Affiliation:
1Department of Oral and Maxillofacial Pathology, Vishnu Dental College, West Godavari 534202, Andhra Pradesh, India
ORCID: https://orcid.org/0000-0002-6286-4029
Affiliation:
2Department of Periodontics and Implantology, Vishnu Dental College, West Godavari 534202, Andhra Pradesh, India
Email: mosups@gmail.com
ORCID: https://orcid.org/0000-0001-7797-1890
Affiliation:
1Department of Oral and Maxillofacial Pathology, Vishnu Dental College, West Godavari 534202, Andhra Pradesh, India
ORCID: https://orcid.org/0000-0001-6952-5014
Affiliation:
1Department of Oral and Maxillofacial Pathology, Vishnu Dental College, West Godavari 534202, Andhra Pradesh, India
ORCID: https://orcid.org/0000-0001-7561-5630
Affiliation:
3Department of Orthodontics, Dentistry Faculty, Trakya University, Edirne 22030, Turkey
ORCID: https://orcid.org/0000-0001-9265-1772
Affiliation:
4Multidisciplinary Department of Medical-Surgical and Dental Specialties, Oral Surgery Unit, University of Campania “Luigi Vanvitelli”, 80138 Naples, Italy
ORCID: https://orcid.org/0000-0002-0915-4642
Affiliation:
5Department of Biomedical and Dental Sciences, Morphological and Functional images, University of Messina, 98125 Messina, Italy
ORCID: https://orcid.org/0000-0003-4619-4691
Affiliation:
6Department of Woman, Child and General and Specialist Surgery, University of Campania “Luigi Vanvitelli”, 80138 Naples, Italy
Email: mariamaddalena.marrapodi@studenti.unicampania.it
ORCID: https://orcid.org/0000-0002-9494-6942
Affiliation:
7Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai 600077, India
8Multidisciplinary Department of Medical-Surgical and Dental Specialties, University of Campania “Luigi Vanvitelli”, 80138 Naples, Italy
Email: giuseppe.minervini@unicampania.it
ORCID: https://orcid.org/0000-0002-8309-1272
Explor Med. 2025;6:1001315 DOI: https://doi.org/10.37349/emed.2025.1001315
Received: January 22, 2025 Accepted: April 14, 2025 Published: May 05, 2025
Academic Editor: Luca Testarelli, Sapienza University of Rome, Italy
The article belongs to the special issue Advances in Oral Cancer: Prevention, Diagnosis, and Therapeutics
Background: Early detection of precancerous oral lesions is crucial for preventing oral cancer. Traditional visual inspections have limitations, prompting the development of advanced technologies to improve detection accuracy. The study focused on evaluating and summarizing recent advancements in optical, molecular, and digital technologies used for the identification of oral potentially malignant disorders (OPMDs).
Methods: A comprehensive literature search was conducted using PubMed, Scopus, and Web of Science, focusing on studies published in English between 2014 and 2024. The search targeted pioneering research addressing key challenges in OPMD detection. Selection criteria prioritized innovative approaches for identifying potentially malignant oral lesions.
Results: The initial search yielded 359 studies, with 10 meeting the inclusion criteria for in-depth analysis. These studies highlight emerging technologies that enhance early detection, including molecular biomarkers for analyzing genetic and protein alterations, liquid biopsy for detecting circulating tumor DNA (ctDNA), and AI-assisted diagnostics. Additionally, fluorescence spectroscopy and optical coherence tomography (OCT) improve detection accuracy, enabling early interventions and better patient outcomes.
Conclusions: The systematic review underscores the growing significance of innovative technologies in the early identification of OPMDs. Advances in optical methods such as fluorescence spectroscopy and OCT, molecular techniques including biomarker analysis and liquid biopsy, and digital innovations like AI-driven diagnostics offer substantial improvements over conventional visual inspection. These technologies not only enhance detection accuracy but also hold promise for earlier diagnosis and improved clinical outcomes. Continued research and validation are essential to translate these emerging tools into routine clinical practice, ensuring timely intervention and effective prevention of oral cancer.
