, which are more difficult to treat and have worse survival rates. Moreover, the healthcare system’s overwhelming focus on COVID-19 cases led to an inadequate allocation of resources for cancer treatment centers, which worsened survival prospects for cancer patients. In some countries, cancer treatment units were repurposed as intensive care units for COVID-19 patients, deprioritizing ongoing cancer care [41, 53, 58]. This has significantly impacted patient outcomes, as the delay in critical treatments, such as immunotherapy or hormone therapy, can substantially alter the course of the disease.
Further investigations into age and cancer treatment outcomes revealed that although older cancer patients experienced worse absolute outcomes, younger cancer patients exhibited a disproportionately higher relative mortality risk when compared to non-cancer patients of the same age [59]. This observation challenges the prevailing belief that older age alone is the most significant factor in severe COVID-19 outcomes. Several factors, including cancer type, treatment intensity, and social interactions, may contribute to the elevated risk observed in younger cancer patients [60].
In regard to vaccination, despite the overall reduction in mortality due to vaccination, the efficacy of the vaccines was found to be lower in cancer patients, especially those with hematological malignancies [13, 29]. The delta variant surge in 2021 further widened the mortality gap between cancer patients and the general population, underscoring the need for targeted protective measures. Data spanning the pandemic, from the initial wave to the early Omicron variants (BA.1 and BA.2), illustrated how shifting variants, societal changes like the relaxation of lockdowns, and varying vaccine effectiveness influenced mortality risks over time [61].
In terms of ICU admissions, cancer patients were generally less likely to be escalated to the ICU compared to non-cancer patients [32]. However, this pattern was not observed in younger cancer patients, who, despite their increased mortality risk, were admitted to the ICU at similar rates to non-cancer patients [30]. This disparity suggests that factors beyond ICU admission rates may contribute to poor outcomes among cancer patients. This highlights the need for a more detailed exploration of ICU decision-making and the underlying causes of these outcomes [62].
The findings highlight the need for continued research into ICU escalation decisions and the specific drivers of poor outcomes for cancer patients admitted to the ICU with COVID-19. A more nuanced understanding of these elements could inform clinical guidelines, ultimately improving care and outcomes for this particularly vulnerable population [63].
Looking ahead, it is clear that cancer patients remain at high risk from COVID-19, necessitating ongoing mitigation efforts. These should include prioritizing vaccination for this group, regular testing of healthcare staff in high-risk environments, and ensuring timely access to antiviral treatments and therapeutic antibodies [64]. In the context of future pandemics, the study emphasizes the importance of systematic data collection for cancer patients, advocating for the use of national protocols to provide a comprehensive understanding of their specific risks. This approach would offer more robust insights than isolated cancer registries and would enhance preparedness and response strategies for high-risk groups.
Future clinical research on the impact of COVID-19 on cancer patients is essential for understanding and addressing their increased vulnerability to severe outcomes. Studies consistently show that cancer patients are at higher risk of adverse effects from COVID-19, influenced by factors such as pre-existing comorbidities, cancer stage, ongoing treatments, and immunosuppression [4, 65].
While the immediate effects of COVID-19 on multiple organ systems have been well documented, there is growing concern about the persistence of symptoms long after recovery, especially among cancer patients. Research draws parallels between long COVID and post-viral syndromes observed after past outbreaks [66, 67]. Long COVID, or post-acute sequelae of SARS-CoV-2 (PASC), is characterized by symptoms persisting for at least four weeks after diagnosis. Estimates of its prevalence in the general population vary widely, from 10% to around 90%, but data specific to cancer patients are limited, and the implications for their care and prognosis remain unclear [68, 69].
The severity of the initial COVID-19 infection appears to be a key factor in the development of long COVID, with patients who required hospitalization more likely to experience lingering symptoms. However, even those with mild or moderate infections have reported prolonged health issues, underscoring the complexity of the condition [70]. A study of few hundreds’ cancer patients found that 60% developed long COVID, with women more likely to report symptoms such as fatigue, sleep disturbances, and muscle pain. Surprisingly, cancer type, age, or initial infection severity did not predict long COVID, although hypertension was inversely associated with its development [33]. Hypertension, a known risk factor for severe acute COVID-19, appears to have a different role in long COVID, suggesting distinct mechanisms between the acute and post-acute phases of the disease. Furthermore, although men typically experience more severe COVID-19, women in this study were more likely to report long-term symptoms, pointing to possible influences from immune responses and hormonal factors [34, 71].
Fatigue was the most commonly reported symptom in cancer patients with long COVID, affecting 82%, consistent with reports from the general population. Other prevalent symptoms included sleep disturbances, muscle pain, and gastrointestinal issues. However, ongoing cancer treatments such as chemotherapy and radiotherapy may complicate symptom attribution, making it difficult to determine whether these are related to COVID-19 or cancer therapy [72, 73].
Long COVID poses significant concerns for cancer patients, particularly women, with fatigue and sleep disturbances being the most frequent symptoms [33, 74]. Although severe acute COVID-19 was associated with higher mortality, it did not reliably predict the development of long COVID [75]. Further research is critical to developing effective long-term management strategies tailored to the specific needs of cancer patients.
SARS-CoV-2, the virus responsible for COVID-19, is a positive-sense, single-stranded RNA (ss-RNA) virus with a genome size of 26–32 kilobases [76]. Unlike retroviruses, which encode reverse transcriptase, SARS-CoV-2 does not have the capacity to convert RNA into DNA. However, it possesses critical molecular components, including the spike (S) protein, which enables viral entry into human cells. The receptor-binding domain (RBD) of the spike protein, located in the S1 subunit, interacts specifically with angiotensin-converting enzyme 2 (ACE2) receptors on human cell surfaces, facilitating viral invasion (Table 2) [77, 78]. Mutations in the spike protein, particularly within the RBD, can impair antibody recognition, increasing the risk of reinfection and diminishing vaccine efficacy.
The molecular mechanisms of SARS-CoV-2 and its potential role in cancer development
| Molecular mechanism | Description | Potential impact on cancer | Reference |
|---|---|---|---|
| Spike (S) protein and ACE2 interaction | The spike protein binds ACE2, facilitating viral entry. | ACE2 downregulation disrupts RAAS, promoting inflammation, fibrosis, and cancer progression. | [79, 80] |
| CircRNAs and miRNA interaction | SARS-CoV-2 circRNAs interact with host miRNAs. | Alters metabolic and tumorigenic pathways, potentially affecting cancer-related processes. | [81–84] |
| Dysregulation of renin-angiotensin-aldosterone system (RAAS) | ACE2 downregulation disrupts Ang-2/AT1R signaling. | Enhances inflammation, cancer stem cell formation, and oncogenic pathways (e.g., MAPK/ERK, TGF-β, IL-6, IL-8), driving tumor growth and metastasis, particularly in NSCLC. | [85–88] |
| Tumor suppressor degradation (pRB, p53) | Viral proteins (e.g., nsp15, nsp3) degrade pRB and p53. | Loss of tumor suppressors leads to uncontrolled cell proliferation and increased cancer risk. | [89–92] |
| Epigenetic modifications | SARS-CoV-2 proteins interact with epigenetic regulators (e.g., nsp7, nsp8, nsp14, SIRT5, and NSD2). | Alters gene expression; NSD2 activates RAS signaling, while SIRT5/HDAC2 modulate p53 and tumor progression. | [93–96] |
| Cytokine storm and inflammatory pathways | Severe COVID-19 triggers elevated IL-6 and other cytokines. | STAT3/NF-κB activation promotes proto-oncogene expression (e.g., c-myc), fueling tumor growth and metastasis. | [88, 97–100] |
| Viral-host protein interactions | SARS-CoV-2 proteins modulate host pathways. | Potential activation of oncogenic Wnt and NF-κB signaling, suggesting therapeutic targets. | [36, 98, 101, 102] |
ACE2: angiotensin-converting enzyme 2; Ang-2: angiopoietin-2; circRNAs: circular RNAs; COVID-19: coronavirus disease 2019; miRNA: microRNA; NSCLC: non-small cell lung cancer; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; pRB: protein retinoblastoma
Beyond its immediate impact on the respiratory system, SARS-CoV-2 may influence broader biological processes that could contribute to cancer development [103]. Recent studies have identified virus-encoded circular RNAs (circRNAs) in various coronaviruses, including SARS-CoV-2, which interact with human microRNAs (miRNAs) (Table 2). These interactions regulate gene networks involved in critical processes such as cancer, metabolism, and autophagy [104]. Specifically, circRNAs derived from MERS-CoV modulate tumorigenic pathways, while SARS-CoV-2-associated circRNAs have been implicated in metabolic and oxidative stress pathways. While oncogenic mechanisms have been observed in SARS-CoV-1, long-term studies have yet to definitively link the virus to cancer development [82, 84]. Although SARS-CoV-2 exhibits characteristics common to oncogenic viruses, definitive evidence connecting it to tumorigenesis remains lacking, necessitating further investigation.
