Affiliation:
1Department of Pathology and Laboratory Medicine, Mount Sinai Hospital and Laboratory Medicine and Pathobiology, University of Toronto, Toronto, ON M5G 1X5, Canada
2Histopathology Unit, Department of Pathology, Hospital Kuala Lumpur, Kuala Lumpur 50586, Malaysia
†
ORCID: https://orcid.org/0000-0002-1465-5812
Affiliation:
3General Internal Medicine, Western University, London, ON N6A 5A5, Canada
†
ORCID: https://orcid.org/0000-0001-7888-676X
Affiliation:
4Department of Pathology and Laboratory Medicine, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA 90095, USA
Affiliation:
5Department of Pathology and Laboratory Medicine, Mount Sinai Hospital, Toronto, ON M5G 1X5, Canada
ORCID: https://orcid.org/0000-0001-5613-2502
Affiliation:
5Department of Pathology and Laboratory Medicine, Mount Sinai Hospital, Toronto, ON M5G 1X5, Canada
Email: nancy.liu@sinaihealth.ca
ORCID: https://orcid.org/0000-0003-1145-3999
Explor Med. 2025;6:1001302 DOI: https://doi.org/10.37349/emed.2025.1001302
Received: January 23, 2025 Accepted: March 18, 2025 Published: April 08, 2025
Academic Editor: Amedeo Lonardo, Azienda Ospedaliero-Universitaria di Modena, Italy
Vanishing bile duct syndrome (VBDS) is a rare condition, representing approximately 0.5% of small bile duct diseases, characterized by progressive destruction of intrahepatic bile ducts, leading to ductopenia. This condition encompasses various etiologies, with drug-induced VBDS (D-VBDS) accounting for 7% of VBDS cases. D-VBDS arises from liver injury due to chemical drugs, traditional medicines, and dietary supplements, often resulting in inflammatory responses and necrosis of bile duct epithelium. Recent years have seen a rise in reported cases, making drug-related injuries a leading cause of acute liver failure in Western countries. This review provides a comprehensive analysis of VBDS, focusing on the histopathological features of acute and chronic D-VBDS, alongside exploring its clinical presentation, prognostic implications, and future research directions. Understanding the diverse etiologies, clinical manifestations, and biochemical parameters associated with D-VBDS is essential for improving diagnosis, treatment strategies, and patient outcomes.
Vanishing bile duct syndrome (VBDS) is a clinically rare condition, accounting for only 0.5% of small bile duct diseases. The term “vanishing bile duct syndrome” encompasses a group of cholestatic liver diseases characterized by progressive destruction of segments of the intrahepatic biliary tree, resulting in ductopenia. Ductopenia is semiquantitatively defined as a marked reduction in intrahepatic bile ducts in at least 50% of portal tracts in an adequate liver specimen with more than ten portal tracts for evaluation [1–5].
VBDS has been linked to numerous etiologies [3]. Among these, drug-induced VBDS (D-VBDS) is particularly significant, accounting for 7% of all VBDS cases [6]. D-VBDS refers to liver injury induced by chemical drugs, traditional medicines, dietary supplements, and their metabolites or excipients. Certain drugs can provoke inflammatory responses and necrosis of bile duct epithelial cells in the liver tissue, leading to persistent damage, bile duct loss, and eventually cholestatic cirrhosis. Reports of D-VBDS have risen in recent years, making it a leading cause of acute liver failure in Western countries [7]. The range of drugs implicated in D-VBDS continues to expand, garnering considerable clinical and research attention. To date, more than 70 drugs have been identified as potential triggers of VBDS, and the condition may persist even after the removal of the culprit agents [1]. Common drugs associated with VBDS include antimicrobials, non-steroidal anti-inflammatory drugs (NSAIDs), antipsychotics, lipid-lowering agents, hypoglycemic drugs, and proton pump inhibitors [1, 3]. Herbal medicines can also induce ductopenia; however, these preparations often contain multiple components, making it challenging to identify the specific elements responsible. In a study by Bonkovsky et al. [8], 3 out of 26 cases of bile duct loss were linked to herbal agents, specifically Artemisia annua (500 mg) and Gluco-Ease Plus (525 mg). One case associated with Hydroxycut™, a popular dietary supplement for weight loss that contains extracts such as Garcinia cambogia, Gymnema sylvestre, Camelia sinensis, and chromium, has also been reported [9].
