Affiliation:
1Department of Pharmacy Practice, National Institute of Pharmaceutical Education and Research (NIPER), Mohali 160062, Punjab, India
ORCID: https://orcid.org/0009-0005-9551-6708
Affiliation:
2Department of Pharmacoinformatics, National Institute of Pharmaceutical Education and Research (NIPER), Mohali 160062, Punjab, India
ORCID: https://orcid.org/0009-0009-4140-2229
Affiliation:
1Department of Pharmacy Practice, National Institute of Pharmaceutical Education and Research (NIPER), Mohali 160062, Punjab, India
Email: ptiwari@niper.ac.in
ORCID: https://orcid.org/0000-0003-0442-7880
Affiliation:
3Department of General Medicine, Government Medical College and Hospital (GMCH), Chandigarh 160030, India
ORCID: https://orcid.org/0000-0001-8831-8002
Affiliation:
4Department of Pathology, Government Medical College and Hospital (GMCH), Chandigarh 160030, India
ORCID: https://orcid.org/0009-0003-8630-6309
Explor Med. 2025;6:1001303 DOI: https://doi.org/10.37349/emed.2025.1001303
Received: December 05, 2024 Accepted: March 20, 2025 Published: April 13, 2025
Academic Editor: Yingyong Zhao, Northwest University, China
Aim: To investigate the impact of socio-demographic characteristics on health-related quality of life (HRQoL) among dialysis (hemodialysis)-dependent chronic kidney disease (DD-CKD) patients. The findings aim to inform strategies for enhancing the overall well-being of CKD patients.
Methods: This cross-sectional study was conducted at the nephrology clinic of GMCH, a public tertiary care hospital in Chandigarh, India, with a random sample of 104 participants. Data were collected using the Kidney Disease Quality of Life-Short Form (KDQOL-SF™) questionnaire and analyzed using SPSS (version 20.0). Baseline characteristics were described with descriptive statistics, and Cronbach’s α measured questionnaire reliability. Independent t-tests and ANOVA were applied to compare HRQoL scores across groups, while simple linear regression, logistic and multiple regression analyses examined associations between the variables.
Results: This study assessed HRQoL in 104 DD-CKD patients (mean age 53.27 ± 13.67 years, HRQoL score 32.70 ± 6.00). Reliability was highest in the effect of kidney disease (EKD) domain (Cronbach’s α = 0.832). Higher incomes were linked to better physical component summary (PCS) (61.33 ± 12.92, p = 0.049), while unmarried patients had higher burden of kidney disease (BKD) scores (28.12 ± 16.47, p = 0.047). Hypertension (74.6%, p = 0.037) and alcohol use (75.0%, p = 0.013) were more common in males > 50 years, while > 50 years females had higher diabetes prevalence (50.0%, p < 0.001). These findings highlight the need for tailored, patient-focused care strategies.
Conclusions: The study concludes that socio-demographics play a crucial role in influencing HRQoL in DD-CKD patients. Higher income levels and marital status were significantly associated with improved HRQoL scores, while age and gender impacted the prevalence of comorbidities and risk behaviors. These findings highlight the need for personalized, patient-centered care strategies to address physical, mental, and social challenges, ultimately improving the HRQoL for this functionally impaired population.
Globally, chronic kidney disease (CKD) affects over 10% of the population, approximately 800 million. In India, adult prevalence is estimated at 17.2%, with diabetic nephropathy leading at 31% of cases [1, 2]. CKD has significant implications for patient health, particularly in its advanced stages, where survival depends on dialysis. This reliance on dialysis, often lifelong, poses substantial physical, emotional, and social challenges [3]. Dialysis affects the patients on multiple dimensions, highlighting the necessity for a comprehensive evaluation of health-related quality of life (HRQoL). Evaluation of HRQoL for a CKD patient provides a patient-centered perspective [4, 5]. In India, approximately 220,000 patients are diagnosed with end-stage renal disease (ESRD) annually, leading to a demand for nearly 34 million dialysis sessions. As of December 2022, approximately 1,350 dialysis facilities have been functional in India. To address the increasing burden, the National Dialysis Program was launched in 2016; this facilitated the addition of 8,000 dialysis stations. Despite these efforts, the unmet demand for dialysis remains highly prevalent, with an estimated 1.75 million individuals projected to be living with end-stage kidney disease (ESKD). Several researchers have emphasised the pressing need for robust public health infrastructure that prioritizes a list of important initiatives that enable early detection, effective management of prevalent risk factors such as diabetes and hypertension (HTN), and the provision of a significant expansion of dialysis facilities to accommodate the rising of CKD patient requiring renal replacement therapy [6–9]. While dialysis is a life-sustaining intervention, it has its own burdens, which include time-intensive procedures, dietary and fluid restrictions, and financial load. These challenges, coupled with the constant need for medical monitoring, disrupt daily routines and impair social interactions. Patients on dialysis often experience symptoms like fatigue, pain, depression, and anxiety, further compromising their well-being. Additionally, socio-demographic factors such as age, gender, marital status, and socioeconomic conditions can have a real impact on these issues, highlighting the multifaceted nature of the challenges faced by dialysis-dependent chronic kidney disease (DD-CKD) patients [10–14]. The interplay between socio-demographic factors significantly shapes HRQoL. Studies have shown that disparities in these variables influence patient experiences, underscoring the importance of tailored care approaches. Clinical parameters, including the type of dialysis, duration of treatment, comorbidities, and disease history, further contribute to variability in HRQoL, emphasizing the need for integrated and personalized care models [15, 16].
It has been well reported that HRQoL among DD-CKD patients is significantly lower compared to the general population. This disparity showcases the urgent need for targeted interventions to improve HRQoL. Dialysis, while essential for managing CKD, often leads to complex health outcomes that influence daily functioning and overall well-being [17–19].
This study, conducted in a tertiary care setting, evaluated HRQoL in DD-CKD patients, focusing on the influence of socio-demographic factors. By examining variables such as past disease history, comorbidities, and social habits, the study attempted to provide actionable insights for enhancing the well-being of this set of patients.
This cross-sectional study was conducted after obtaining ethical clearance from the Institutional Ethics Committee of the National Institute of Pharmaceutical Education and Research, Mohali (IEC/78/2023-RI) and the Government Medical College and Hospital, Chandigarh (GMCH/IEC/2024/1137R). This original research was carried out at a Government Medical College and Hospital (GMCH) located in Chandigarh, India, between April to November 2024, focusing on patients with DD-CKD.
A total of 104 DD-CKD patients were included based on predefined inclusion and exclusion criteria. The inclusion criteria required participants to be between 18 and 70 years old, have completed at least three months of maintenance hemodialysis, and have provided written informed consent to participate in the study. Patients typically underwent twice- or thrice-weekly hemodialysis sessions, with each session lasting 4–5 hours.
The risks and benefits of participation were thoroughly explained to each participant before enrolment, ensuring a clear understanding of the study’s purpose, objectives, and procedures. The exclusion criteria prohibited those patients with a history of malignancy, recent or active blood loss, pregnant or breastfeeding women, psychiatric disorders, and sensory impairments (hearing or speech limitations) [20–23] from participating in this study.
Data collection was done using a structured questionnaire. This included personal identifiers such as unit name and Unique Health Identification (UHID) number, along with demographic information, including age, gender, educational level, occupation, marital status, income, social habits, and health status. These factors were meticulously documented to facilitate a robust analysis. The methodology emphasized rigorous participant selection and systematic data collection, enabling a comprehensive understanding of the factors influencing CKD patients in a tertiary care setting.
This study has utilized a standardized self-administered questionnaire to collect data, incorporating the Kidney Disease Quality of Life-Short Form (KDQOL-SF™) survey. This comprehensive tool consists of two distinct sections: one addressing issues specific to ESRD and another based on the SF-36. Participants primarily completed the questionnaire independently, but assistance was offered by researchers for those who required help, ensuring completeness and accuracy.