The molecular interactions between SARS-CoV-2 proteins and tumor suppressors or oncogenes represent a crucial area of investigation. Research into how proteins like nsp15 and nsp3 degrade critical tumor suppressors like pRB and p53 could offer valuable insights into the mechanisms of viral oncogenesis [105]. Furthermore, exploring the role of virus-encoded circRNAs in regulating cancer-related gene networks could uncover novel therapeutic targets. The association between SARS-CoV-2 and metabolic and oxidative stress pathways, particularly through circRNAs, also warrants further exploration.
A major area of concern in the context of SARS-CoV-2 infection is its effect on the renin-angiotensin-aldosterone system (RAAS). SARS-CoV-2 downregulates ACE2, a key regulatory protein in RAAS, leading to an imbalance that enhances angiopoietin-2 (Ang-2)/AT1R signaling [106]. This dysregulation contributes to inflammation, fibrosis, and oxidative stress, all of which are recognized as key factors in cancer progression. Ang-2, for instance, has been implicated in the formation of cancer stem cells, particularly in non-small cell lung cancer (NSCLC) (Table 2) [107, 108]. Moreover, Ang-2 activates the MAPK/ERK pathway, resulting in TGF-β production, which further promotes cancer cell growth and migration [109]. Additionally, dysregulated RAAS stimulates the release of inflammatory cytokines like IL-6 and IL-8, as well as vascular endothelial growth factor (VEGF), crucial for tumor angiogenesis [110, 111].
A promising research direction involves understanding how SARS-CoV-2-induced dysregulation of the RAAS influences cancer development, for instance, by enhancing store-operated calcium entry [112, 113]. This line of investigation could provide further insights into the mechanistic links between RAAS disruption and cancer progression. Given the established connection between RAAS and cancer progression, exploring how RAAS imbalances affect cancer stem cell formation and tumor growth could lead to innovative therapeutic strategies. Additionally, assessing inflammatory cytokine levels, such as IL-6 and IL-8, in cancer patients with a history of SARS-CoV-2 infection may improve our understanding of cancer outcomes in COVID-19 survivors.
SARS-CoV-2 also appears to interfere with critical tumor suppressors, such as pRB and p53, through viral proteins like nsp15 and nsp3. These proteins promote the degradation of these tumor suppressors, potentially increasing the risk of cancer (Table 2) [105, 114]. Moreover, viral infections such as SARS-CoV-2 can disrupt the cell cycle and activate pro-apoptotic mechanisms, including the production of caspase-8 (Cas8), further contributing to oncogenesis [88].
The interaction between SARS-CoV-2 and epigenetic or metabolic regulators is emerging as a critical area of research. SARS-CoV-2 proteins such as nsp7 and nsp8 have been shown to interact with epigenetic modifiers, while nsp14 engages with SIRT5, a protein associated with lung cancer progression [94, 115]. Another epigenetic regulator, NSD2, upregulates the RAS signaling pathway, directly linked to cancer progression (Table 2). Simultaneously, HDAC2, an enzyme that activates the tumor suppressor p53, has been implicated in various cancers, suggesting that the virus may modulate epigenetic pathways influencing tumorigenesis [116, 117].
Epigenetic alterations induced by viral infections represent a critical avenue for further exploration. Specifically, investigating DNA methylation changes linked to cancer risk can yield important insights. By utilizing bisulfite sequencing to profile DNA methylation changes in leukocytes from COVID-19 patients and comparing these profiles to those of healthy controls [118], researchers can identify specific epigenetic modifications that may predispose individuals to cancer.
The potential influence of SARS-CoV-2 proteins on epigenetic regulators like SIRT5 and HDAC2 also presents an exciting frontier in cancer research. Since epigenetic modifications significantly impact gene expression and tumor behavior, investigating how SARS-CoV-2 proteins affect these pathways could provide critical insights into cancer therapy and prevention.
Furthermore, functional studies are needed to determine the effect of these methylation changes on gene expression related to cancer. This research will advance our understanding of how viral infections may influence gene regulation and contribute to oncogenesis.
A hallmark feature of severe COVID-19 is the cytokine storm, characterized by elevated levels of pro-inflammatory cytokines such as IL-6. These elevated levels correlate with poor disease outcomes in COVID-19 and may also promote cancer progression (Table 2) [97, 119]. IL-6 activates the STAT3 and NF-κB pathways, which in turn promote the expression of proto-oncogenes like c-myc, driving growth and metastasis in cancers such as pancreatic and breast cancer [87, 98]. This connection between inflammatory responses and cancer progression underscores the need for ongoing research into the long-term oncogenic potential of SARS-CoV-2, especially in individuals who have experienced chronic infection or severe inflammatory responses.
Comparative studies of SARS-CoV-2 alongside established oncogenic viruses such as Epstein-Barr virus (EBV) and human T-cell lymphotropic virus (HTLV) offer valuable opportunities to explore shared and distinct mechanisms of oncogenesis (Table 2) [89]. Identifying common pathways and unique interactions may uncover potential therapeutic targets and inform cancer prevention strategies specific to SARS-CoV-2.
Additionally, the application of integrative omics approaches is crucial for unraveling the virus’s impact on cellular processes. By analyzing genetic, transcriptomic, proteomic, and metabolomic profiles in SARS-CoV-2-infected cells, researchers can identify novel biomarkers and pathways linked to the virus’s oncogenic potential. Such discoveries are essential for building a comprehensive understanding of the long-term health implications of COVID-19, including its possible contributions to cancer development [120].
A key area of focus is investigating the role of specific viral proteins in modulating cancer-related cellular pathways. Using advanced molecular biology techniques such as CRISPR/Cas9 and RNA interference (RNAi) to knock down viral proteins in cell lines allows for the assessment of their influence on crucial oncogenic pathways, including Wnt and NF-κB signaling. Concurrently, proteomic analyses can identify host proteins that interact with viral components, shedding light on the mechanisms through which these interactions drive tumorigenic processes. These insights may reveal novel targets for therapeutic interventions aimed at cancer prevention in the context of SARS-CoV-2.
The mutagenic effects of SARS-CoV-2 provide a unique insight into the complex relationships between viral infections, immune responses, and oncogenesis [121]. A key tool for studying these connections is Mendelian randomization (MR), an epidemiological method that uses genetic variants as instrumental variables to infer causal relationships between exposures, such as viral infections, and outcomes like cancer risk [122, 123]. MR helps minimize confounding factors and reverse causality, offering a more robust method to establish causality in epidemiological studies. This approach has been previously applied to identify associations between COVID-19 and various chronic conditions, including type 2 diabetes, and cancer risk factors like alterations in gut microbiota and autoimmune diseases such as rheumatoid arthritis [124, 125]. Recent applications of MR to explore potential links between COVID-19 and cancer have provided important insights into how viral infections might contribute to oncogenesis [123].