Oral cancer is a serious global health issue, causing around 180,000 deaths and over 350,000 new cases every year. Catching it early is key to keeping patients happy and boosting survival rates. We really need some fresh tech to help spot oral cancer faster, since the usual biopsies and visual checks often slow things down and aren’t always efficient in diagnosing and treating it [1–6].
Oral potentially malignant disorders (OPMDs) such as erythroplakia and leukoplakia precede oral cancer, making their accurate detection and ongoing monitoring critical to prevent malignant transformation. Recent advancements in digital, molecular, and optical technologies promise enhanced early intervention and improved detection accuracy [7–10].
The present review thoroughly assessed the cutting-edge technologies used for detecting OPMDs. Data related to digital technologies [including machine learning models and artificial intelligence (AI)-powered algorithms], biological biomarkers (such as genetic and epigenetic markers), and optical technologies [like fluorescence spectroscopy and optical coherence tomography (OCT)] were examined. The review aimed to elucidate how these innovations had the potential to improve the prevention, treatment, and early detection of oral cancer [11–15].
Innovative tools for detecting OPMDs are revolutionizing early detection methods. AI systems are being utilized to detect precancerous changes in oral lesions using effective image processing, representing a considerable improvement in mouth cancer screening. OCT, which provides high-resolution imaging for assessing subsurface tissues, is another important method [16–19]. Fluorescence spectroscopy studies tissue fluorescence to detect metabolic changes that indicate precancerous lesions, whereas Tissue autofluorescence imaging visualizes fluorescence to identify lesions. Furthermore, Narrow Band Imaging (NBI) highlights vascular patterns, making it easier to detect OPMDs [18, 20–23].
High-resolution micro-endoscopy allows for real-time, high-magnification imaging, making thorough examinations of oral tissues possible. Testing for saliva biomarkers can also detect molecular alterations suggestive of OPMDs [24, 25]. Although Raman spectroscopy examines the molecular composition of tissues to look for OPMDs, diagnostic devices are quick and portable instruments for early detection [26, 27]. Real-time, high-resolution images are provided for a comprehensive evaluation by confocal laser endomicroscopy. Bringing these technologies together really boosts the specificity, sensitivity, and accuracy of spotting OPMDs, making it easier to get timely treatment and jump on things early [28–31].
This research is all about giving policymakers, educators, and healthcare professionals a solid rundown of the latest and greatest in detecting and preventing oral cancer. It examines more of the relevant literature, and provides an overview of some broader advantages, issues, and concerns of new potential methodologies to provide direction on how the knowledge can be potentially applied within clinical and educational systems.
The research question was developed using the PICO (Population, Intervention, Comparison, Outcomes) framework to enhance clarity and focus. A preliminary search of relevant databases was initially conducted to ensure that the research question had not been previously addressed. This search also facilitated the refinement of the study scope and helped identify existing gaps in the literature. Employing the PICO framework allowed for a structured and targeted approach to the research problem, while the preliminary search helped to avoid duplication and ensured that the study contributed meaningfully to the existing body of knowledge.
To guarantee openness, the study protocol was registered on September 16th, 2024, with the International Prospective Register of Systematic Reviews (PROSPERO), using registration ID CRD42024587327 described the initial steps of the review, including the data extraction process, inclusion and exclusion criteria, and the analytic approach.
In this review, only articles published in English were included for consistency, access, and complete analysis. We included randomized controlled trials on new tech for spotting precancerous oral lesions, like optical, molecular, and digital technologies. We also looked at original research papers, case reports, and systematic reviews focused on these innovative methods. On the flip side, we excluded non-English publications, and studies that did not align with the objectives of this review, as well as technical comments, editorial letters, brief communications, and mini-reviews. Limitations: one limitation of this review was the inclusion of only English-language publications. While this approach ensured consistency and facilitated comprehensive analysis, it may have led to the exclusion of relevant studies published in other languages, potentially introducing language bias, and limiting the global scope of the findings.
We launched a search strategy for this systematic review to uncover the most relevant studies related to our topic. The review focused on articles published between 2014 and 2024. The final literature search was completed in November 2024. We narrowed down the search parameters to include the most recent technologies for identifying OPMDs, such as digital, molecular, and optical techniques.