The ESRD-specific section included various domains: 12 items measuring symptoms and problem (SP) list, 8 items evaluating the effect of kidney disease (EKD), 4 items assessing the burden of kidney disease (BKD), 11 items covering the physical component summary (PCS), and 8 items addressing the mental component summary (MCS). The SF-36 component examined across multiple domains, with 10 items on physical functioning (PF), 4 on role physical (RP), 2 on bodily pain (BP), 5 on general health (GH), 5 on emotional well-being (EWB), 3 on role emotional (RE), 2 on social function (SF), and 4 on energy and fatigue (EF) [24, 25].
Responses were transformed to a standardized 0–100 scale, where higher scores indicated better health outcomes. However, certain pre-coded items were scored inversely, with lower scores representing more favorable health conditions. This standardized approach enabled a robust and detailed analysis of the HRQoL measures among the patients.
The data collected was analysed using SPSS software (version 20.0). Descriptive statistics, including mean ± standard deviation, percentages, and frequencies, were applied to describe socio-demographic variables, health-related characteristics, and categorical data. Responses from the KDQOL-SF™ survey were examined to determine the proportion of participants encountering difficulties in specific dimensions. Cronbach’s α coefficient was employed to assess the internal consistency and reliability of the subscales.
For statistical comparisons, student t-tests were used to evaluate differences between two groups, while ANOVA was utilized to examine variations across three or more groups. In addition, statistical models, including simple linear regression, logistic regression, and multiple regression analyses, were used to investigate associations between variables. A significance level of p < 0.05 was set to ensure the statistical validity of the results.
The study evaluated HRQoL among 104 dialysis patients with CKD. The average age of the participants was 53.27 ± 13.67 years, and the mean HRQoL score was 32.70 ± 6.00. Males were 67.30% (n = 70), while females were 32.69% (n = 34). Additionally, 87.5% (n = 91) of the patients were from the In-Patient Department (IPD), while 12.5% (n = 13) were from the Out-Patient Department (OPD).
The internal consistency of the variables was assessed using Cronbach’s α, which is a measure of reliability. The findings revealed that the EKD demonstrated the highest reliability (Cronbach’s α = 0.832). The BKD showed acceptable reliability (Cronbach’s α = 0.660). The SP scale and the PCS both demonstrated moderate reliability (Cronbach’s α = 0.513 and 0.524), respectively.
Figure 1 illustrates the multidimensional impact of DD-CKD on HRQoL across various domains, emphasizing differences in physical, mental, and social well-being among patients undergoing dialysis. The physical complications associated with dialysis, such as fatigue, reduced mobility, and musculoskeletal discomfort, profoundly affect overall health and daily functioning. The psychological burden of CKD, including depression, anxiety, and emotional distress, further diminishes patients’ HRQoL. Additionally, the economic burden and the disease’s impact on family life contribute to challenges in daily activities and overall life satisfaction [26, 27].
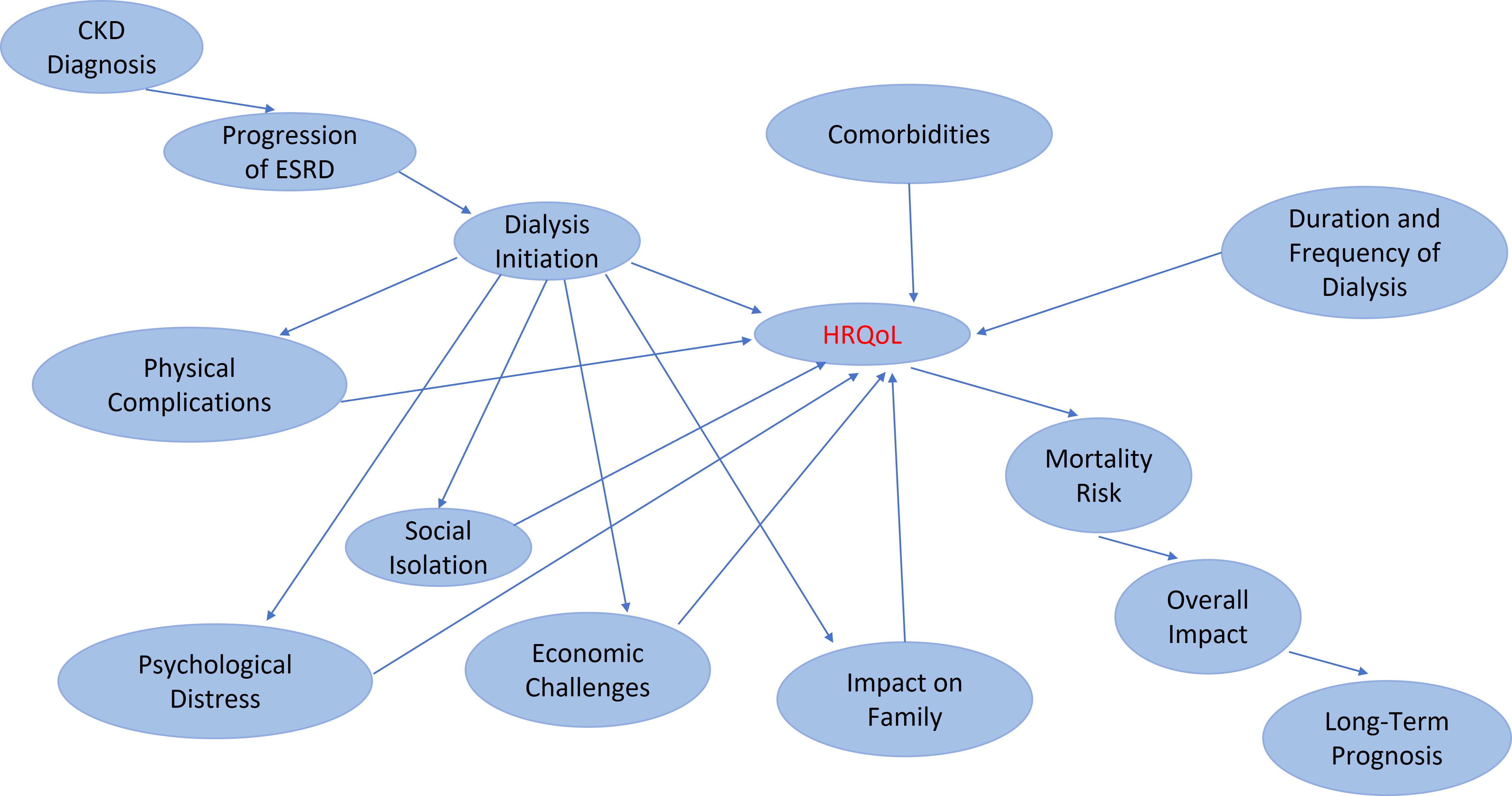
Impact of dialysis-dependent chronic kidney disease (DD-CKD) on health-related quality of life (HRQoL). ESRD: end-stage renal disease
The baseline demographic analysis of ESRD patients using binary logistic regression in Table 1 showed that SP was slightly higher in IPD patients (41.66 ± 13.28) compared to OPD patients (40.56 ± 14.61, p = 0.164). EKD was higher in IPD patients (44.78 ± 9.84) than OPD patients (36.93 ± 15.83, OR = 1.047, p = 0.093), while BKD was lower in IPD patients (13.46 ± 13.46) compared to OPD patients (19.91 ± 16.50, p = 0.369). PCS was statistically significantly higher in IPD patients (58.75 ± 14.66) than in OPD patients (52.12 ± 9.58, OR = 1.066, p = 0.038), whereas MCS showed no statistically significant difference (OR = 0.988, p = 0.765).