Recent MR studies have begun to identify genetic predispositions that link COVID-19 with specific cancers (Table 3). For instance, lung adenocarcinoma has emerged as one of the cancers potentially associated with SARS-CoV-2 infection. Genetic data from critically ill and hospitalized COVID-19 patients suggest that cancer risk may increase with the severity of the disease [126, 127]. Critically ill patients demonstrate an elevated risk for cancers such as HER2-positive breast cancer, esophageal cancer, CRC, stomach cancer, and colon cancer [128]. Notably, even patients with less severe disease—those hospitalized but not requiring intensive care—show increased risks for HER2-positive breast cancer, esophageal cancer, and stomach cancer [129].
Genetic associations between COVID-19 and cancer with underlying mechanisms
| Cancer type | Genetic link to COVID-19 | Mechanisms/Pathophysiology | Reference |
|---|---|---|---|
| Lung cancer | Increased risk, particularly in severe cases. | - SARS-CoV-2 induces pulmonary fibrosis.- Activates oncogenic pathways (e.g., PI3K/AKT), promoting cell proliferation.- Depletes immune cells (NK cells, CTLs), impairing immune defense. | [130–132] |
| Breast cancer | Linked to HER2-positive cases in severe infections. | - Viral hyperglycosylation disrupts protein interactions.- E-Cadherin downregulation facilitates EMT and invasiveness.- NF-κB activation drives inflammation and tumor progression. | [112, 133, 134] |
| Pancreatic cancer | Potential modulation of tumorigenesis-related genes. | - Upregulation of PTEN, CREB1, CASP3, and SMAD3.- Chronic inflammation fosters oncogenesis and delays diagnosis. | [135–137] |
| CRC | COVID-19-induced gut microbiota dysregulation. | - Dysbiosis weakens mucosal immunity.- Chronic inflammation exacerbates mucosal damage, increasing CRC risk. | [138–141] |
| Oral cancer | Altered angiogenesis and extracellular matrix regulation. | - SARS-CoV-2 modulates angiopoietin-2 and EMMPRIN, promoting tumor growth and metastasis. | [88, 142, 143] |
| Gastric cancer | Higher incidence in critically ill patients. | - Prolonged viral replication in the GI tract via ACE2 receptors. | [144–147] |
| Head and neck cancer | Lower risk, potentially due to reduced TMPRSS2 expression. | - Limited viral entry minimizes cellular damage and oncogenesis. | [148–151] |
ACE2: angiotensin-converting enzyme 2; COVID-19: coronavirus disease 2019; CRC: colorectal cancer; CTLs: cytotoxic T lymphocytes; EMMPRIN: extracellular matrix metalloproteinase inducer; EMT: epithelial-mesenchymal transition; NK: natural killer; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
An intriguing discovery is that individuals with milder SARS-CoV-2 infections, who do not require hospitalization, exhibit a higher risk of stomach cancer, while their risk of head and neck cancer is relatively lower [152, 153]. These variations suggest that the immune response to SARS-CoV-2, even in milder cases, may paradoxically foster an inflammatory environment that supports oncogenesis.
The relationship between COVID-19 severity and cancer risk is multifaceted and, at times, counterintuitive. Milder COVID-19 cases have been linked to an increased risk of gastric cancer, possibly due to immune responses that manage viral replication but cause chronic inflammation in the gastric mucosa [154, 155]. Chronic inflammation is known to promote mutagenic environments, increasing the likelihood of DNA damage and cellular changes that predispose tissues to malignant transformation [99, 156]. Furthermore, the immune dysregulation triggered by SARS-CoV-2 infection may interfere with normal tumor-suppressive mechanisms, contributing to cancer development (Table 3).
Lung cancer risk among COVID-19 patients is especially concerning due to the direct impact of the virus on the lungs. SARS-CoV-2 induces pulmonary interstitial fibrosis, a well-known precursor to lung malignancies [157]. The virus also activates oncogenic signaling pathways, including the PI3K/AKT pathway, which promotes abnormal proliferation of alveolar epithelial cells. This leads to pathological changes such as hyperplasia and fibrosis, which are risk factors for lung cancer [158, 159].
Additionally, SARS-CoV-2 suppresses immune surveillance by depleting natural killer (NK) cells and cytotoxic T lymphocytes (CTLs), both critical for eliminating cancer cells [160]. This immune impairment, coupled with cytokine storms and chronic inflammation in severe COVID-19, creates a tumor-promoting environment. The generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) further damages DNA, fueling mutations that drive lung cancer [161, 162]. Elevated cytokines, particularly IL-6, are implicated in metastasis and epithelial-mesenchymal transition (EMT), both hallmarks of aggressive lung cancer.
SARS-CoV-2 has been linked to an increased risk of HER2-positive breast cancer, potentially through viral hyperglycosylation mechanisms that disrupt cellular protein interactions, downregulating E-cadherin, a molecule crucial for epithelial cell integrity [163, 164]. Loss of E-cadherin facilitates EMT, promoting invasiveness and metastasis. Additionally, the NF-κB pathway, central to inflammation and cancer progression, is activated by SARS-CoV-2, which may enhance breast cancer risk. Interactions between the virus and the ACE2 receptor, a viral entry point, might also unintentionally suppress immune responses [134], further supporting tumor progression in patients with underlying breast cancer.
Severely ill or hospitalized COVID-19 patients are also at increased risk of developing HER2-positive breast cancer, potentially due to elevated ACE2 expression in these tissues [165]. The connection between ACE2 and poor outcomes in HER2-positive breast cancer, coupled with its role in SARS-CoV-2 infection, further complicates the prognosis for affected individuals.
Pancreatic adenocarcinoma, a highly lethal cancer, may be influenced by SARS-CoV-2’s modulation of gene expression in pancreatic tissues [136]. Key genes involved in tumorigenesis, such as PTEN, CREB1, CASP3, and SMAD3, may be upregulated in response to the virus, accelerating pancreatic cancer development. Chronic inflammation driven by immune responses to SARS-CoV-2 could further facilitate oncogenesis, often allowing pancreatic cancer to progress undetected until advanced stages [166].
CRC development in COVID-19 patients might be linked to disturbances in the gut microbiota caused by viral interference with ACE2 function. SARS-CoV-2 induces gut dysbiosis, characterized by a loss of beneficial bacteria and an increase in pathogenic strains [138]. This dysbiosis weakens gut immunity, contributing to chronic inflammation, a known risk factor for CRC [167]. The gastrointestinal symptoms commonly observed in COVID-19, such as diarrhea and inflammation, may exacerbate CRC risk by causing persistent mucosal damage [168].
Viral-induced changes in angiogenesis and extracellular matrix regulation may contribute to oral cancer. SARS-CoV-2 modulates levels of Ang-2 and extracellular matrix metalloproteinase inducer (EMMPRIN), which are key to tumor growth and metastasis [111]. Increased Ang-2 levels, driven by ACE2 downregulation, have been associated with oral cancer, suggesting a mechanism linking the infection to oral carcinogenesis [169].
Critically ill, hospitalized, and SARS-CoV-2-infected individuals exhibit a notably higher risk of gastric cancer. This may be attributed to prolonged viral replication in the gastrointestinal tract, where ACE2 and TMPRSS2 receptors are more highly expressed, enabling greater viral entry and infection [146, 170].
Interestingly, COVID-19 patients appear to have a lower risk of head and neck cancer, which could be explained by reduced TMPRSS2 expression in these tissues [149]. This limits viral entry and minimizes cellular damage in these areas.
MR offers a powerful framework to study the causal links between SARS-CoV-2 infection and cancer [126]. The observed associations between COVID-19 severity, immune dysregulation, and cancer risk highlight the importance of understanding the long-term oncogenic effects of viral infections. Advancing our knowledge in this area could lead to the development of novel therapeutic strategies to counteract the cancer-promoting consequences of viral infections like COVID-19.
Viral infections have long been recognized as significant contributors to cancer development, with approximately 15% of all cancer cases worldwide linked to carcinogenic viruses [15]. Research exploring the relationship between viruses and cancer began in the mid-1960s, following the discovery of the first human oncogenic virus [171]. This breakthrough led to extensive investigations into how viruses contribute to tumorigenesis. Today, several viruses are strongly associated with specific cancer types, underscoring the profound impact of viral infections on cancer risk [172].