The pathogenesis of D-VBDS remained unclear until a comprehensive review by Bessone et al. [1] in 2021, which highlighted that the mechanisms underlying drug-induced ductopenia are not mutually exclusive and these mechanisms may involve immunologically mediated bile duct damage, particularly when accompanied by immunoallergic features. Other, less common mechanisms may include direct drug or reactive metabolite toxicity or alterations in bile composition, impairing the bile ducts’ protective functions against bile salt cytotoxicity. Factors such as ischemia during hepatic allograft rejection or increased biliary acidity in primary biliary cirrhosis could also amplify the immune response [5].
This review provides a comprehensive analysis of VBDS, focusing on the histopathological features of both acute and chronic forms of D-VBDS. Additionally, we explore its clinical perspectives, prognosis, and key future research directions.
VBDS is associated with a wide range of etiologies, all characterized by the progressive loss of intrahepatic bile ducts. These causes can be categorized into eight distinct groups: toxin and drug-related factors, immunologic conditions, infectious agents, transplant-related diseases, neoplastic disorders, ischemic cholangiopathy, pediatric and young adult diseases (including congenital, developmental, and genetic disorders), and idiopathic conditions, as detailed in Table 1. Notably, D-VBDS represents a significant contributor to this condition, often precipitated by using medications such as antimicrobials, NSAIDs, and antipsychotics. Identifying the underlying etiology is crucial for guiding treatment strategies and improving patient outcomes [3, 8–18].
Causes of ductopenia
| Causes | Examples |
|---|---|
| Toxins and drugs | Anti-microbials (amoxicillin/clavulanate, azithromycin, erythromycin, flucloxacillin, quinolones, sulfamethoxazole-trimethoprim, terbinafine, thiabendazole, etc.) |
| NSAIDs (ibuprofen, diclofenac) | |
| Psychotropics (chlorpromazine, amitriptyline, imipramine, carbamazepine, etc.) | |
| Herbal and dietary supplements (Artemisia annua, Gluco-Ease Plus, Hydroxycut) | |
| Immunologic | Primary biliary cholangitis |
| Immune cholangitis | |
| Primary sclerosing cholangitis | |
| Sarcoidosis | |
| Infectious | Cytomegalovirus |
| Rubella | |
| Hepatitis B and C viruses | |
| Epstein-Barr virus | |
| COVID-19 | |
| Human immunodeficiency virus | |
| Transplant-related diseases | Chronic graft-versus-host disease |
| Chronic ductopenic rejection | |
| Neoplastic disorders | Hodgkin disease |
| Langerhans cell histiocytosis | |
| Ischemic cholangiopathy | Surgical procedures (liver transplantation, cholecystectomy with arterial injury) |
| Treatments (transarterial chemotherapy or chemoembolization) | |
| Systemic disease with microvascular involvement | |
| Intensive care unit cholangiopathy | |
| Congenital, developmental, and genetic diseases | Late feature of extrahepatic biliary atresia |
| Genetic diseases: Alagille syndrome, polycystic liver diseases, fibropolycystic liver diseases (Caroli disease and congenital hepatic fibrosis), cystic fibrosis, progressive familial intrahepatic cholestasis, α1-antitrypsin deficiency | |
| Idiopathic | Non-syndromic paucity of bile ducts in infancy without identifiable etiology |
| Idiopathic adulthood ductopenia |
NSAIDs: non-steroidal anti-inflammatory drugs
Clinical features of VBDS vary depending on the etiology. Among a patient cohort of D-VBDS, all patients except one developed jaundice, and just under half had nausea, fatigue, fever, and abdominal pain. The onset of these symptoms ranged widely between days to months after exposure to the offending agent [12]. Hence, a detailed medication and exposure history is critical in diagnosing D-VBDS. On the other hand, a viral infection like hepatitis B, hepatitis C, or cytomegalovirus (CMV) can be largely asymptomatic and detected only due to abnormal liver enzymes [8, 19, 20].