Baseline demographics of ESRD-targeted patients analysed using binary logistic regression
| Variables | N | SP | EKD | BKD | PCS | MCS |
|---|---|---|---|---|---|---|
| Patient type | ||||||
| OPD | 91 | 40.56 ± 14.61 | 36.93 ± 15.83 | 19.91 ± 16.50 | 52.12 ± 9.58 | 48.26 ± 8.67 |
| IPD | 13 | 41.66 ± 13.28 | 44.78 ± 9.84 | 13.46 ± 13.46 | 58.75 ± 14.66 | 48.36 ± 5.64 |
| OR | -- | 0.961 | 1.047 | 0.979 | 1.066 | 0.988 |
| p-value | -- | 0.164 | 0.093 | 0.369 | 0.038 | 0.765 |
| Age (years) | ||||||
| ≤ 50 | 35 | 42.32 ± 14.10 | 38.67 ± 16.82 | 17.85 ± 15.69 | 55.70 ± 11.26 | 49.19 ± 7.59 |
| > 50 | 69 | 39.88 ± 14.57 | 37.53 ± 14.73 | 19.74 ± 16.59 | 51.56 ± 9.88 | 47.82 ± 8.70 |
| OR | -- | 0.994 | 1.006 | 1.008 | 0.966 | 0.987 |
| p-value | -- | 0.769 | 0.731 | 0.549 | 0.104 | 0.638 |
| Gender | ||||||
| Male | 70 | 39.82 ± 14.50 | 36.22 ± 15.20 | 20.98 ± 16.20 | 52.22 ± 10.24 | 48.66 ± 8.38 |
| Female | 34 | 42.52 ± 14.20 | 41.39 ± 15.44 | 15.25 ± 15.85 | 54.46 ± 10.99 | 47.50 ± 8.32 |
| OR | -- | 1.001 | 1.019 | 0.981 | 1.018 | 0.975 |
| p-value | -- | 0.942 | 0.283 | 0.189 | 0.417 | 0.350 |
| Marital status | ||||||
| Married | 94 | 40.09 ± 14.69 | 37.46 ± 15.27 | 18.15 ± 16.00 | 52.61 ± 10.67 | 48.17 ± 8.42 |
| Unmarried | 10 | 46.45 ± 9.92 | 42.14 ± 16.81 | 28.12 ± 16.47 | 56.12 ± 8.47 | 49.25 ± 7.82 |
| OR | -- | 1.025 | 1.013 | 1.040 | 1.030 | 0.982 |
| p-value | -- | 0.467 | 0.654 | 0.047 | 0.379 | 0.690 |
| Residential status | ||||||
| Rural | 48 | 41.40 ± 11.99 | 38.40 ± 13.93 | 20.31 ± 17.07 | 53.18 ± 10.88 | 47.42 ± 8.52 |
| Urban | 56 | 40.10 ± 16.25 | 37.50 ± 16.66 | 18.08 ± 15.57 | 52.75 ± 10.25 | 49.01 ± 8.18 |
| OR | -- | 0.993 | 0.996 | 0.989 | 1.033 | 0.641 |
| p-value | -- | 0.682 | 0.793 | 0.389 | 0.794 | 0.209 |
| History of past disease | ||||||
| Yes | 67 | 38.80 ± 14.41 | 36.89 ± 15.15 | 18.75 ± 15.34 | 52.00 ± 10.60 | 47.61 ± 7.76 |
| No | 37 | 44.14 ± 13.89 | 39.76 ± 15.88 | 19.76 ± 17.95 | 54.66 ± 10.22 | 49.49 ± 9.27 |
| OR | -- | 1.024 | 0.997 | 1.003 | 1.014 | 1.014 |
| p-value | -- | 0.218 | 0.839 | 0.818 | 0.508 | 0.605 |
| Recommended for fistula | ||||||
| Yes | 62 | 39.81 ± 14.21 | 39.91 ± 14.99 | 19.05 ± 17.40 | 53.71 ± 10.47 | 48.56 ± 8.78 |
| No | 42 | 42.01 ± 14.73 | 34.95 ± 15.69 | 19.19 ± 14.56 | 51.83 ± 10.56 | 47.86 ± 7.71 |
| OR | -- | 0.954 | 1.046 | 1.006 | 1.024 | 1.009 |
| p-value | -- | 0.021 | 0.015 | 0.643 | 0.275 | 0.729 |
OPD: Out-Patient Department; IPD: In-Patient Department; OR: odds ratio; SP: symptoms and problems; EKD: effects of kidney disease; BKD: burden of kidney disease; PCS: physical component summary; MCS: mental component summary; ESRD: end-stage renal disease. --: no applicable
The HRQoL was also studied with age as a variable. Patients (≤ 50 years) had higher SP (42.32 ± 14.10 vs. 39.88 ± 14.57, OR = 0.994, p = 0.769), EKD (38.67 ± 16.82 vs. 37.53 ± 14.73, OR = 1.006, p = 0.731), and PCS (55.70 ± 11.26 vs. 51.56 ± 9.88, OR = 0.966, p = 0.104), though none were statistically significant different than patients > 50 years of age.
HRQoL among the two-gender indicated that females had higher EKD (41.39 ± 15.44 vs. 36.22 ± 15.20, OR = 1.019, p = 0.283) and PCS (54.46 ± 10.99 vs. 52.22 ± 10.24, OR = 1.018, p = 0.417), while males had higher BKD (20.98 ± 16.20 vs. 15.25 ± 15.85, OR = 0.981, p = 0.189). Further, unmarried patients had significantly higher BKD (28.12 ± 16.47 vs. 18.15 ± 16.00, OR = 1.040, p = 0.047) but statistically insignificant differences in SP (OR = 1.025, p = 0.467), PCS (OR = 1.030, p = 0.379), or MCS (OR = 0.982, p = 0.690). Rural and urban comparisons, as well as past disease history, showed no significant variations across any variables. However, fistula recommendations showed significant variations in SP and EKD (p < 0.05).
Multinomial logistic regression Table 2 demonstrated a significant relationship between monthly income and PCS. Patients earning ₹100,000 had significantly better PCS scores (61.33 ± 12.92, OR = 0.892, p = 0.049) compared to those earning below ₹10,000. Other income groups showed no significant differences across SP, EKD, BKD, PCS, or MCS (p > 0.05). Similarly, education, occupation, and diagnosis type did not demonstrate statistically significant effects on any variable of HRQoL.