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has added new complexities to understanding the relationship between viral infections and long-term health outcomes, including potential cancer risks [105]. With increasing concerns about long COVID—where individuals experience prolonged symptoms months after the acute phase of infection—questions have arisen regarding its possible role in elevating long-term cancer risk [99].
Furthermore, the relationship between viral infections and cancer risk is complex, with significant public health implications [172]. Understanding how viral infections, such as SARS-CoV-2, contribute to cancer development is essential for developing effective interventions to mitigate this risk (Table 4).
Viral mechanisms contributing to cancer development, with a focus on SARS-CoV-2
| Mechanism | Description | Implications for cancer | Reference |
|---|---|---|---|
| Oncogenic potential of RNA viruses | RNA viruses, such as hepatitis C, produce viral proteins that disrupt cellular homeostasis, posing long-term oncogenic risks. | Persistent SARS-CoV-2 proteins may drive immune dysregulation, increasing cancer susceptibility. | [173, 174] |
| SARS-CoV-2 specific mechanisms | The spike protein enables viral entry, while nucleocapsid proteins facilitate replication. ACE2 receptor interactions influence tissue susceptibility. | SARS-CoV-2 may trigger cellular alterations linked to tumorigenesis, necessitating further investigation. | [77, 88, 175] |
| Chronic inflammation | Persistent infections induce prolonged inflammatory responses, promoting a pro-tumorigenic environment. | Post-COVID-19 inflammation may enhance cancer risk through sustained immune activation. | [100, 176–178] |
| Immunosuppression | Viral infections compromise immune function, fostering a tumor-permissive microenvironment. | Impaired immune surveillance facilitates tumor initiation and progression. | [179, 180] |
| Cellular senescence (CS) | Viral infections can induce CS, characterized by growth arrest and a senescence-associated secretory phenotype (SASP). | CS may exert dual effects, initially preventing tumors but later promoting chronic inflammation and cancer. | [181–183] |
| DNA modification | Viruses can introduce oncogenes, activate proto-oncogenes, or suppress tumor suppressor genes. | These genetic disruptions drive oncogenesis, underscoring the need for further molecular studies. | [184, 185] |
| Autophagy dysregulation | Viral proteins disrupt autophagy, leading to cellular damage accumulation and immune evasion. | Impaired autophagy fosters tumor development by promoting cell survival and immune resistance. | [186–188] |
ACE2: angiotensin-converting enzyme 2; COVID-19: coronavirus disease 2019; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
The mechanisms through which viruses contribute to cancer development are diverse. Chronic inflammation, immunosuppression, and DNA modification are key processes in viral oncogenesis [189]. Viruses can initiate cancer by introducing foreign oncogenes, overactivating human oncogenes, or inhibiting tumor suppressor genes [184]. Understanding these mechanisms is vital for unraveling the complex relationship between viral infections and cancer (Table 4).
In the context of long COVID, several hypotheses have emerged regarding its long-term effects, including the persistence of viral antigens and RNA, chronic inflammation, autoimmunity, dysbiosis of the microbiome, and tissue damage across multiple organs [190]. These factors may contribute to ongoing health complications that potentially elevate cancer risk.
Key structural components of SARS-CoV-2 play crucial roles in viral entry and replication, further complicating health outcomes. The spike glycoprotein facilitates the virus’s entry into host cells, while the nucleocapsid protein is essential for genome replication [191]. The membrane and envelope proteins contribute to virus assembly and structural integrity. Importantly, SARS-CoV-2 binds to the ACE2 receptor, found in various tissues, including the lungs, kidneys, and immune cells, which may lead to widespread effects on human health and potential cancer risks [192, 193].
One area of interest in exploring the link between COVID-19 and cancer risk is cellular senescence (CS), a process associated with aging and several diseases, including cancer [194, 195]. CS is triggered by stressors such as DNA damage and oxidative stress, which alter cell proliferation [196]. While CS can act as a tumor-suppressive mechanism, it also contributes to chronic inflammation through the senescence-associated secretory phenotype (SASP) [197]. Studies suggest that viral infections, including COVID-19, can induce CS, with SARS-CoV-2 shown to enhance SASP and promote CS in infected cells. Additionally, persistent DNA methylation changes have been observed in leukocytes following COVID-19 infection [172].
A pivotal area for exploration is the role of CS in the oncogenic potential of viral infections. Specifically, it is essential to examine how SARS-CoV-2-induced CS may contribute to cancer development [198]. Longitudinal studies should be initiated to assess CS markers, such as p16INK4a and p21CIP1, in patients recovering from COVID-19 and other viral infections [199]. By correlating these markers with cancer incidence over time, we can better understand the long-term oncogenic risks associated with viral infections.
In addition to longitudinal studies, in vitro models of human epithelial cells infected with SARS-CoV-2 can be employed to study the induction of CS and the SASP. This approach will allow researchers to evaluate changes in cytokine profiles and cellular proliferation rates post-infection, providing insights into the mechanisms by which viral infections might facilitate cancer progression.
Chronic inflammation is a key mechanism linking viral infections to cancer risk. Persistent viral infections and immune evasion by tumor-associated viruses often lead to prolonged inflammatory responses that facilitate tumor development [177, 200]. Individuals recovering from COVID-19 may experience lasting inflammatory changes, increasing their susceptibility to cancer. Elevated levels of inflammatory markers and dysfunctional neutrophils have been observed in COVID-19 survivors, emphasizing the potential role of inflammation in heightening cancer risk [201, 202].
Chronic inflammation triggered by viral infections represents a critical pathway that may enhance cancer susceptibility [203, 204]. Investigating inflammatory markers associated with long COVID could provide insights into this relationship. Initiating cohort studies to track inflammatory markers such as C-reactive protein (CRP), IL-6, and TNF-α in long COVID patients compared to non-infected controls is essential. By examining the associations between elevated inflammatory markers and subsequent cancer diagnoses, we can better understand how persistent inflammation might contribute to oncogenesis.
RNA viruses, such as hepatitis C, are well-established contributors to cancer. These viruses often promote cancer by continuously expressing viral gene products that alter normal cellular functions [205]. Notably, recent studies have detected residual SARS-CoV-2 proteins in various tissues up to six months post-recovery, suggesting continued immune interactions that could be associated with an increased risk of cancer [206].
Animal models simulating chronic viral infections such as HCV, can also provide valuable insights [207]. These models can be utilized to assess the effects of sustained inflammation on tumor development and progression, allowing for a deeper understanding of the interplay between chronic inflammation and cancer.
Immunosuppression is a critical factor in understanding the link between viral infections and cancer. The immune system plays a crucial role in eliminating tumor cells, and individuals with weakened immune responses are more prone to developing tumors [208, 209]. Studies show that SARS-CoV-2 can infect but not replicate in monocytes and macrophages, leading to immunoparalysis, which exacerbates COVID-19 progression [210–212]. The virus’s ability to alter macrophage function—shifting them towards a tumor-promoting M2 macrophages phenotype—further highlights its potential role in cancer development.
Research on long COVID has also shown that patients exhibit distinct immunological profiles, including highly activated innate immune cells, reduced numbers of naive T and B cells, and elevated levels of type I and III interferons for up to eight months post-infection [190, 213]. SARS-CoV-2 additionally disrupts epigenetic regulation, impairing the host’s ability to mount effective immune responses, which may contribute to chronic inflammation and increased cancer risk. The virus appears to evade recognition by Toll-like receptor 4 (TLR4), leading to diminished immune responses during the early stages of infection [102, 214].
A vital area of research involves examining the mechanisms through which viral infections, particularly SARS-CoV-2, cause immunosuppression and their implications for tumorigenesis [215]. Immune profiling through the immunophenotyping of peripheral blood mononuclear cells from long COVID patients could reveal changes in immune cell populations, with specific focus on naive T and B cells. Such profiling is crucial for understanding how viral infections may alter immune surveillance mechanisms that typically protect against cancer [216].