Three reviewers hit the literature hard, looking for papers on innovative tech for identifying these lesions. Digital databases (PubMed, Web of Science, and Scopus) were utilized to identify relevant publications for this focused scoping review, as they are widely recognized for their extensive coverage of biomedical and health sciences literature and provide access to high-quality, pertinent studies. These databases offer access to peer-reviewed research and include a vast amount of journals that are important to our study. To reduce bias, we carried out a comprehensive search using a variety of terms, and MeSH terms to ensure we retrieved all appropriate literature.
Based on our knowledge of the subject and the research publications that were available, we created a list of keywords. The use of the term “innovative technology/advanced technology”, “oral potentially malignant disorders”, and “oral lesions” were used in the early detection of OPMDs that play a crucial role in preventing oral cancer.
This combination of phrases was then used as a search query in databases, including PubMed, Web of Science, and Scopus. By utilizing optical, molecular, and digital technologies to identify OPMDs, this strategy made it easier to reduce the key search criteria. In PubMed, Boolean operators like AND, OR, and NOT were employed to either narrow or broaden the search to include all possible publications. The following search phrases were used in the databases of Scopus, Web of Science, and PubMed.
Innovative [All Fields] AND “technology” [MeSH Terms] OR AND identifying [All Fields] AND precancerous [All Fields] AND “mouth” [MeSH Terms] OR “mouth” [All Fields] OR “oral” [All Fields] AND lesions [All Fields] AND (“systematic review” [All Fields] OR “systematic reviews as topic” [MeSH Terms] OR “systematic review” [All Fields]). Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards as a reference, a flow chart (Figure 1) explains the search strategy’s progression.
After retrieving the articles, relevant studies were carefully selected for inclusion in the systematic review through a meticulous screening process. This review identified and included study designs based on their relevance to the research question, methodological rigor, and ability to provide a more thorough insight into emerging diagnostic technologies for OPMDs. The reviewers prioritized clinical trials, cohort studies, case-control studies, and systematic reviews evaluating the effectiveness, accuracy, and feasibility of these technologies. Any studies rated lower than the acceptable methodological quality or underwent location-based income comparison assessment were not included in the review to safeguard the integrity of these findings. Study selection was performed in duplicate to enhance the reliability and validity of the review.
Authors MKP and SS screened titles and abstracts, while authors RM and SP assessed full-text eligibility, resolving discrepancies with author GM. This data was then used to help develop data collection forms and to generate outlines for the figures and tables that will be used to present the evaluation.
To conduct a systematic review, the two reviewers painstakingly created a pre-tested data extraction form on “Innovative technologies for identifying oral potentially malignant disorders”. As part of the data extraction procedure, a comprehensive form was created to document study specifics such as identification, design, population, intervention, outcomes, results, and quality assessment. For the pre-testing of the data extraction form, five studies were chosen to examine whether the data extraction form adequately captured all the information needed. The studies used a variety of designs and reporting styles to ascertain the form’s comprehensiveness.
Two reviewers separately extracted the data, compared it, and then utilized a consensus meeting to resolve any discrepancies. In the case of non-consented manuscripts, a third reviewer was involved to address any discrepancies that could not be resolved. If an agreement still could not be reached, the reviewer continued to discuss the matter until they were able to arrive at an agreement. Subsequently, the extracted data were systematically entered into a centralized database. This methodical process ensured consistency and reliability, thereby providing a robust foundation for the systematic review. In order to increase the accuracy and consistency of the information obtained, we carried out data extraction in duplicate. This strategy limits the risk of possible errors and biases by having two independent reviewers extract and analyze data for each study.
Statistical analyses were conducted using statistical software (RevMan, version 5.4; Cochrane Collaboration, London, UK) for meta-analysis and pooled effect size calculations. The quality of the included studies was assessed using a critical appraisal tool [Critical Appraisal Skills Programme (CASP); CASP UK, Oxford, UK]. We rolled out the Cochrane Collaboration’s Risk of Bias Tool in this scoping analysis to sniff out any potential risks. By using this risk assessment method, we managed to churn out top-notch articles with solid conclusions. We ran the data analysis using JiraTM software (9.5.0). To evaluate the study’s quality, we leaned on checklists from the CASP, done independently. The final data extraction was handled by two independent observers, and their findings were properly documented. The present study demonstrated strong inter-observer agreement, with a Kappa score of 0.75.