Baseline demographics of ESRD-targeted patients analysed using multinomial logistic regression
| Variables | N | SP | OR(p-value) | EKD | OR(p-value) | BKD | OR(p-value) | PCS | OR(p-value) | MCS | OR(p-value) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Education | |||||||||||
| Illiterate | 38 | 41.61 ± 15.37 | Reference. | 39.20 ± 14.85 | Reference. | 18.25 ± 13.96 | Reference. | 52.05 ± 10.14 | Reference. | 48.23 ± 9.24 | Reference. |
| Matric pass | 48 | 38.97 ± 11.82 | 0.995 (0.788) | 34.30 ± 14.39 | 0.980 (0.227) | 22.26 ± 18.59 | 0.993 (0.800) | 52.41 ± 9.97 | 0.300 (1.015) | 47.66 ± 7.36 | 1.011 (0.645) |
| Graduate | 12 | 46.35 ± 17.68 | 0.966 (0.274) | 49.70 ± 17.46 | 1.051 (0.113) | 13.54 ± 12.45 | 1.040 (0.382) | 59.49 ± 11.86 | 0.982 (0.490) | 52.01 ± 8.79 | 1.058 (0.110) |
| Beyond graduate | 6 | 37.50 ± 19.85 | 0.998 (0.964) | 35.11 ± 12.45 | 0.979 (0.580) | 10.41 ± 11.63 | 0.986 (0.799) | 49.92 ± 11.87 | 0.959 (0.263) | 46.04 ± 8.73 | 0.986 (0.787) |
| Occupation | |||||||||||
| Student | 5 | 45.83 ± 11.12 | Reference. | 40.71 ± 21.36 | Reference. | 22.50 ± 12.96 | Reference. | 57.81 ± 4.61 | Reference. | 48.08 ± 5.80 | Reference. |
| Employed | 27 | 40.58 ± 12.83 | 0.978 (0.655) | 36.24 ± 15.80 | 0.992 (0.841) | 21.52 ± 16.29 | 0.965 (0.437) | 53.22 ± 10.67 | 1.030 (0.644) | 48.25 ± 8.24 | 0.991 (0.764) |
| Unemployed | 72 | 40.39 ± 15.21 | 0.972 (0.549) | 38.34 ± 15.00 | 1.003 (0.928) | 17.96 ± 16.48 | 0.957 (0.311) | 52.51 ± 10.72 | 1.034 (0.585) | 48.30 ± 8.60 | 0.979 (0.450) |
| Monthly income (₹) | |||||||||||
| 10,000 | 66 | 39.99 ± 15.49 | Reference. | 38.37 ± 15.78 | Reference. | 19.98 ± 17.27 | Reference. | 53.41 ± 10.43 | Reference. | 48.01 ± 8.61 | Reference. |
| 25,000 | 22 | 42.42 ± 8.96 | 1.034 (0.149) | 36.68 ± 12.18 | 0.978 (0.283) | 19.31 ± 13.89 | 1.006 (0.855) | 50.55 ± 9.64 | 0.993 (0.676) | 48.16 ± 6.85 | 0.962 (0.167) |
| 50,000 | 10 | 37.70 ± 16.29 | 1.007 (0.816) | 32.50 ± 15.65 | 0.968 (0.256) | 21.25 ± 14.79 | 1.041 (0.391) | 50.19 ± 9.75 | 1.002 (0.938) | 49.50 ± 8.91 | 0.967 (0.378) |
| 100,000 | 6 | 47.22 ± 15.57 | 1.008 (0.855) | 46.42 ± 20.70 | 0.995 (0.910) | 5.20 ± 10.01 | 1.028 (0.647) | 61.33 ± 12.92 | 0.892 (0.049) | 49.65 ± 11.00 | 1.063 (0.188) |
| Diagnosis | |||||||||||
| Freshly diagnosed | 66 | 41.57 ± 14.53 | Reference. | 38.41 ± 14.85 | Reference. | 19.60 ± 16.18 | Reference. | 52.86 ± 10.65 | Reference. | 49.24 ± 8.17 | Reference. |
| Known case | 35 | 38.80 ± 14.44 | 0.994 (0.769) | 35.82 ± 16.38 | 0.994 (0.740) | 18.39 ± 17.21 | 0.957 (0.117) | 52.14 ± 9.74 | 0.997 (0.841) | 46.15 ± 8.57 | 1.003 (0.882) |
| Referred case | 3 | 43.75 ± 12.67 | 0.921 (0.186) | 51.19 ± 11.48 | 1.096 (0.135) | 16.66 ± 3.60 | 1.016 (0.849) | 64.39 ± 12.71 | 1.007 (0.883) | 51.80 ± 5.68 | 1.115 (0.081) |
The value in parentheses represents the p-values across the different groups. Reference category 1. OR: odds ratio; SP: symptoms and problems; EKD: effects of kidney disease; BKD: burden of kidney disease; PCS: physical component summary; MCS: mental component summary; ESRD: end-stage renal disease
The baseline characteristics of the SF-36 domains for ESRD patients analysed in Table S1 using binary logistic regression showed statistically insignificant differences in PF between IPD and OPD patients (22.31 ± 11.10 vs. 22.69 ± 14.47, OR = 0.999, p = 0.956). Role physical (RP) was higher in IPD patients (11.54 ± 19.40 vs. 9.07 ± 20.24, OR = 1.009, p = 0.568), while BP was lower (31.15 ± 14.38 vs. 39.36 ± 15.68, OR = 0.957, p = 0.058). GH, EWB, RE, and SF showed statistically insignificant differences between IPD and OPD patients (p > 0.05). For age, ≤ 50 years had significantly better GH (29.74 ± 10.72 vs. 20.65 ± 12.74, OR = 0.927, p < 0.001) and SF (38.57 ± 15.27 vs. 34.42 ± 15.37, OR = 0.962, p = 0.041), while > 50 years of patients had higher EWB (49.04 ± 9.20 vs. 46.29 ± 8.47, OR = 1.076, p = 0.017). Gender comparisons showed males had higher PF (24.07 ± 14.37 vs. 19.71 ± 13.08, OR = 0.971, p = 0.100), while females had marginally better GH (24.59 ± 12.17 vs. 23.28 ± 13.15, OR = 1.015, p = 0.382). Unmarried patients had significantly higher RP (20.00 ± 25.82 vs. 8.24 ± 19.18, OR = 1.034, p = 0.049), with statistically insignificant differences in other domains. Patients without a history of past disease had higher GH (29.76 ± 11.00 vs. 20.37 ± 12.56, OR = 1.085, p < 0.001) and SF (41.21 ± 14.69 vs. 32.83 ± 15.04, OR = 1.068, p = 0.002), but slightly lower EF (39.05 ± 9.63 vs. 41.34 ± 12.32, OR = 0.945, p = 0.030). Fistula recommendations were not significantly associated with most variables, except for SF, which was higher in patients not recommended for fistula (39.88 ± 15.67 vs. 33.06 ± 14.68, OR = 0.963, p = 0.018). Patients from rural or urban settings were found to match across SF-36 domains (p > 0.05).
Table S2 (the multinomial logistic regression analysis) showed statistically insignificant differences across education, occupation, or monthly income categories for any SF-36 domains (p > 0.05). Diagnosis type revealed no major differences, although known cases had slightly lower BP (35.64 ± 14.97) compared to freshly diagnosed patients (40.15 ± 15.94, OR = 0.982, p = 0.259).
The correlation analysis of key variables in ESRD patients, as presented in Table 3, shows that the PCS showed a weak positive correlation with EKD (r = 0.211, p = 0.032), SP (r = 0.321, p < 0.001), and MCS (r = 0.203, p = 0.038). EKD exhibited a moderate correlation with SP (r = 0.576, p < 0.001) and a weak correlation with MCS (r = 0.202, p = 0.039), highlighting its influence on social and mental health dimensions. SP was weakly correlated with MCS (r = 0.269, p = 0.006), while BKD showed no significant correlations with other variables (p > 0.05). These findings highlight the interconnectedness of physical, social, and mental health aspects in ESRD patients, guiding integrated care strategies.
Correlation analysis of ESRD
| Variables | Pearson correlation (r) | PCS (N = 104) | EKD (N = 104) | SP (N = 104) | MCS (N = 104) | BKD (N = 104) |
|---|---|---|---|---|---|---|
| PCS | r | 1 | 0.211* | 0.321** | 0.203* | 0.036 |
| p-value | -- | 0.032 | < 0.001 | 0.038 | 0.717 | |
| EKD | r | 0.211* | 1 | 0.576** | 0.202* | 0.132 |
| p-value | 0.032 | -- | < 0.001 | 0.039 | 0.181 | |
| SP | r | 0.321** | 0.567** | 1 | 0.269** | 0.024 |
| p-value | < 0.001 | < 0.001 | -- | 0.006 | 0.807 | |
| MCS | r | 0.203* | 0.202* | 0.269** | 1 | 0.103 |
| p-value | 0.038 | 0.039 | 0.006 | -- | 0.296 | |
| BKD | r | 0.036 | 0.132 | 0.024 | 0.103 | 1 |
| p-value | 0.717 | 0.181 | 0.807 | 0.296 | -- |
*Correlation is significant at the 0.05 level (2-tailed). ** Correlation is significant at the 0.01 level (2-tailed). SP: symptoms and problems; EKD: effects of kidney disease; BKD: burden of kidney disease; PCS: physical component summary; MCS: mental component summary; ESRD: end-stage renal disease. --: not applicable
The correlation among SF-36 health survey domains in ESRD patients Table 4 showed that PF was statistically significantly correlated with GH (r = 0.248, p = 0.011) and BP (r = 0.219, p = 0.026), suggesting that better PF is associated with improved GH and BP levels. RP showed a moderate correlation with RE (r = 0.334, p < 0.001), indicating a close relationship between the PF and EWB in daily functioning. Additionally, SF showed weak correlations with EWB (r = 0.228, p = 0.020) and BP (r = 0.207, p = 0.035). These results emphasise the comprehensive nature of physical, emotional, and social health in ESRD management.