Additionally, functional assays should be performed to assess the cytotoxic activity of immune cells co-cultured with cancer cell lines. Evaluating the functional capacity of these immune cells in the context of tumor surveillance will help identify potential deficits in immune responses that may permit tumor development.
Autophagy, a cellular process critical for maintaining homeostasis, is also disrupted by SARS-CoV-2 and other viruses. Several viral proteins interfere with autophagy, preventing its normal induction and causing the accumulation of damaged proteins and organelles, which can create a pro-cancerous environment [217, 218]. Conversely, SARS-CoV-2 can exploit autophagy to degrade major histocompatibility complex I (MHC-I), aiding immune evasion—a strategy also employed by cancer cells [219, 220].
The dysregulation of autophagy by viral proteins warrants further investigation in the context of cancer development. Biochemical assays, such as LC3-II quantification and p62 degradation analysis, should be used to measure autophagy flux in cells infected with SARS-CoV-2 [221, 222]. These assessments will provide insights into how viral infections may disrupt cellular homeostasis and potentially contribute to tumorigenesis.
Moreover, exploring pharmacological agents that enhance autophagic processes in viral-infected models could illuminate potential therapeutic strategies for mitigate cancer risk. Such interventions might restore normal autophagic function and counteract the oncogenic effects of viral infections.
The outcomes of COVID-19 in cancer patients vary significantly based on the type of cancer and the treatments they have undergone (Table 5) [223]. While cancer patients generally face higher mortality rates from COVID-19, these data must be interpreted with caution due to variability in reporting methods. Some studies report overall mortality rates, while others focus on short-term mortality (e.g., 30-day mortality) [27]. Additionally, the strain on healthcare systems has varied across regions, complicating direct comparisons. Despite these discrepancies, the general trends indicate that cancer patients, particularly those undergoing recent or active treatments, remain at heightened risk [224, 225].
COVID-19 outcomes in cancer patients: impact of cancer type and treatment
| Category | Details | Reference |
|---|---|---|
| Overall COVID-19 outcomes in cancer patients | - Cancer patients face higher COVID-19 mortality, with outcomes varying by cancer type and treatment.- Studies report on both overall and short-term mortality rates. | [27, 223, 225] |
| Impact of cancer type on COVID-19 outcomes | Hematologic malignancies:- Worse COVID-19 outcomes, with a 33% mortality rate in a European study.Solid tumors:- High risk in lung, gastrointestinal, and CNS cancers, with lung cancer patients being particularly vulnerable. | [226–231] |
| Cancer treatment and COVID-19 outcomes | Chemotherapy & chemoimmunotherapy:- Increased risk of severe outcomes due to immunosuppression.Immunotherapy, targeted, & endocrine therapies:- Endocrine therapies linked to fewer complications. | [232–235] |
| Role of recent treatments on COVID-19 outcomes | Chemotherapy:- Conflicting data on risk; a UK study found no increased risk, while others reported higher mortality, especially in hematologic cancers.Endocrine & other therapies:- Endocrine therapies are linked to fewer complications. | [236–239] |
| COVID-19 outcomes in cancer patients: research directions | Preventative therapeutics:- Development of agents to counter SARS-CoV-2’s oncogenic effects.Cancer surveillance:- Trials needed to assess long-term cancer risks in COVID-19 survivors.Omics approaches:- Genomic and proteomic research essential for understanding long-term oncogenic effects. | [73, 120, 240] |
COVID-19: coronavirus disease 2019; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
Patients with hematologic cancers, such as leukemia, face significantly worse outcomes when infected with SARS-CoV-2 [226]. Studies have consistently shown higher mortality rates in this group. For instance, a European study reported a 33% mortality rate among patients with hematologic cancers and COVID-19, emphasizing the vulnerability of these patients (Table 5) [227, 228].
Among patients with solid tumors, those with lung, gastrointestinal, and central nervous system cancers are at the highest risk of severe COVID-19 [229]. Lung cancer patients, in particular, show poor outcomes due to the combined impact of underlying respiratory conditions, a history of smoking, and compromised pulmonary function. This is supported by multinational registries like the Thoracic Cancers International COVID-19 Collaboration (TERAVOLT), which noted high hospitalization and mortality rates among lung cancer patients [230, 231].
Patients undergoing recent systemic therapies, especially chemotherapy and chemoimmunotherapy, are at increased risk for severe COVID-19 outcomes. The immunosuppressive effects of chemotherapy likely contribute to worse outcomes in cancer patients [232, 233]. In light of this, improved risk stratification is essential to guide clinical decision-making. Healthcare systems must also allocate resources carefully to manage the higher burden imposed by this vulnerable population (Table 5).
Other cancer therapies, such as immunotherapy, targeted therapy, and endocrine therapy, exhibit more nuanced effects on COVID-19 outcomes [234]. Endocrine treatments, which are often prescribed to healthier patients, have been associated with fewer complications. In contrast, therapies like immunotherapy and targeted therapy need further investigation, as their impacts on COVID-19 outcomes are less clear.
The findings underscore the importance of tailoring cancer treatments to minimize the risk of severe COVID-19 outcomes while ensuring patients continue to receive essential care [235]. Cancer care strategies should consider both the type of cancer and the patient’s overall health, including their immune status and recent treatments. This personalized approach will be critical for optimizing care during pandemics (Table 5) [241].
While some studies have reported an association between recent chemotherapy treatment and worse COVID-19 outcomes, others have not found a clear link [232]. For example, the UK Coronavirus Cancer Monitoring Project found no significant increase in risk from chemotherapy. In contrast, data from the COVID-19 and Cancer Consortium suggested that chemotherapy could elevate mortality risks, particularly in patients with hematologic malignancies (Table 5) [236, 237]. This inconsistency highlights the complexities of assessing cancer treatment risks during COVID-19 and calls for further research to clarify these relationships.
Endocrine therapies are associated with fewer complications in COVID-19 outcomes. This could be attributed to the fact that endocrine treatments are often prescribed to patients with fewer comorbidities, potentially leading to a more favorable response to the virus [238, 239]. However, more research is needed to assess how therapies like immunotherapy and targeted treatments may affect COVID-19 outcomes.
The treatment of cancer in the presence of COVID-19 presents significant challenges, necessitating innovative therapeutic approaches. Nanotechnology-based drug delivery systems have shown great potential in enhancing cancer treatment efficacy while simultaneously addressing SARS-CoV-2 infection. The application of folate-functionalized PLGA-PEG nanoparticles (NPs) loaded with metformin (Met) has demonstrated promising anticancer effects against breast cancer cells, effectively inducing apoptosis and inhibiting tumor growth [242]. Similarly, the co-encapsulation of Artemisinin (Art) and Chrysin (Chr) in PEGylated PLGA NPs has exhibited synergistic anti-proliferative effects on cancer cells [243]. These nanocarriers not only improve drug bioavailability and targeted delivery, but they also offer a potential dual therapeutic strategy. This strategy provides anticancer benefits while mitigating the complications associated with COVID-19 in cancer patients. Given the increased vulnerability of cancer patients to viral infections, integrating nanotechnology into treatment regimens could optimize therapeutic outcomes, enhancing both cancer therapy and viral infection management.
The development of therapeutics to mitigate the oncogenic effects of SARS-CoV-2 or modulate inflammatory responses offers an exciting avenue for research [73]. Such agents could help reduce cancer risks in patients recovering from COVID-19 and improve their overall health outcomes.
Establishing clinical trials focused on cancer surveillance in patients with a history of SARS-CoV-2 infection is crucial [240]. By monitoring these patients for early signs of specific cancers, researchers can better understand the long-term implications of COVID-19 and provide early intervention strategies (Table 5).
Integrative omics approaches (genetic, transcriptomic, proteomic, and metabolomic analyses) are essential for understanding the long-term health impacts of COVID-19 on cancer patients [120, 244]. These approaches can uncover novel biomarkers and pathways, facilitating a better understanding of the virus’s potential oncogenic effects.