We spotlighted the findings from each study and laid out the unique features of the research papers included in this scoping review.
The review was conducted in accordance with the PRISMA guidelines, along with the PRISMA-S extension, with a focus on emerging technologies for the detection of OPMDs. These cutting-edge technologies have really shaken up the dental scene. This review aims to provide a comprehensive overview of recent innovations in dentistry designed to enhance detection accuracy.
A quick keyword search dug up 359 results. After some initial filtering, we narrowed it down to 125 publications that matched our review’s goals. Out of those, 28 were considered relevant to our standards or objectives. Only ten papers providing systematic and comparative reviews of cutting-edge technology for detecting OPMDs were chosen from among the eligible articles (Figure 1).
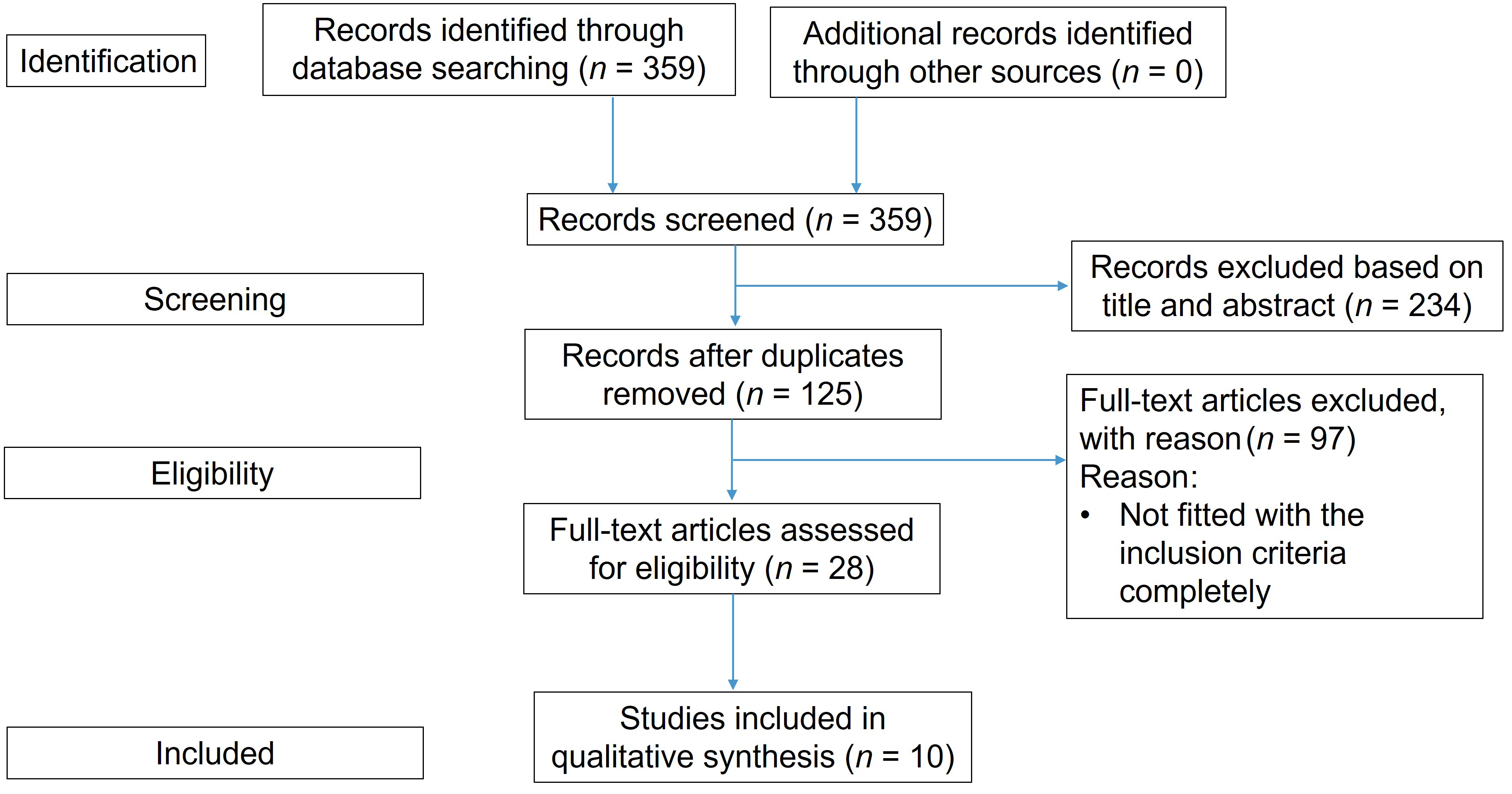
Demonstrates flow diagram of the study selection process as indicated by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)
The research papers and systematic reviews included in this review are categorized based on their purpose and significance. The details about the features of these publications were shown in Table 1.