Autoimmune conditions such as primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) may be initially asymptomatic, but patients commonly develop debilitating fatigue and pruritus [21, 22]. In hepatic graft-versus-host disease (GvHD), which includes VBDS, patients can develop acute or slowly progressive jaundice accompanied by other features of GvHD, such as rash and diarrhea [23]. Hepatic sarcoidosis has a variety of presentations, including asymptomatic enzyme elevation, only constitutional symptoms, as well as painful or painless jaundice [24]. Finally, idiopathic adulthood ductopenia will typically present in young to middle-aged adults as progressive or episodic jaundice and pruritic that remains without a specific etiology after an extensive workup [4, 25].
Other clinical features and sequelae may develop depending on the chronicity and stage of the disease. In long-standing cholestasis, patients can develop gallstones, intestinal malabsorption, and dyslipidemia [8]. Decompensated liver failure can additionally occur either acutely in the setting of severe disease or gradually as patients develop cirrhosis. This manifests with various complications, including hepatic encephalopathy, ascites, and variceal bleeding.
Biochemically, patients with VBDS typically have marked elevation in conjugated bilirubin and cholestatic liver enzymes, including alkaline phosphatase (ALP) and γ-glutamyl transferase (GGT), with a milder elevation in transaminases [3]. Other liver function tests, such as albumin and international normalized ratio (INR), may also be abnormal depending on the chronicity and stage of the disease. Additional disease-specific markers may be present in bloodwork. Autoimmune markers, including antimitochondrial antibodies (AMA), anti-smooth muscle antibodies (ASMA), and antinuclear antibodies (ANA), are seen in autoimmune and D-VBDS [8, 21, 22]. Viral serologies can be used to diagnose viral hepatitis, and seroconversion typically occurs concurrently with liver enzyme elevation. Imaging studies, including ultrasound, rule out extrahepatic biliary obstruction but are typically normal since VBDS primarily affects small intrahepatic bile ducts [3]. Magnetic resonance cholangiopancreatography can be used to detect biliary strictures if there is a high suspicion for PSC [22].
A comprehensive approach to evaluating ductopenia involves obtaining a detailed medical history, performing a thorough clinical assessment, and systematically analyzing biochemical patterns alongside diagnostic imaging studies (Figure 1 and Figure S1).
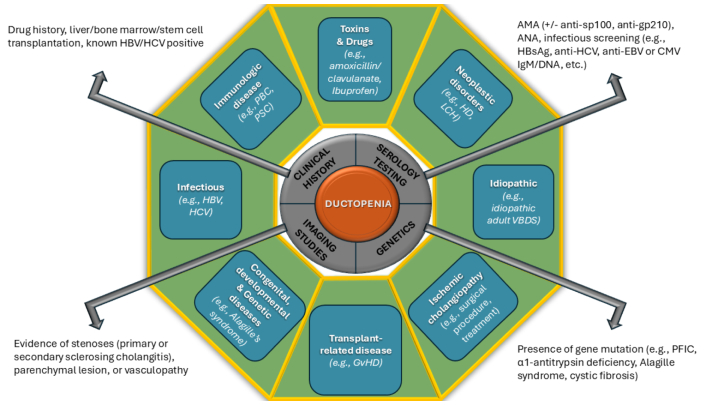
An approach to identifying the causes of ductopenia, integrating clinical, laboratory, genetic, and imaging findings. ANA: antinuclear antibodies; AMA: antimitochondrial antibodies; CMV: cytomegalovirus; EBV: Epstein-Barr virus; GvHD: graft-versus-host disease; HBsAg: hepatitis B surface antigen; HD: Hodgkin disease; HBV: hepatitis B virus; HCV: hepatitis C virus; LCH: Langerhans cell histiocytosis; PBC: primary biliary cholangitis; PFIC: progressive familial intrahepatic cholestasis; PSC: primary sclerosing cholangitis; VBDS: vanishing bile duct syndrome
The histological changes in drug-induced liver injury (DILI) vary based on the severity of the lesions and the stage of progression. These changes can be categorized into acute and chronic phases, although some overlap may occur.