Correlation analysis of SF-36 items targeted area
| Variables | Pearson correlation (r) | PF (N = 104) | RP (N = 104) | RE (N = 104) | SF (N = 104) | BP (N = 104) | EWB (N = 104) | EF (N = 104) | GH (N = 104) |
|---|---|---|---|---|---|---|---|---|---|
| PF | r | 1 | 0.182 | 0.213* | 0.158 | 0.219* | 0.114 | 0.005 | 0.248* |
| p-value | -- | 0.064 | 0.030 | 0.109 | 0.026 | 0.250 | 0.961 | 0.011 | |
| RP | r | 0.182 | 1 | 0.334** | 0.007 | 0.007 | 0.074 | 0.147 | 0.043 |
| p-value | 0.064 | -- | < 0.001 | 0.941 | 0.940 | 0.453 | 0.136 | 0.667 | |
| RE | r | 0.213* | 0.334** | 1 | 0.199* | 0.031 | 0.040 | 0.171 | 0.054 |
| p-value | 0.030 | < 0.001 | -- | 0.043 | 0.757 | 0.688 | 0.082 | 0.588 | |
| SF | r | 0.158 | 0.007 | 0.199* | 1 | 0.207* | 0.228* | 0.157 | 0.137 |
| p-value | 0.109 | 0.941 | 0.043 | -- | 0.035 | 0.020 | 0.112 | 0.164 | |
| BP | r | 0.219* | 0.007 | 0.031 | 0.207* | 1 | 0.155 | 0.037 | 0.100 |
| p-value | 0.026 | 0.940 | 0.757 | 0.035 | -- | 0.115 | 0.707 | 0.314 | |
| EWB | r | 0.114 | 0.074 | 0.040 | 0.228* | 0.155 | 1 | 0.131 | 0.054 |
| p-value | 0.250 | 0.453 | 0.688 | 0.020 | 0.115 | -- | 0.185 | 0.588 | |
| EF | r | 0.005 | 0.147 | 0.171 | 0.157 | 0.037 | 0.131 | 1 | 0.058 |
| p-value | 0.961 | 0.136 | 0.082 | 0.112 | 0.707 | 0.185 | -- | 0.555 | |
| GH | r | 0.248* | 0.043 | 0.054 | 0.137 | 0.100 | 0.054 | 0.058 | 1 |
| p-value | 0.011 | 0.667 | 0.588 | 0.164 | 0.314 | 0.588 | 0.555 | -- |
*Correlation is significant at the 0.05 level (2-tailed). **Correlation is significant at the 0.01 level (2-tailed). PF: physical functioning; RP: role physical; RE: role emotional; SF: social function; BP: bodily pain; EWB: emotional well-being; EF: energy and fatigue; GH: general health. --: not applicable
The simple linear regression analysis depicted statistically significant associations between variables Table 5. SP showed a strong relationship (B = 0.263, t = 8.207, p < 0.001), accounting for 39.8% of the variance (R² = 0.398, F = 67.35). Similarly, the EKD demonstrated a significant impact (B = 0.184, t = 5.418, p < 0.001, R² = 0.223, F = 29.35), while the BKD contributed moderately (B = 0.131, t = 3.831, p < 0.001, R² = 0.126, F = 14.67).
Simple linear regression results of key variables related to ESRD and the SF-36 item health survey
| Variables | B (Beta) coefficients | t-Value | p-Value | R Square (R²) | F-value |
|---|---|---|---|---|---|
| SP | 0.263 | 8.207 | < 0.001 | 0.398 | 67.35 |
| EKD | 0.184 | 5.418 | < 0.001 | 0.223 | 29.35 |
| BKD | 0.131 | 3.831 | < 0.001 | 0.126 | 14.67 |
| PCS | 0.192 | 3.593 | < 0.001 | 0.112 | 12.90 |
| MCS | 0.355 | 5.726 | < 0.001 | 0.243 | 32.79 |
| PF | 0.252 | 7.380 | < 0.001 | 0.348 | 54.46 |
| RP | 0.142 | 5.419 | < 0.001 | 0.224 | 29.36 |
| RE | 0.173 | 5.639 | < 0.001 | 0.238 | 31.79 |
| SF | 0.102 | 2.734 | 0.007 | 0.068 | 17.47 |
| BP | 0.167 | 4.912 | < 0.001 | 0.191 | 24.13 |
| EWB | 0.126 | 1.947 | 0.054 | 0.036 | 13.79 |
| EF | 0.175 | 3.582 | < 0.001 | 0.112 | 12.83 |
| GH | 0.216 | 5.250 | < 0.001 | 0.213 | 27.56 |
PF: physical functioning; RP: role physical; RE: role emotional; SF: social function; BP: bodily pain; EWB: emotional well-being; EF: energy and fatigue; GH: general health; SP: symptoms and problems; EKD: effects of kidney disease; BKD: burden of kidney disease; PCS: physical component summary; MCS: mental component summary; ESRD: end-stage renal disease
PCS (B = 0.192, t = 3.593, p < 0.001, R² = 0.112, F = 12.90) and MCS (B = 0.355, t = 5.726, p < 0.001, R² = 0.243, F = 32.79) were both significant, with MCS showing a stronger association. PF (B = 0.252, t = 7.380, p < 0.001, R² = 0.348, F = 54.46) and RP (B = 0.142, t = 5.419, p < 0.001, R² = 0.224, F = 29.36) also exhibited substantial impacts.
Other key variables such as RE (B = 0.173, t = 5.639, p < 0.001, R² = 0.238, F = 31.79), BP (B = 0.167, t = 4.912, p < 0.001, R² = 0.191, F = 24.13), and GH (B = 0.216, t = 5.250, p < 0.001, R² = 0.213, F = 27.56) were statistically significantly associated with HRQoL. SF (B = 0.102, t = 2.734, p = 0.007, R² = 0.068, F = 17.47) and EF (B = 0.175, t = 3.582, p < 0.001, R² = 0.112, F = 12.83) also showed notable contributions. However, EWB (B = 0.126, t = 1.947, p = 0.054) was statistically insignificant. These findings confirm that physical, mental, and social health factors significantly influence HRQoL in ESRD patients, emphasising the need for comprehensive targeted interventions to improve the HRQoL.
The HRQoL data was analysed with respect to comorbidity and social habits.
The comorbidity patterns of DD-CKD patients in Table 6 were categorized by age and gender. In males, HTN was significantly higher in male patients > 50 years (74.6%) compared to ≤ 50 years (25.4%, p = 0.037) as was polycystic kidney disease (PKD) (76.5% vs. 23.5%, p = 0.028) and pyelonephritis (76.0% vs. 24.0%, p = 0.029). In females, HTN was evenly distributed across age groups (50.0%, p = 0.034), while diabetes showed a significant age-related difference (50.0% in ≤ 50 years vs. 50.0% in > 50 years females, p < 0.001). Glomerulonephritis was present among the female patients (50.0%, p < 0.001). These results indicate a higher prevalence of certain comorbidities, particularly HTN and PKD, in > 50 years males.