While cancer patients face higher mortality rates from COVID-19, there is still much to learn about the complex interplay between cancer types, treatments, and COVID-19 outcomes. Ongoing research into the molecular mechanisms, treatment-related risks, and long-term health impacts of SARS-CoV-2 will be essential in developing evidence-based strategies to manage cancer care after the pandemic (Table 5).
The COVID-19 pandemic has highlighted vulnerabilities in global healthcare systems, particularly in cancer care. It has disrupted cancer treatments, delayed early detection, and impeded preventive measures, leading to lasting impacts on patient outcomes [41]. Looking ahead, research must focus on strategies to ensure the resilience of cancer care systems during global crises (Figure 1). Several key research areas focused on mitigating the long-term effects of healthcare disruptions, improving treatment accessibility, and addressing the challenges posed by long COVID in cancer patients are outlined below and in Figure 1.
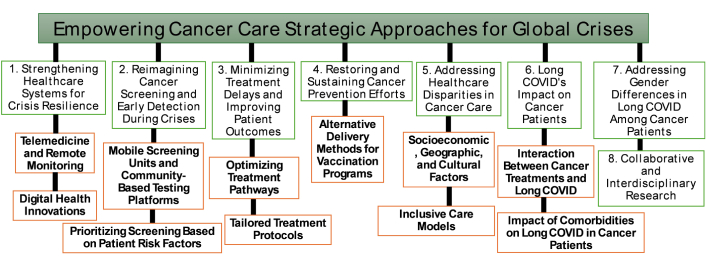
Key research areas in response to the challenges posed by the COVID-19 pandemic on cancer care. COVID-19: coronavirus disease 2019
A fundamental aspect of preparing for future global crises is to build healthcare systems that can withstand and adapt to sudden disruptions [245]. Research should aim to identify and implement strategies that ensure continuity in essential services, such as cancer screening and treatment. Key areas of research include:
Telemedicine and remote monitoring: Telemedicine, remote monitoring, and digital health tools have proven invaluable in maintaining access to healthcare during the pandemic [246]. These technologies can play a crucial role in cancer care during future emergencies. Research should explore the effectiveness of these tools for cancer patients, particularly those at high risk of complications from delayed care. For example, telehealth platforms can facilitate consultations, monitoring of symptoms, and follow-up care, ensuring that cancer patients continue to receive timely medical attention during crises [247].
Digital health innovations: Beyond remote consultations, research should focus on digital health innovations, such as wearable devices that monitor vital signs or artificial intelligence (AI)-based tools that assist in the diagnosis and treatment of cancer. These tools can help provide continuous care even when traditional healthcare facilities are overwhelmed, thus ensuring that cancer patients remain connected to their healthcare providers and receive timely assessments [248, 249].
During periods of healthcare strain, it is critical to reimagine how cancer screening is conducted to ensure early detection and timely intervention. Research should focus on developing new, adaptable screening methods that can be deployed during emergencies, with a focus on:
Mobile screening units and community-based testing platforms: Innovative approaches like mobile screening units or community-based testing platforms can be rapidly mobilized during crises [250]. Research should explore the logistical feasibility, accessibility, and accuracy of these methods, ensuring they reach diverse populations, including those in remote or underserved areas.
Prioritizing screening based on patient risk factors: During periods of crisis, not all cancer screenings can be performed on the same scale as during normal times. Research should focus on how to prioritize screenings based on individual patient risk factors. This will ensure that the most vulnerable populations, such as those with a family history of cancer or other risk factors, receive timely care. This strategy could help mitigate the impact of missed diagnoses and reduce treatment delays.
The impact of treatment delays on cancer progression and mortality is well-established, highlighting the need for future research to prioritize strategies that minimize such delays during crises. Key areas for investigation include:
Optimizing treatment pathways: Research should focus on streamlining diagnostic procedures and treatment protocols to reduce the time to intervention [251]. This could involve implementing triage systems to prioritize high-risk cancer cases and evaluating the feasibility of expediting treatments such as adjuvant chemotherapy. By optimizing treatment pathways, healthcare systems can better manage patient care during times of crisis and reduce the risk of worsened outcomes.
Tailored treatment protocols: Some cancer types may be more vulnerable to the effects of delayed care than others [241, 252]. Research should investigate whether treatment protocols can be adjusted for specific cancer types to mitigate risks during healthcare disruptions. For instance, studies could examine whether certain treatments can be adjusted or delivered more quickly without compromising patient safety or treatment efficacy.
Global crises like the COVID-19 pandemic have disrupted critical cancer prevention programs, such as vaccinations for HPV [253]. These disruptions can lead to an increase in preventable cancers, particularly those linked to viral infections. To ensure continued cancer prevention efforts during emergencies, future research should explore:
Alternative delivery methods for vaccination programs: Research should explore alternative methods of delivering cancer prevention vaccines during crises. For example, school-based or community-wide vaccination initiatives could be developed to ensure that vaccination coverage is maintained even when traditional healthcare settings are compromised [254]. These models would make vaccines more accessible and help maintain high vaccination rates during emergencies.
Long-term impact of disrupted vaccination schedules: Studies should assess the long-term effects of disrupted vaccination schedules, particularly regarding the risk of cancer in populations that miss doses or are delayed in receiving critical vaccines [255]. Understanding these effects will help in developing strategies to recover from gaps in vaccination coverage and to prevent future cancer cases that might have been avoidable.
The COVID-19 pandemic has exacerbated existing healthcare disparities, particularly among underserved populations, making access to timely cancer care even more challenging [225, 256]. Research must identify and address the systemic barriers that contribute to unequal access and treatment delays. Key areas of focus include:
Socioeconomic, geographic, and cultural factors: Research should investigate how socioeconomic, geographic, and cultural factors contribute to unequal access to cancer care. Understanding these factors will help identify the populations most at risk of being disproportionately affected by delays in cancer care and treatment during a global crisis [257].
Inclusive care models: Targeted interventions, such as community-specific outreach programs and pilot models of inclusive care, should be developed to reduce inequities in cancer care [258]. These programs can ensure that vulnerable populations, particularly those in remote or underserved areas, are not left behind during crises and receive the care they need.
The COVID-19 pandemic has resulted in persistent health complications, collectively termed long COVID, which pose significant challenges for vulnerable populations, including cancer patients. Given their compromised immune systems and the physiological strain imposed by oncological treatments, cancer patients may experience heightened susceptibility to the prolonged effects of COVID-19 [259]. Understanding the interplay between long COVID and cancer is essential for developing effective clinical management strategies.
A critical area of investigation involves the interaction between cancer treatments and long COVID. Therapies such as chemotherapy, radiotherapy, and immunotherapy exert profound immunomodulatory effects, which could either exacerbate or mitigate long COVID symptoms [202, 260]. Chemotherapy, by suppressing immune function, may prolong viral persistence, delay viral clearance, and exacerbate systemic inflammation, thereby intensifying long COVID symptoms such as fatigue, cognitive impairment, and organ dysfunction [232]. Additionally, chemotherapy-induced lymphopenia could increase susceptibility to secondary infections, further complicating recovery from long COVID [261]. Radiotherapy, particularly in patients with thoracic malignancies, can cause pulmonary fibrosis and chronic lung injury, potentially compounding respiratory complications associated with long COVID [262]. The impact of immunotherapy, including immune checkpoint inhibitors, on long COVID remains complex. While these agents enhance anti-tumor immunity, they may also contribute to hyperinflammatory states, increasing the risk of autoimmune-like manifestations of long COVID [263]. Furthermore, corticosteroids, commonly used in oncology to manage treatment-related adverse effects, may suppress immune responses to COVID-19 [264], leading to prolonged viral shedding and delayed resolution of symptoms.