Figure 2 highlights key histological changes in the acute phase, showing parenchymal cholestasis and acute cholangitis. Parenchymal cholestasis affects hepatocytes, canaliculi, and Kupffer cells. Acute cholangitis is characterized by degenerative changes in the interlobular bile ducts, including cholangiocyte apoptosis with karyorrhectic debris formation, cholangiocyte necrosis, cytoplasmic alteration (e.g., eosinophilia, vacuolation), nuclear hyperchromasia, nuclear disarray (crowding, loss of polarity), and increased mitotic activity. Portal tracts often exhibit varying densities of inflammatory cell infiltrates, including neutrophils, lymphocytes, histiocytes, eosinophils, and occasional plasma cells, along with possible granulomas and mild parenchymal cell damage [2, 26, 27]. However, there is no bile ductular reaction in acute ductopenia, a phenomenon occurring during bile duct response to the injury. The tissue copper histochemical stain, i.e. Rhodanine stain, is negative, a unique feature often associated with chronic VBDS.
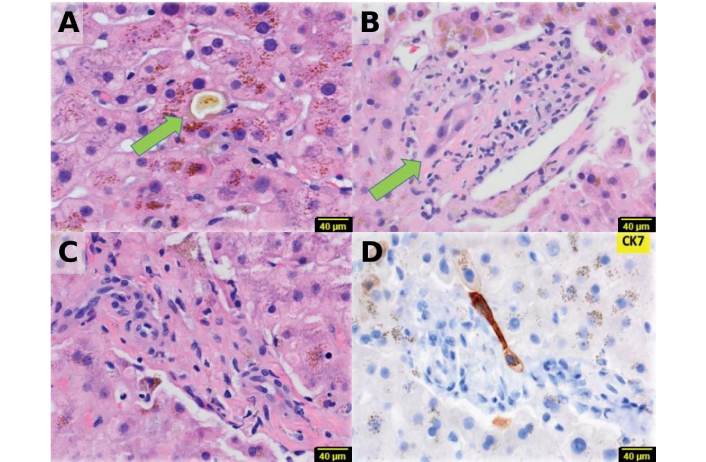
Histopathological features of the acute stage of the disease. (A) Zone three cholestasis is evident (arrow, H&E × 40), (B) bile duct injury is observed (arrow, H&E × 40), (C) the portal tract lacks a bile duct (H&E × 40), (D) CK7 immunostain confirms the absence of bile ducts and ductules (× 40). Adapted with permission from [26], © 2022 British Pharmacological Society
In the chronic phase, ductopenia is the hallmark lesion and may occur with or without parenchymal cholestasis (Figure 3). Advanced cases can develop chronic cholestasis, characterized by feathery degeneration of periportal hepatocytes, Mallory-Denk bodies, increased periportal copper deposition, infiltration by chronic inflammatory cells, ductular reaction, and pericellular fibrosis. The extent of ductopenia varies, determined by the proportion of portal tracts lacking interlobular ducts. Severe ductopenia is defined as a reduction of at least 50% of portal tracts or a ratio below 0.5 (normal typically ranges from 0.9 to 1.8), though less severe cases can also occur. In rare instances, progressive fibrosis may lead to biliary-type fibrosis and cirrhosis [2, 27].
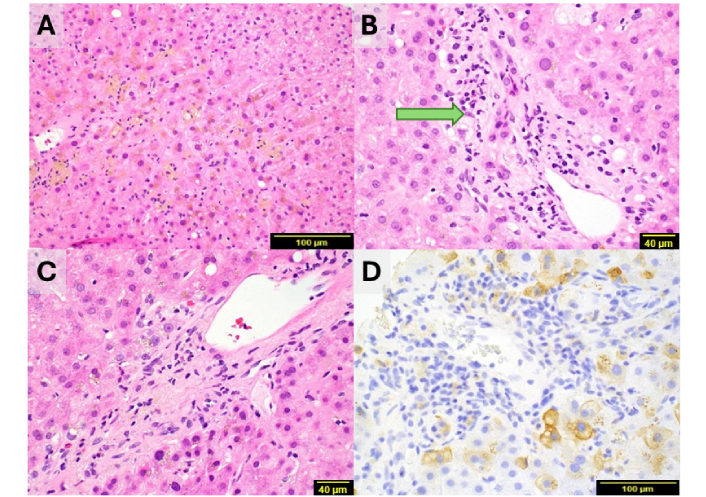
Histopathological characteristics of lobular and portal tract injury. (A) Lobular injury shows scattered lymphocyte infiltration, canalicular cholestasis, and pigmented macrophages at centrilobular regions (H&E × 20), (B) the bile duct in this portal tract exhibits injury/degeneration (arrow, H&E × 40), (C) absence of a bile duct in this portal tract, with mild lymphocytic infiltrates and rare eosinophils, ductular reaction is absent (H&E × 40), (D) CK7 immunostaining confirms the absence of bile duct and ductular reaction, with biliary metaplasia in periportal hepatocytes (H&E × 20). Images A to D contributed by Dr. Hanlin L. Wang
Bile duct injury or loss can be difficult to identify in H&E-stained sections. Occasionally, identification of bile ducts and ductular reaction may have to be facilitated by immunohistochemical stains for the biliary cytokeratins (CKs), CK7 or CK19, to highlight the bile duct or its remnants. The absence of CK7 stained bile duct further confirms the diagnosis of bile duct loss. In contrast, the absence of reactive ductules favors an acute episode of bile duct injury and loss [26]. When a duct is truly lost, periportal hepatocytes may undergo biliary metaplasia, resulting in the formation of ‘intermediate hepatobiliary cells’ that express cytoplasmic CK7. Additionally, histochemical stains like Victoria Blue, Rhodanine, and Orcein can effectively reveal periportal copper accumulation associated with cholate stasis resulting from chronic duct loss [28, 29].