Description of the qualitative characteristics and conclusive statements of innovative technologies for identifying oral potentially malignant disorders
| Type of study | Authors and journal | Intervention | Outcome measures and summary | Quality assessment score | Research setting |
|---|---|---|---|---|---|
| Review | Tatehara et al. [32], Cancers | Non-invasive diagnostic system based on light | In order to objectively distinguish between high-risk and low-risk OPMDs, it has been demonstrated that the 5-aminolevulinic acid-photodynamic diagnostic (ALA-PDD system) in conjunction with basic imaging processing has a greater sensitivity and specificity for detecting high-risk dysplasia and malignancy. | 7 | Department of Oral Medicine and Stomatology |
| Original Research | Kujan et al. [33], Journal of Oral Science | Oral brush | According to the study’s findings, oral liquid-based cytology with the Orcellex brush may hold great promise for the early diagnosis of precancerous and cancerous oral tissues. | 9 | University Dental Hospital of Manchester, United Kingdom; St Mary’s Hospital, Manchester, United Kingdom |
| Review | Abdul [34], Cureus | Advanced diagnostic aids | The most cutting-edge, forward-thinking diagnostic clinical approaches for advanced oral diagnostics include a multispectral digital microscope, time-resolved, laser-induced fluorescence spectroscopy, spectroscopy of diffuse reflectance, terahertz imaging, hyperspectral imaging, confocal laser endomicroscopy, quantum dots and nanoparticles, bionic sensor, and diagnostic molecular pathology. | 7 | Department of Oral Maxillofacial Surgery (OMFS) and Diagnostic Sciences at the College of Dentistry, Riyadh Elm University, Riyadh, Saudi Arabia |
| Review | Cui et al. [38], Science and Technology of Advanced Materials | Advanced materials and technologies | In this review, the comparison of new and old materials and methodically outlined application techniques in advanced materials and technologies for oral disorders were explained based on the diseases’ etiology. | 7 | Science and Technology Innovation Center, Guangzhou University of Chinese Medicine |
| Systematical Review and Meta-Analysis | Kim et al. [39], Cancers (Basel) | Artificial intelligence (AI) | AI-powered automated lesion identification of malignant lesions in the mouth would be a quick, non-invasive diagnostic technique that might yield instantaneous results on the diagnostic work-up of oral cancer. | 8 | College of Medicine at the Catholic University of Korea |
| Original Research | Liu et al. [42], Oral Oncology | Convolutional neural networks | According to the study’s findings, it is possible to detect premalignant or precancerous oral lesions with computer-assisted detection, setting the stage for potential therapeutic application. | 10 | Rutgers, The State University of New Jersey; Department of Pathology, University of Pittsburgh School of Dental Medicine |
| Original Research | Gattuso et al. [43], Noncoding RNA | Liquid biopsy and circulating biomarkers | This study described the most promising biomarkers and the existing procedures to give an updated overview of the possible use of liquid biopsy as an additional tool for the management of oral lesions. | 9 | Department of Biomedical and Biotechnological Sciences, University of Catania, Catania, Italy |
| Original Research | Malone et al. [44], Cancers (Basel) | In vivo endoscopic optical coherence tomography | The study’s findings indicated that the stratification biomarkers show subsurface alterations, which may be useful in the future for determining therapy margins or choosing biopsy sites. | 9 | Department of Integrative Oncology, British Columbia Cancer Research Institute, Vancouver, BC, Canada |
| Original Research | Rebaudi et al. [45], Front Immunol | Non-invasive cyto-salivary sampling and rapid-highly sensitive ELISA immunoassay | According to the study’s findings, a non-invasive rapid phenotyping technique could be helpful as a screening tool for phenotyping oral lesions and support clinical practice by providing precise indications on the lesion’s characteristics. It could also be used to help patients with OSCC receive new anti-tumor treatments like immunotherapy. | 9 | University of Genova, Genova, Italy |