Comorbidity profile analysis by age and gender
| Variables | Age (years) | HTN, N (%) | Diabetes, N (%) | PKD, N (%) | Glomerulonephritis, N (%) | Pyelonephritis, N (%) | Others, N (%) |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Male | ≤ 50 | 17 (25.4) | 11 (21.6) | 4 (23.5) | 7 (33.3) | 6 (24.0) | 11 (23.9) |
| > 50 | 50 (74.6) | 40 (78.4) | 13 (76.5) | 14 (66.7) | 19 (76.0) | 35 (76.1) | |
| p-value | 0.037 | 0.154 | 0.028 | 0.113 | 0.029 | 0.057 | |
| Female | ≤ 50 | 17 (50.0) | 14 (50.0) | 5 (45.5) | 3 (50.0) | 10 (58.8) | 10 (43.5) |
| > 50 | 17 (50.0) | 14 (50.0) | 6 (54.5) | 3 (50.0) | 7 (41.2) | 13 (65.5) | |
| p-value | 0.034 | < 0.001 | 0.063 | < 0.001 | 0.174 | 0.185 | |
Other include urinary tract infection (UTI), pancreatitis, drug use, hepatitis, pedal edema, and obstruction. HTN: hypertension; PKD: polycystic kidney disease
In males, smoking was more prevalent in > 50 years (80.6%) than ≤ 50 years (19.4%, p = 0.146), while alcohol consumption was significantly greater in > 50 years males (75.0% vs. 25.0%, p = 0.013). Tobacco use was also elevated in > 50 years males (78.9% vs. 21.1%, p = 0.065), and other risk behaviors (poor dietary habits, physical inactivity, inadequate hydration, drug use, and non-adherence to medication) were more common (72.4% vs. 27.6%, p = 0.036). Among females, smoking and alcohol use were higher in > 50 years (71.4% vs. 28.6%, p = 0.338; 73.3% vs. 26.7%, p = 0.383, respectively), with similar trends in tobacco use (66.7% vs. 33.3%, p = 0.152) and other risk behaviors (61.5% vs. 38.5%, p = 0.179) (Table 7).
Behavioral risk factors distribution across age and gender categories
| Variables | Age (years) | Smokers, N (%) | Alcoholic, N (%) | Tobacco users, N (%) | Others, N (%) |
|---|---|---|---|---|---|
| Gender | |||||
| Male | ≤ 50 | 7 (19.4) | 7 (25.0) | 4 (21.1) | 8 (27.6) |
| > 50 | 29 (80.6) | 21 (75.0) | 15 (78.9) | 21 (72.4) | |
| p-value | 0.146 | 0.013 | 0.065 | 0.036 | |
| Female | ≤ 50 | 4 (28.6) | 4 (26.7) | 2 (33.3) | 5 (38.5) |
| > 50 | 10 (71.4) | 11 (73.3) | 4 (66.7) | 8 (61.5) | |
| p-value | 0.338 | 0.383 | 0.152 | 0.179 | |
Other include poor dietary habits, physical inactivity, inadequate hydration, drug use, and non-adherence to medication
Table S3 illustrates a statistically significant association (p < 0.05) in the distribution of past disease history across diagnostic categories. Among freshly diagnosed cases, 44.8% (N = 30) reported a history of past diseases, whereas 97.3% (N = 36) did not; this comprised 63.5% (N = 66) of the total sample studied. In the ‘known’ case group, 52.2% (N = 35) reported a past disease history, with none reporting an absence, accounting for 33.7% (N = 35) of the total. Referred cases represented 2.9% (N = 3) of the total patient set, with 3.0% (N = 2) having a history of past diseases and 2.7% (N = 1) without.
The findings indicate that the most common singular social habit among the patients in the study is smoking, observed in 48.07% (N = 50) of patients. This was followed by alcohol use, reported in 41.34% (N = 43) of patients. The least prevalent habit is tobacco use, noted in 24.03% (N = 25) of patients. Additionally, 40.38% (N = 42) of patients fall into the “Others” category, which encompasses various distinct habits. This distribution highlights smoking as the most pervasive habit among DD-CKD patients, with alcohol being the second most prevalent.
Figure 2 is a detailed analysis of the frequency distribution of various social habits and their combinations among the patients in the study. The most prevalent combination is smoking and alcohol consumption (S+A), reported in 33.65% (N = 35) of patients, followed by smoking with other habits (S+O), observed in 28.84% (N = 30) of patients. Moderate frequencies were recorded for smoking and tobacco use (S+TU), found in 17.30% (N = 18) of patients, and alcohol combined with other habits (A+O), observed in 22.11% (N = 23) patients. More complex combinations, such as smoking, alcohol, and tobacco use (S+A+TU), were reported in 9.61% (N = 10) of patients, and all four habits combined (S+A+TU+O), found in 7.69% (N = 8) of patients, were less frequent.
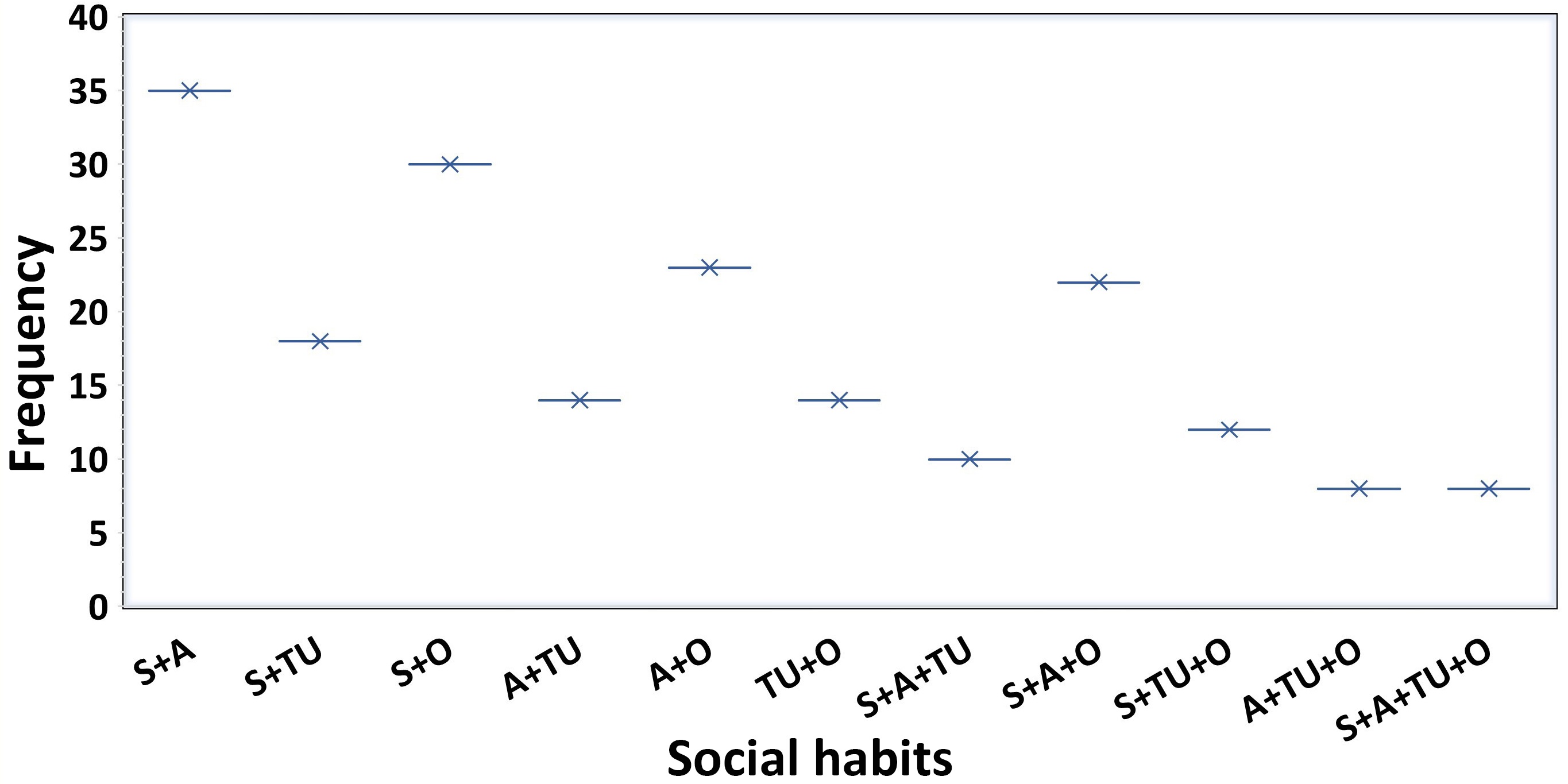
Frequency distribution of social habits among patients. Smoker (S), tobacco user (TU), alcohol consumer (A), and others (O). Other include poor dietary habits, physical inactivity, inadequate hydration, drug use, and non-adherence to medication
The distribution of comorbidities in the study (Figure 3) shows significant trends. HTN was the most prevalent comorbidity, affecting 97.11% (N =101) of patients, highlighting its dominance as a comorbid condition in the patient. Diabetes follows, impacting 75.96% (N = 79) of patients, reflecting its substantial role as a comorbidity. Other noteworthy conditions include pyelonephritis, present in 40.38% (N = 42) patients, PKD in 26.92% (N = 28) patients, and glomerulonephritis in 25.96% (N = 27) patients. Additionally, 66.34% (N = 69) of patients fall under the category of “Others”. These findings underscore the prevalent and diverse health challenges to improve patient outcomes.