The treatment for VBDS largely depends on the treatment of the underlying cause, including withdrawal of the offending drug or toxin. Treatment with ursodiol is often recommended for the relief of pruritic symptoms in patients with severe disease. Various mechanisms of action for ursodiol have been proposed that may be helpful, including inhibition of apoptosis and cell protection from bile acids [30]. However, while it seems to help relieve symptoms, its efficacy in treating the underlying cholestasis is based only on a few case reports [31–33]. It is generally well tolerated, with only rare side effects, including diarrhea, rash, and cytopenias with prolonged use [34]. Other treatments that have been trialed to target cholestasis include rifampin and cholestyramine, although their efficacy is unclear as they were combined with ursodiol [35, 36]. Additionally, there are published cases of successfully using immunosuppressants, such as tacrolimus and infliximab, and steroids in treating refractory D-VBDS [37–39]. There is at least one report of using immunomodulators in idiopathic adulthood ductopenia [3]. Although these above agents have been reported to show some efficacy, the risks and side effects of these medications remain largely understudied, and routine clinical use is therefore limited. In all cases, the mainstay treatment is supportive care with close monitoring and management of complications. If VBDS progresses to decompensated cirrhosis, liver transplantation (LT) may be necessary [40].
D-VBDS ranges from gradual recovery after cessation of drug in some cases, to progression to liver failure and death. In a review of 21 cases with reported outcomes after D-VBDS, 57% achieved symptom resolution within a median of 11 weeks following drug discontinuation, while 29% required LT, and 14% died [41]. Similarly, a multicenter retrospective cohort study conducted in China by Lv et al. [42], involving 183 VBDS patients, found that VBDS rarely led to poor outcomes. Half of the patients either recovered or maintained their condition, while 23% progressed to end-stage liver disease or required LT. Notably, despite over 50% of portal tracts lacking bile ducts, only one-third of patients exhibited jaundice, potentially due to a compensatory biliary network. Histological analysis in this cohort revealed that features such as hepatocellular cholestasis, foam cells, and advanced fibrosis were associated with poor prognosis, with advanced fibrosis identified as a critical factor. Additionally, the same study indicated that the underlying etiology of VBDS did not significantly affect outcomes, although D-VBDS had a more favorable prognosis than PBC-associated VBDS. Consistent with this finding, a retrospective study of a large cohort of patients with early-stage PBC and ductopenia in China, despite some limitations, also found worse biochemical profiles and poorer treatment responses in these patients [43]. While COVID-19-associated VBDS is often transient and resolves with the resolution of the disease, some cases of COVID-19-related ductopenia can be severe [42]. Bonkovsky et al. [8] identified the extent of bile duct loss as the strongest predictor of poor outcomes in a cohort of 363 patients with DILI. Among the 26 patients (7%) with bile duct loss, 94% developed chronic liver injury, often severe and cholestatic, compared to 47% of those without bile duct loss. These studies suggest that D-VBDS presents a variable clinical course, ranging from gradual full recovery after cessation of drug in some cases, to progression to liver failure and death. While a significant proportion of patients experience symptom resolution after drug discontinuation, others may require LT or succumb to the disease. Factors such as the extent of bile duct loss and histological features, including advanced fibrosis, may predict poor outcomes.