| Original Research | Saputra et al. [46], International Journal of Medical Science and Clinical Research Studies | VELScope | VELScope has an 83% 5-year survival rate, which contributes to a decrease in oral cancer mortality. Although it has limitations, further technological advancements may increase its precision, making it an essential instrument for precise early diagnosis and a reduction in the death rate from oral cancer. | 9 | Bachelor of Dental Surgery, Faculty of Dentistry, Universitas Jember |
OSCC: oral squamous cell carcinoma; OPMDs: oral potentially malignant disorders
Using the CASP checklist as a guide, we assessed potential biases like publication bias and selective reporting that could mess with the overall data. Once we pulled the data, we did a quality check with the CASP checklist to figure out the pros and cons of the methods used in the studies we found. This evaluation was done independently, focusing on how consistent, transparent, and reliable the research was. The following articles were eliminated from consideration for the study based on specific criteria. Articles numbered 1, 2, 3, 9, 13, 24, and 25 were excluded because they described traditional or antiquated diagnostic methods and did not mention any other technologies for figuring out OPMDs.
As for the results, about 70.0% of the ratings were marked as low risk, 15.0% as uncertain, and 15.0% as high risk, based on the different domains assessed throughout this scoping review (Figure 2).
The review examined 359 different studies, selecting 125 articles. Of these, 10 were significant for their involvement with innovative diagnostic technologies aimed at facilitating OPMDs, namely AI-assisted detection, non-invasive imaging, liquid biopsy, and spectroscopy. These technologies have the ability to improve early detection, sensitivity, and specificity. 70.0% of studies were recognized for having a low risk of bias, while 15.0% were recognized as being uncertain, and only 15.0% were recognized as being high risk. Limitations were noted, such as publication bias and variability among studies.
Timely recognition of OPMDs in the oral cavity is vital to enhancing the likelihood of patient survival and minimizing the impact of oral cancer. Traditional diagnostic procedures using visual examination and biopsy, often involve subjectiveness, invasiveness, or delayed results. Recent advancements in emerging non-invasive diagnostic technologies, such as optical imaging, AI, and liquid biopsy, have been shown to improve accuracy, sensitivity, and specificity when detecting oral lesions. These advances provide exciting new options for improving early detection and clinical decision-making [25–29].
In recent times, we have seen developments in non-invasive optical imaging modalities, for example, the operative efficacy of the 5-aminolevulinic acid-photodynamic diagnostic (ALA-PDD) system, and in vivo endoscopic OCT, among others, to help differentiate high-risk from low-risk oral lesions. AI-based tools, such as convolutional neural networks (CNNs), have appeared as valuable tools in the detection of lesions, and post-detection, high accuracy and efficiency have been reported, particularly with salivary measurement methods [30–32].
In addition to non-invasive imaging and AI tools, cytological and biochemical techniques, like liquid biopsy and cyto-saliva have also shown promise to objectively and non-invasively identify associated biomarkers of early-stage malignancy. Ultimately, spectroscopy-based diagnostic tools are yet another contributor to the rapidly evolving scope of oral cancer detection, by providing valuable molecular information about tissue variability. While embracing these technologies is welcomed, further research, clinical validation and standardization are necessary before routine clinical application can occur [31–33].