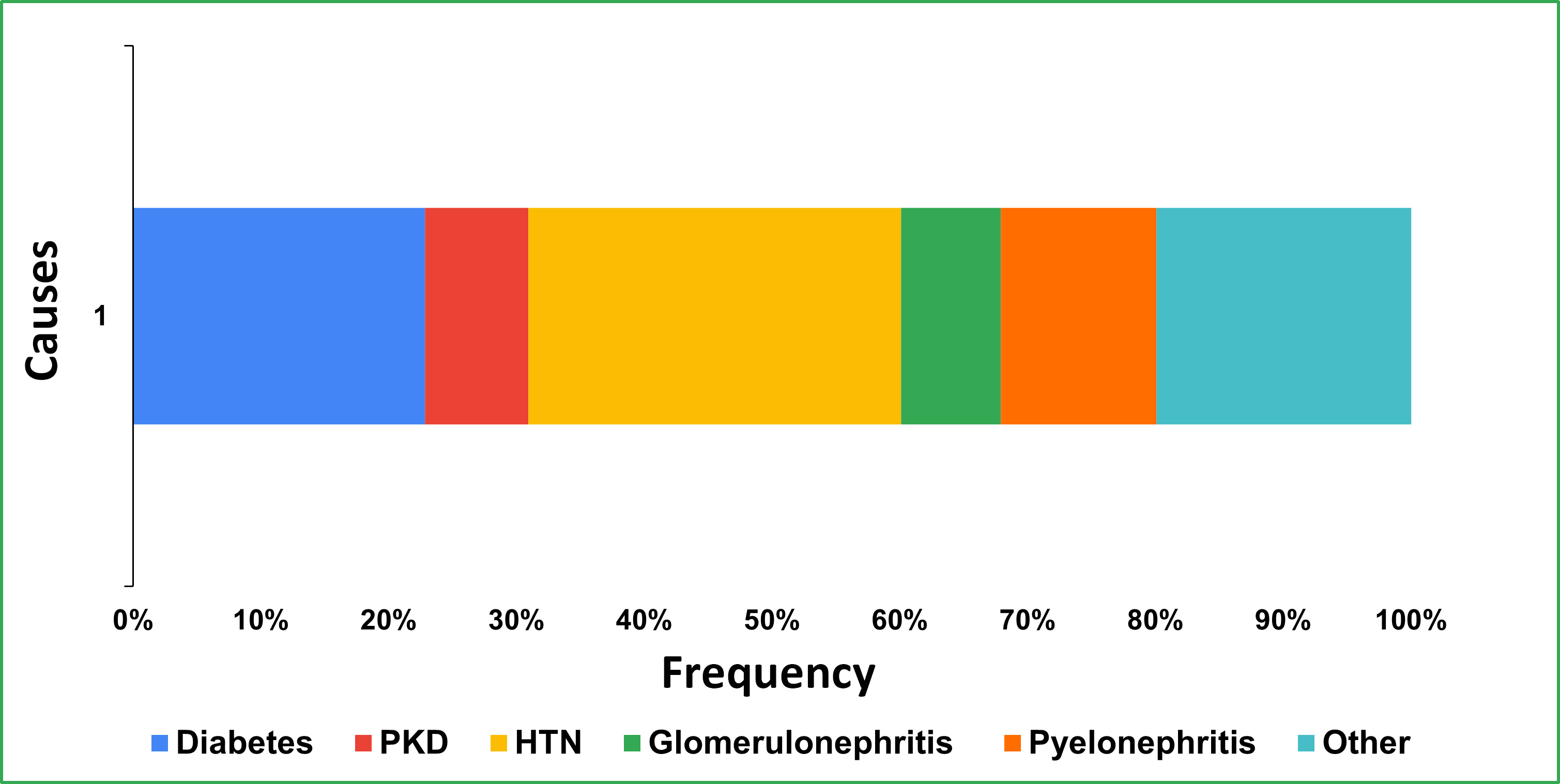
Distribution of underlying causes among patients. PKD: polycystic kidney disease; HTN: hypertension
This study emphasizes the complex interplay of socio-demographic and behavioral factors influencing the HRQoL in DD-CKD patients. The findings reveal a mean HRQoL score of 32.70 ± 6.00, substantially lower than that of the general population, reflecting the profound physical, emotional, and social burdens of dialysis dependency. Rincon Bello et al. [3] studied HRQoL in dialysis patients, correlating higher HRQoL scores with prolonged survival and decreased hospitalization rates. Pei et al. [28] suggested that patients with ESRD could experience diminished HRQoL in comparison to the general population in Sweden.
Socio-demographic factors, particularly income and marital status, emerged as significant determinants of HRQoL. Patients with higher incomes had better PCS scores (61.33 ± 12.92, p = 0.049) compared to lower-income groups, emphasizing the role of financial capability in affording better healthcare access and coping mechanisms. Conversely, unmarried patients experienced a higher BKD, likely due to reduced emotional and social support. These observations align with those obtained from a previous study by Molsted et al. [29], who demonstrated the impact of marital and socioeconomic status on HRQoL. Kim and Park [30] emphasized that HRQoL is significantly affected by a combination of household income, educational attainment, and social class.
The study also revealed gender- and age-specific disparities in comorbidities among CKD patients. Older males were found to have higher rates of HTN and behavioral risk factors, such as smoking and alcohol use, whereas older females exhibited a greater prevalence of diabetes.
Tong et al. [31] have shown that women with CKD often do not give due priority to their own health due to caregiving responsibilities, experience social and financial barriers in accessing healthcare, and experience discriminatory or unfair treatment based on preconceived stereotypes that impact clinical decision-making.
Further research suggests that CKD is more prevalent in women, yet men tend to experience a more rapid progression to ESRD and have higher mortality rates. These disparities highlight the necessity of sex-specific approaches in CKD management to ensure equitable care [32].
The results of Lopes et al. [33] found that women on maintenance hemodialysis reported lower HRQoL scores than men, with findings suggesting that depression may partially explain these gender differences.
Additionally, a longitudinal study demonstrated that women with advanced CKD had lower baseline HRQoL scores, but men experienced a more rapid decline over time [26].
These differences align with addressing the gender disparities in HRQoL among hemodialysis patients, which requires a multifaceted approach that incorporates gender-sensitive HRQoL assessments, mental health screening, and personalized interventions such as cognitive therapy. Additionally, socioeconomic support, nutritional counselling, and telehealth-based programs should be explored to improve accessibility and adherence to care. Policy-driven studies focusing on universal healthcare and mobile dialysis units could help enhance healthcare delivery for underserved patients. Moreover, personalized pharmacological strategies for anemia management in women and behavioral risk reduction programs for men should be prioritized to mitigate disease progression and improve overall well-being. A multidisciplinary approach, integrating mental health support, lifestyle modifications, and policy reforms, is crucial to effectively addressing these disparities and improving HRQoL in CKD patients [26, 31–33].