Additionally, the presence of comorbidities—including hypertension, diabetes, and cardiovascular disease—compounds the complexity of long COVID in cancer patients. These conditions not only influence acute and post-acute COVID-19 outcomes but may also alter the trajectory of recovery in oncological populations [265, 266]. Chronic systemic inflammation, a hallmark of both long COVID and cancer, may create a tumor-promoting microenvironment by sustaining elevated levels of pro-inflammatory cytokines such as IL-6, TNF-α, and CRP [267]. These inflammatory mediators can contribute to immune evasion, tumor growth, and metastasis, thereby accelerating cancer progression in affected patients. Moreover, endothelial dysfunction and microvascular damage observed in long COVID may impair tissue oxygenation and nutrient supply to tumors, potentially influencing treatment responses and disease progression [268]. Long COVID-associated metabolic disturbances, such as insulin resistance and mitochondrial dysfunction, could exacerbate cancer-associated cachexia, reduce treatment tolerance, and negatively impact overall survival [269]. Identifying predictive biomarkers, such as circulating inflammatory markers or metabolic signatures, could help stratify cancer patients at the highest risk for adverse long COVID outcomes, enabling more targeted and individualized therapeutic approaches.
Future research should prioritize a comprehensive assessment of these factors to inform evidence-based strategies for mitigating long COVID’s impact on cancer patients. A multidisciplinary approach incorporating oncology, immunology, infectious diseases, and precision medicine will be essential in developing targeted interventions. Clinical trials should investigate the long-term impact of COVID-19 on cancer progression, treatment efficacy, and patient survival, particularly in the context of novel immunotherapies and emerging antiviral treatments. Additionally, prospective cohort studies should explore whether specific cancer subtypes or treatment regimens predispose patients to more severe or prolonged manifestations of long COVID.
There is emerging evidence that gender-based differences in immune response and hormonal fluctuations may influence the prevalence and severity of long COVID symptoms, particularly among female cancer patients [270]. Research should explore how these gender differences contribute to the higher incidence of symptoms such as fatigue and sleep disturbances in women. Understanding these differences, alongside the effects of cancer treatments, could lead to more personalized and effective management strategies for long COVID in female cancer patients.
Addressing the complex interplay between cancer care, long COVID, and healthcare disruption requires the formation of interdisciplinary research teams. These teams should bring together expertise from oncology, virology, immunology, neurobiology, and other relevant fields to develop targeted interventions for cancer patients affected by long COVID [1, 271]. Such collaborative efforts will address the immediate challenges faced by cancer patients during times of crisis. They will also provide valuable insights into enhancing the resilience of cancer care systems, particularly in maintaining continuity of care during public health emergencies.
The impact of global crises, such as the COVID-19 pandemic, has underscored the urgent need for comprehensive research into cancer care during periods of disruption [41]. This research must address both the direct and indirect effects of global health crises on cancer patients. It should include disruptions in diagnosis, treatment delays, and the psychological and social impacts of these crises. Future research should prioritize strengthening healthcare system resilience and reimagining cancer screening protocols to adapt to evolving risks. It should also focus on minimizing treatment delays through innovative solutions, restoring prevention efforts that may have been sidelined during crises, addressing healthcare disparities, and understanding the specific impacts of long COVID on cancer treatment outcomes [44, 272]. In this context, global health organizations could play a critical role in developing international frameworks for preparedness. Their efforts could focus on building rapid response capabilities to address cancer care disruptions during emergencies. Additionally, they should ensure equitable access to cancer treatment across diverse populations and foster global collaboration among researchers, healthcare professionals, and policymakers.
Specific recommendations for future preparedness should include the establishment of global databases to monitor cancer patient outcomes during public health crises [273]. This would facilitate data-sharing across borders, helping them to better understand the challenges faced by cancer patients in different regions. Additionally, creating scalable telemedicine platforms to ensure continued access to cancer care remotely, especially in resource-limited settings, will be crucial [274]. Telemedicine can also facilitate remote consultations and follow-up care, alleviating the burden on healthcare facilities during high-demand periods. Another key recommendation is the development of flexible healthcare policies that allow for rapid adaptation in times of emergency. These policies should include adjusting cancer screening protocols, treatment regimens, and prevention efforts based on real-time data and emerging evidence [275].
Through these initiatives, we can ensure that cancer care remains robust and accessible, even in the face of future emergencies. Ultimately, this will safeguard vulnerable populations, improve patient outcomes, and fortify healthcare systems to be more resilient against future global challenges.
The COVID-19 pandemic and previous public health crises have underscored the critical role of policy in sustaining resilient cancer care systems [56, 273]. Effective policymaking ensures that disruptions to oncology treatment are minimized, healthcare infrastructure is reinforced, and equitable access to care is maintained even in times of crisis. By analyzing past interventions, we can identify key strategies for strengthening cancer care in future emergencies.
In response to the pandemic, governments worldwide implemented emergency measures, including expedited telehealth approvals, regulatory flexibility for cancer treatments, and financial support for affected patients [276]. While these adaptations helped mitigate immediate challenges, their long-term effectiveness and integration into routine care remain key areas for evaluation.
Collaboration between governments, pharmaceutical companies, healthcare providers, and research institutions played a crucial role in sustaining cancer care during the pandemic [277]. These partnerships facilitated the rapid development of treatments, stabilized supply chains, and introduced innovative care delivery models [278]. Moving forward, policy efforts should focus on optimizing these collaborations through streamlined clinical trials, improved data-sharing frameworks, and mechanisms that ensure equitable access to oncology therapeutics. Analyzing successful and unsuccessful partnerships can provide valuable insights into improving coordination and efficiency in future health crises.
The pandemic exacerbated existing healthcare disparities, disproportionately affecting vulnerable populations. Examining national and global policies on healthcare equity can help inform more inclusive approaches to oncology care [279, 280]. Key areas of focus include evaluating the effectiveness of subsidized treatment programs, flexible insurance policies, and community-based outreach initiatives. Additionally, research into alternative care delivery models and resource allocation strategies can help ensure that equitable access to cancer treatment [257] remains a priority during future crises.
By integrating lessons from past public health responses, policymakers can develop evidence-based strategies to strengthen cancer care systems. A forward-thinking approach—centered on adaptability, collaboration, and inclusivity—will be essential in ensuring uninterrupted oncology services in the face of future global health challenges.
The COVID-19 pandemic has profoundly impacted global health systems, not only through the direct effects of the virus but also by disrupting essential healthcare services, including cancer prevention, diagnosis, and treatment [1, 2]. Recent epidemiological data suggest a temporal association between the pandemic and an increase in cancer incidence across various regions, with this association being multifactorial (Figure 2). Factors such as delays in screening, interruptions in treatment, and reduced access to healthcare services have all contributed to this rise.
Temporal association between the COVID-19 pandemic and increased cancer incidence. COVID-19: coronavirus disease 2019
A key factor driving the increase in cancer incidence is the delay in diagnoses resulting from the strain placed on healthcare systems. As hospitals became overwhelmed with COVID-19 patients, many elective medical procedures, including routine cancer screenings, were postponed or canceled. This was observed across several countries, including the United States, Italy, Spain, and the United Kingdom, where screening programs for breast, colorectal, and cervical cancers experienced significant declines during the early phases of the pandemic [3–5]. For example, a dramatic drop in cancer screenings was reported in the United States in 2020, with CRC screenings decreasing by more than 80% in some states [6]. This delay in diagnosis led to the identification of cancers at more advanced stages, contributing to higher incidence rates in the years following the pandemic.
Patient behavior also played a crucial role in the observed temporal association. Fear of contracting COVID-19 in healthcare settings caused many individuals to avoid medical consultations and screenings, particularly in regions with high COVID-19 mortality rates [7]. In countries like Brazil and India, where healthcare systems were significantly strained by the pandemic, the number of cancer diagnoses initially fell. However, the postponement of treatments and failure to diagnose cancers early likely resulted in a rise in mortality rates in the years that followed [8, 9]. Vulnerable populations, such as the elderly and individuals with preexisting comorbidities, were particularly affected, as they are at higher risk for certain cancers. As a result, delays in diagnosis and treatment were compounded, and the full impact on cancer incidence may not be fully understood until comprehensive post-pandemic data is available.