The present study demonstrated that emerging technology has the potential to diagnose OPMDs at early stages. Early detection of oral cancer was made possible by using techniques like fluorescence spectroscopy, OCT, and photodynamic diagnostics that have proven to be better than traditional approaches. The molecular biomarkers, such as genetic and epigenetic markers like DNA methylation and miRNA expression, offer helpful insights for pinpointing individuals at high risk for oral cancer [32–37].
Plus, the integration of digital technologies, especially AI-driven algorithms and machine learning models, enhances the analysis of images and data, making it easier to detect issues earlier. Furthermore, liquid biopsies present a non-invasive approach to detecting circulating biomarkers in saliva or blood, adding another layer of convenience and precision [38–41].
A recent study aimed to develop a CNN model capable of identifying regions within whole-slide pathology images that may be indicative of oral epithelial dysplasia (OED). This approach highlights the growing potential of AI in assisting pathologists by providing rapid, consistent, and objective assessments of histopathological features. By accurately pinpointing suspect areas, such models could enhance early detection and improve diagnostic accuracy, ultimately contributing to better patient outcomes in the context of potentially malignant oral disorders. The results of the study concluded that a CNN-based deep learning model was successfully used to identify and classify OED. OPMDs will be diagnosed accurately by computer technology and prove that they can set the stage for future treatment possibilities [42].
Gattuso et al. [43] in 2022 conducted a study to provide an update on how liquid biopsy could be used as a supplementary technique for treating oral cancer. They described existing procedures and highlighted the most promising biomarkers, giving a comprehensive review of liquid biopsy’s potential for controlling oral lesions.
Malone et al. [44] in 2022 conducted a study to investigate imaging biomarkers for oral dysplasia and cancer using in vivo endoscopic optical coherence tomography. The results of the study stated that stratification biomarkers can identify subsurface changes, which could be truly vital for future uses like selecting the best biopsy sites and defining treatment margins. The new technological advances in imaging techniques will help health professionals to become more knowledgeable and can make better decisions about patient care.
Rebaudi et al. [45] in 2023 conducted a study to explore a new method for spotting oral cancer biomarkers. The sample used in this study to diagnose oral cancer was cyto-salivary samples along with a quick, ultra-sensitive ELISA immunoassay. The initial results showed that this quick, non-invasive phenotyping technique could be used in anti-tumor therapies in patients with oral squamous cell carcinoma (OSCC). Moreover, it may enhance the screening and characterization of oral cancer, providing health professionals with detailed insights into oral cancer characteristics that could improve diagnostic accuracy and treatment planning. Overall, this research opens new avenues for integrating cutting-edge technologies into clinical practice.
Saputra et al. [46] in 2024 reviewed the potential of the VELScope as a future dental diagnostic tool for identifying oral cancer. Their study found that the VELScope could reduce oral cancer mortality by up to 83% over five years. Though it has some limitations, further advancements in technology could boost its accuracy, making it an essential device for early diagnosis and lowering oral cancer death rates.
Eshraghi et al. [47] in 2024 conducted a study to know geometry and clinical differences of manually and digitally designed sockets. They found that there were no differences both geometrically and clinical comfort scores between sockets that were designed manually or digitally. Therefore, the feasibility of the research study was found to be successful.
In a study conducted by Simonato et al. [48] in 2019 to evaluate the detection of oral cancer and OPMDs with and without the use of fluorescence visualization (FV) in a population screening program. The study found that FV has high diagnostic values for GPD and increased detection of OPMD in population screening. FV has the potential to be utilized as an adjunctive method for the early diagnosis of oral high-risk lesions.
Tomo et al. [49], in a 2019 review, indicated that the use of FV has increased as an adjunctive tool for the early identification of oral mucosal changes associated with malignancy or pre-malignancy. Currently, the available scientific evidence shows that FV may enhance the early detection of OSCC and OPMDs. As such, we recommended a role for FV in primary health care by general dental practitioners, oral hygienists, and oral health therapists, however further investigation in population screening parameters is still needed.
In 2024, Tomo et al. [50] carried out an investigation on ChatGPT’s ability in the differential diagnosis of oral and maxillofacial diseases. The findings of the study noted that ChatGPT-4 has an accuracy comparable to specialists for providing a differential diagnosis of oral and maxillofacial diseases. The consistency of ChatGPT to offer diagnostic hypotheses for cases of oral disease is moderate, which is a significant limitation for clinical use. The quality of case documentation and case description plays a substantial role in ChatGPT’s ability.