Kamal et al. [16] further supported these findings, noting that such disparities significantly impact daily living, leading to physical and psychological impairments. These challenges disrupt marital, family, and social life, underscoring the need for comprehensive and gender-specific healthcare strategies to improve HRQoL and clinical outcomes of DD-CKD patients. According to Elhadad et al. [27], depression and anxiety disorders are the most frequently observed psychiatric conditions in patients. Moreover, patients with ESRD were noted to have markedly diminished HRQoL. Psychiatric conditions, particularly depression and anxiety, have a profound impact on HRQoL in DD-CKD patients. In clinical practice, routine mental health screening (e.g., PHQ-9, GAD-7), integrated multidisciplinary care, cognitive-behavioral therapy, judicious pharmacologic management, and peer support initiatives can enhance patient well-being. Further research should focus on longitudinal studies, intervention trials, biopsychosocial models, digital health innovations, and policy-driven strategies to improve the integration of mental health care in nephrology [23, 34]. The KDQOL-SF™ questionnaire provided robust insights into HRQoL domains, with the EKD domain showing the highest reliability (Cronbach’s α = 0.832). The moderate reliability of the KDQOL-SF™ domains, particularly the SP and PCS scores, underscores the robustness of this tool in capturing the multifaceted aspects of HRQoL in DD-CKD patients. However, variability in reliability across other domains indicates the complexity of capturing HRQoL comprehensively. Chow and Tam [35] demonstrated that the Chinese version of the KDQOL-SF™ exhibits good reliability and modest validity, reinforcing its utility in evaluating HRQoL in specific populations. Similarly, Joshi et al. [36] reported that the KDQOL-SF™ demonstrated acceptable reliability, strong construct validity, and significant discriminatory ability in a representative cohort of ESRD patients in Singapore, further establishing its effectiveness as a comprehensive tool for HRQoL assessment.
Behavioral risk factors, such as smoking and alcohol consumption, were notably prevalent among males undergoing dialysis, exacerbating both the physical and psychological challenges of the treatment process. These habits have been linked to lower MCS scores, emphasizing the importance of implementing lifestyle modification programs. Li et al. [37] demonstrated that smoking significantly increases the likelihood of death and hospitalization in patients undergoing hemodialysis, with younger patients and those with diabetes being at the greatest risk. Similarly, Schouten et al. [38] emphasized that anxiety symptoms pose a critical risk for both morbidity and mortality in dialysis patients, highlighting the urgent need for further investigation into effective interventions to manage these psychological issues.
The correlation analysis further demonstrated the interconnectedness of HRQoL domains, revealing positive correlations between the PCS and EKD (r = 0.211, p = 0.032), as well as between SP and the MCS (r = 0.321, p < 0.001; r = 0.203, p = 0.038, respectively). These findings highlight that physical health influences mental and social well-being. Pei et al. [28] highlighted that a decline in the PCS score is linked to factors such as advanced age, CVD, diabetes mellitus (DM), reduced hemoglobin levels, lower sodium concentrations, and an elevated risk of mortality. Notably, the MCS score did not exhibit a significant association with mortality. Similarly, Pagels et al. [4] observed that the presence of comorbid conditions, including inflammation and CVD, serves as a strong predictor of diminished HRQoL in patients with CKD.
Despite the study’s robust methodology and valuable findings, several limitations warrant consideration. The cross-sectional design limits causal inferences, and the single-center setting may affect generalizability. Future research should explore longitudinal designs across diverse populations to better understand the dynamic interplay between socio-demographic factors, clinical characteristics, and HRQoL.
This study emphasizes the critical need for personalized, patient-centered care strategies that account for socio-demographic and clinical diversity in DD-CKD patients. Targeted interventions to address financial, social, and health-related challenges can significantly enhance the overall well-being and HRQoL of functionally impaired patients.
The findings of this study indicate a clear association between increased symptom burden and lower HRQoL scores among DD-CKD patients. This emphasizes the importance of timely identification and management of patient-reported symptoms. Additionally, future research should explore the integration of electronic patient-reported outcome (ePRO) tools with electronic health records, as this approach may facilitate real-time symptom monitoring and timely clinical interventions, thereby enhancing the accuracy of HRQoL assessments and improving patient outcomes [39].
The key findings of the study are as follows: first, financial capability positively influences physical health outcomes. Second, unmarried patients experience a higher BKD, likely due to reduced social and emotional support. Third, gender and age-specific patterns, such as a higher prevalence of HTN and alcohol use in older males and diabetes in older females, emphasize the need for tailored interventions targeting these subgroups. Fourth, behavioral risk factors, including smoking and alcohol consumption, were found to exacerbate the challenges faced by these patients, further underscoring the importance of comprehensive lifestyle management programs. The use of the KDQOL-SF™ questionnaire provided a detailed assessment of HRQoL, though variability in reliability across its domains suggests the need for a multifaceted approach to evaluating and addressing the concerns in DD-CKD patients.
While this study provides valuable insights into HRQoL among dialysis patients, certain limitations must be considered when interpreting the findings. The cross-sectional design enables the identification of associations but does not establish causality, highlighting the need for longitudinal studies to confirm these relationships over time. Additionally, as this study was conducted in a single-center setting, the findings primarily reflect the characteristics of the studied patients. Conducting multi-center studies would enhance the generalizability of these results.
Furthermore, the reliance on self-reported measures introduces the possibility of response bias. However, standardized assessment tools were employed to minimize subjectivity and improve data reliability. Despite these considerations, the study’s robust methodology and adequate sample size provide a strong foundation for understanding gender- and age-specific disparities in HRQoL. Patients on peritoneal dialysis were excluded from this study; therefore, the findings are specific to hemodialysis patients and may not be generalizable to the peritoneal dialysis population. This study may be prone to recall bias due to the self-reported nature of data collection, which should be considered a methodological limitation. To address these limitations, future research incorporating longitudinal follow-ups, multi-center cohorts, and larger, more diverse populations will be crucial in validating and expanding upon these findings.
A notable strength of this study is its adequate sample size, which enhances the reliability and robustness of the results. The focus on socio-demographic variables offers a detailed understanding of the factors influencing HRQoL in DD-CKD patients. This cross-sectional analysis holds significance in its ability to inform strategies for improving HRQoL through tailored, patient-centered interventions. Prior studies have underscored the impact of clinical and treatment-related factors on HRQoL outcomes. For instance, the HEMO study by Unruh et al. [40] demonstrated that variations in dialysis dose and membrane flux can influence patient-reported outcomes. Similarly, Weisbord et al. [41] showed that addressing symptoms such as pain, depression, and sexual dysfunction can meaningfully enhance the quality of life in patients receiving chronic hemodialysis. In this context, our study contributes valuable evidence that can support the development of targeted approaches to improve patient well-being.
Future studies should adopt longitudinal and multi-center designs, explore the impact of mental health and social support on HRQoL, and integrate qualitative methods for deeper patient insights.
BKD: burden of kidney disease
BP: bodily pain
DD-CKD: dialysis-dependent chronic kidney disease
EF: energy and fatigue
EKD: effect of kidney disease
ESRD: end-stage renal disease
EWB: emotional well-being
GH: general health
HRQoL: health-related quality of life
HTN: hypertension
IPD: In-Patient Department
KDQOL-SF™: Kidney Disease Quality of Life-Short Form
MCS: mental component summary
OPD: Out-Patient Department
OR: odds ratio
PCS: physical component summary
PF: physical functioning
PKD: polycystic kidney disease
RE: role emotional
RP: role physical
SF: social functioning
SP: symptoms and problem
The supplementary tables for this article are available at: https://www.explorationpub.com/uploads/Article/file/1001303_sup_1.pdf.
We sincerely acknowledge the invaluable support provided by the National Institute of Pharmaceutical Education and Research (NIPER), Mohali, and the Government Medical College and Hospital (GMCH), Chandigarh. We are also deeply grateful to the study participants for their significant contributions to this research.
GS: Conceptualization, Data curation, Methodology, Formal analysis, Writing—original draft. PA: Supervision, Validation, Writing—review & editing, Formal analysis. PT: Conceptualization, Supervision, Validation, Writing—review & editing. SD and AT: Supervision, Validation, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Ethical approval for the study was obtained from the Institutional Ethics Committee of the National Institute of Pharmaceutical Education and Research, Mohali (IEC/78/2023-RI), as well as from the Ethics Committee of the Government Medical College and Hospital, Chandigarh (GMCH/IEC/2024/1137R).
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The datasets used and/or analyzed during the current study are available from the corresponding author (Pramil Tiwari, ptiwari@niper.ac.in) upon reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