The pandemic has also been associated with significant changes in lifestyle behaviors, such as increased smoking, alcohol consumption, and physical inactivity, all of which are well-established risk factors for several types of cancer [10, 11]. Studies conducted in countries like the United States, the United Kingdom, and Canada have shown an increase in tobacco use during the pandemic, likely due to heightened stress and the closure of smoking cessation programs [12, 13]. Additionally, disruptions in dietary habits, increased sedentary behavior, and reduced physical activity were observed worldwide, further contributing to an increased cancer risk. For example, the incidence of lung and CRCs during the pandemic may reflect the compounded effects of these lifestyle changes [14, 15]. In Italy, for instance, a sharp rise in alcohol consumption in 2020 has been linked to an increase in alcohol-related cancers, such as liver and esophageal cancers.
Certain types of cancer appear to have been disproportionately impacted by the COVID-19 pandemic. In countries such as the United Kingdom and Italy, the incidence of lung cancer—strongly associated with smoking—and CRC, linked to dietary and lifestyle factors, increased in the years following the onset of the pandemic [16, 17]. On the other hand, some cancer types, particularly those reliant on early detection programs such as breast cancer, experienced a temporary decline in incidence due to postponed screenings [18, 19]. However, an anticipated surge in diagnoses is expected in the future, as delayed cases are detected. In countries like France and Germany, for example, breast cancer diagnoses sharply fell in 2020, but these declines are expected to reverse as delayed diagnoses are made in the years ahead [20, 21].
The interplay between cancer and COVID-19 has revealed significant challenges, particularly for immunocompromised patients who face increased risks from both acute infection and long-term complications [72, 281]. The pandemic has exacerbated vulnerabilities in cancer care, disrupting treatments and delaying diagnoses, thereby contributing to poorer outcomes [41]. These challenges underscore the urgent need to understand the mechanisms through which SARS-CoV-2 may influence cancer progression and to develop robust mitigation strategies tailored to protect this high-risk group. Additionally, the long-term health consequences of COVID-19, including the emerging phenomenon of long COVID, highlight the necessity of sustained monitoring and comprehensive healthcare planning for cancer patients during and after pandemics [66, 282]. Future research should focus on identifying personalized intervention strategies to mitigate the long-term consequences of SARS-CoV-2 in oncology settings, ensuring that affected individuals receive continuous and adaptive care.
Addressing the unique vulnerabilities of cancer patients requires a multifaceted approach that integrates vaccination, timely treatment, and ongoing support for individuals experiencing persistent symptoms [283, 284]. Given the potential oncogenic risks associated with SARS-CoV-2, continued research into its long-term effects on cancer patients is essential. Such research will not only enhance our understanding of COVID-19’s broader health impacts but will also inform future public health strategies, ensuring that cancer patients receive appropriate protection and care during future pandemics. Effective data collection, alongside the refinement of clinical guidelines, will be crucial for improving patient outcomes and strengthening healthcare system preparedness. Furthermore, integrating AI and machine learning models to predict cancer risk in post-COVID-19 patients could provide valuable insights into early detection and preventive strategies.
A growing body of research investigating the role of viral infections in cancer development underscores the significance of viruses, such as SARS-CoV-2, in modulating long-term health risks, including cancer susceptibility [91, 285]. While traditional oncogenic viruses, such as HPV and HBV, have well-established links to cancer, emerging evidence suggests that SARS-CoV-2 may also exhibit oncogenic potential. This may occur through mechanisms such as chronic inflammation, CS, immune dysregulation, and autophagy disruption [88, 156]. These findings raise critical concerns regarding the potential for SARS-CoV-2 to increase cancer risk, particularly as long COVID becomes an area of significant clinical focus. However, the precise molecular pathways linking COVID-19 to oncogenesis remain poorly understood, necessitating further investigation into the cellular and molecular consequences of SARS-CoV-2 infection. Exploring the interactions between viral proteins and key oncogenic pathways, such as the p53 and PI3K-AKT signaling axes, will be essential for elucidating the mechanistic basis of SARS-CoV-2-driven tumorigenesis.
Longitudinal studies are essential to clarify the relationship between COVID-19 and cancer risk, with an emphasis on monitoring immune responses, inflammatory markers, and cellular stress in recovered patients. Investigating mechanisms such as CS, chronic inflammation, and immunosuppression will provide valuable insights into how SARS-CoV-2 may alter tumor-suppressive pathways, potentially fostering an environment conducive to cancer development [177]. Additionally, MR studies can help identify populations with a genetic predisposition to increased cancer risk following SARS-CoV-2 infection [126]. As the world continues to navigate the pandemic’s aftermath, the potential long-term oncogenic risks posed by COVID-19 highlight the need for interdisciplinary research to inform preventive strategies and therapeutic interventions aimed at mitigating future cancer incidences linked to viral infections. Future efforts should include large-scale biobanking and genome-wide association studies (GWAS) to uncover genetic susceptibilities that may predispose individuals to post-COVID-19 malignancies, facilitating early intervention measures.
The molecular impact of SARS-CoV-2 on cancer development presents a complex and multifaceted challenge. The virus has been implicated in dysregulating key cellular pathways, such as the RAAS and epigenetic mechanisms, both of which are recognized contributors to oncogenesis [286]. SARS-CoV-2’s interactions with tumor suppressors and oncogenes, as well as its role in promoting inflammation and oxidative stress, align with established cancer-promoting mechanisms [287]. However, definitive evidence directly linking SARS-CoV-2 to tumorigenesis remains elusive. Continued exploration of virus-encoded circRNAs, inflammatory cytokines, and viral proteins is critical for understanding their impact on cancer-related gene networks and for developing novel therapeutic strategies to mitigate the potential oncogenic effects of COVID-19 [81, 104]. Additionally, single-cell transcriptomics and spatial proteomics could provide unprecedented insights into how SARS-CoV-2 reshapes the tumor microenvironment, potentially driving malignancies in predisposed individuals.
Future research should prioritize investigating the long-term oncogenic potential of SARS-CoV-2, particularly in cancer survivors and individuals with a history of chronic infection. Comparative studies examining SARS-CoV-2’s oncogenic mechanisms alongside those of established oncogenic viruses will be valuable in identifying shared and unique pathways. Integrative omics approaches, coupled with mechanistic studies of viral protein function, will aid in the identification of biomarkers and pathways relevant to both cancer progression and prevention [288, 289]. Additionally, research into SARS-CoV-2-induced epigenetic changes and molecular interactions between viral proteins and host cell factors will be pivotal in unveiling the full spectrum of its influence on cancer development [102]. This research will ultimately guide personalized care and cancer surveillance strategies. Developing predictive models based on multi-omics data will be crucial for stratifying patients at higher risk for post-viral malignancies and tailoring precision oncology approaches.
Ensuring the continuity and resilience of cancer care during global crises is paramount, as demonstrated by the widespread disruptions experienced during the COVID-19 pandemic [273]. Future research must prioritize strategies that mitigate healthcare interruptions, improve access to screenings and treatments, and address the unique challenges cancer patients face in the context of long COVID. Advancing telemedicine, developing adaptable screening methods, and leveraging digital health innovations will help sustain essential services during crises [290]. Furthermore, targeted efforts to reduce healthcare disparities and explore the long-term effects of COVID-19 on cancer patients are crucial to improving patient outcomes [291]. By integrating real-time epidemiological surveillance with AI-driven predictive modeling, healthcare systems can proactively identify emerging risks and implement timely interventions. Establishing an international research consortia focused on post-viral oncology will be instrumental in accelerating discoveries and translating findings into clinical practice, ensuring a more resilient and equitable healthcare future.
ACE2: angiotensin-converting enzyme 2
AI: artificial intelligence
Ang-2: angiopoietin-2
circRNAs: circular RNAs
COVID-19: coronavirus disease 2019
CRC: colorectal cancer
CS: cellular senescence
EMMPRIN: extracellular matrix metalloproteinase inducer
EMT: epithelial-mesenchymal transition
HPV: human papillomavirus
miRNAs: microRNAs
MR: Mendelian randomization
pRB: protein retinoblastoma
RAAS: renin-angiotensin-aldosterone system
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
SASP: senescence-associated secretory phenotype
MMN and OAA equally contributed to: Conceptualization, Writing—original draft, Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