There is no question that innovative technologies can help identify oral premalignant disorders, but their true value will only be reached when these technologies can be made useful for those who will benefit from them most, those who are at the highest risk. By addressing the socioeconomic and infrastructural barriers with a focus on equitable access in mind, we can avoid a missed opportunity of underutilization. Future research should have priority on convenable early detection solutions that are both scalable and cost-effective. Innovative technologies can be used to identify precancerous oral lesions, however, their impact will be contingent upon producing effective approaches to reach high-risk groups. Addressing socioeconomic and infrastructural barriers to the goal of equitable access will be very important to avoid missed opportunity modeling underutilization. Future research should prioritize solutions that focus on early detection that is inexpensive and equitable to benefit the individual while in turn benefitting society [50–52].
The increased accuracy and the ability to identify the early stages of oral cancer lesions are the main characteristics of newer technologies used to identify lesions early on, enabling prompt intervention and maybe better patient outcomes [51–53].
Several limitations must be noted in this systematic review, including the heterogeneity of the studies themselves, making it difficult to draw direct comparisons from the differences in study designs, sample sizes, and diagnosis of the studies. In addition, the findings in this review have limited implications for generalizability because of the lack of large-scale randomized controlled trials and long-term validation of the proposed diagnostic approach.
Future research should focus on developing multimodal techniques that combine many technologies to improve detection accuracy even further. Personalized medicine could play a critical role in adapting detection and treatment procedures to individual patient needs, hence increasing overall efficacy. Raising public and professional knowledge of these breakthrough technologies and their benefits will be critical in promoting their adoption [54–56].
The clinical implications of using novel technologies for detecting OPMDs are significant. These technologies facilitate early identification and prevention, allowing for prompt therapies that can greatly lower the incidence and mortality from oral cancer. These technologies contribute to better patient outcomes by improving detection accuracy and enabling personalized treatment methods, such as higher quality of life and perhaps more successful disease management.
This research deepens the understanding of new diagnostic technologies, including AI-assisted imaging and salivary biomarkers for OPMDs, and allows people to evaluate the evidence critically, whilst clarifying the study limitations, and the applicability of findings to the clinical environment. It also allows the learner to identify practical barriers, such as cost, access, and operator dependence, as well as ethical and regulatory considerations. Gaps are identified that could inspire research and innovation in oral cancer diagnostics.
The systematic review emphasizes the using role of emerging technologies-optical methods (e.g., fluorescence spectroscopy, OCT), molecular methods (e.g., biomarkers, liquid biopsy) and AI-based diagnostics-in the context of earlier OPMD detection. These tools improve accuracy and allow for earlier diagnosis, which may improve outcomes. However, further research and validation is required for adoption into clinical practice to further enhance oral cancer prevention efforts.
AI: artificial intelligence
CNNs: convolutional neural networks
FV: fluorescence visualization
OCT: optical coherence tomography
OED: oral epithelial dysplasia
OPMDs: oral potentially malignant disorders
OSCC: oral squamous cell carcinoma
PICO: Population, Intervention, Comparison, Outcomes
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
SS: Writing—original draft. MKP: Conceptualization, Investigation. RM: Conceptualization. SP: Investigation. HU: Writing—original draft. DR: Writing—original draft. GC: Conceptualization, Writing—original draft, Writing—review & editing, Supervision. MMM: Writing—original draft. GM: Writing—review & editing, Supervision. All authors read and approved the submitted version.
Giuseppe Minervini who is the Guest Editor of Exploration of Medicine had no involvement in the decision-making or the review process of this manuscript. Gabriele Cervino who is the Editorial Board Member of Exploration of Medicine had no involvement in the decision-making or the review process of this manuscript. The other authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
This study does not involve original data, all data analysed are publicly available and have been appropriately cited.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 4235
Download: 77
Times Cited: 0
Yang Liu ... Xinwen Wang
Mohd Javed Naim
Chie Ching Tan ... Spoorthi Ravi Banavar