Key future research areas in DILI include the development of better diagnostic and prognostic biomarkers, the creation of more reliable causality assessment tools, and the conducting of comprehensive epidemiological studies. Many critical aspects of disease pathogenesis and predisposing factors remain poorly understood and warrant further exploration. Enhancing our understanding of the molecular pathogenesis of DILI is essential to reduce morbidity and mortality and identify potential therapeutic targets [42, 44].
Genomic research offers promising insights into genetic susceptibilities associated with DILI. For instance, genetic variation of the CYP 450 enzyme, missense variant (rs2476601) in protein tyrosine phosphatase non-receptor type 22, and HLA alleles (e.g., DRB1*15:01, B*57:01, A*31:01) have been associated with an increased risk of DILI related to specific drugs [45–47]. However, the currently reported genetic polymorphisms (HLA or non-HLA) associated with an increased risk of drug-specific DILI may also be associated with drug-related factors and/or nongenetic host-related factors (e.g., physiologic, lifestyle modifications, underlying diseases); thus, further validation in large, prospective cohorts is crucial for translating genetic discoveries into clinical applications for predicting and preventing DILI [44, 47].
Current serum markers of liver injury [e.g., aspartate transaminase (AST), alanine transaminase (ALT), and ALP] lack the sensitivity and specificity for early DILI detection or reliable outcome prediction. Emerging biomarkers, including glutamate dehydrogenase, micro-RNA-122, CK18, glutathione S-transferase, sorbitol dehydrogenase, bile acids, cytochrome P450, and osteopontin, hold significant potential for improving diagnostic precision and aiding in prognostication. Although not yet routinely used in clinical practice, these biomarkers represent exciting avenues for future research [48, 49].
Another critical area is understanding the impact of novel therapeutic modalities, particularly immune-modulating drugs used in oncology, which pose unique challenges in hepatotoxicity. For instance, a case of VBDS was documented following combination therapy with nivolumab and cabozantinib in a patient with renal carcinoma, illustrating the potential for these treatments to induce D-VBDS [50]. This highlights the importance of clinicians remaining vigilant about such risks and utilizing resources like LiverTox to guide management strategies and optimize patient outcomes [51].
Collectively, these efforts will advance our understanding of DILI and D-VBDS, improve clinical outcomes, and pave the way for personalized approaches in hepatotoxicity management.
VBDS remains a complex and multifaceted condition with diverse underlying causes. Among these, D-VBDS has emerged as a significant concern, particularly due to its increasing incidence and association with various medications. The clinical presentation of VBDS can vary widely, influenced by the specific etiology and patient factors, highlighting the importance of a thorough diagnostic approach.
Understanding the histopathological features and mechanisms underlying D-VBDS is essential for effective management and treatment strategies. However, liver biopsy remains the golden standard for diagnosing and confirming bile duct loss or ductopenia. Continued research is imperative to elucidate pathogenesis and improve diagnostic precision, which may lead to better patient outcomes. Furthermore, as the list of drugs associated with VBDS expands, ongoing surveillance and awareness within the medical community are crucial to prevent and mitigate the risks associated with DILI.
In summary, enhancing our knowledge of drug-induced VBDS, including its causes, clinical features, diagnosis, and treatment options, is essential for enhancing patient care and outcomes. Clinicians must remain vigilant about this entity, as early liver biopsies for pathological examination are crucial for its timely recognition and management. Future research should focus on developing more precise and efficient diagnostic tools and targeted therapies, which could significantly transform the treatment landscape for individuals affected by this rare yet serious condition.
ALP: alkaline phosphatase
AMA: antimitochondrial antibodies
CKs: cytokeratins
DILI: drug-induced liver injury
D-VBDS: drug-induced vanishing bile duct syndrome
EBV: Epstein-Barr virus
GvHD: graft-versus-host disease
LT: liver transplantation
NSAIDs: non-steroidal anti-inflammatory drugs
PBC: primary biliary cholangitis
VBDS: vanishing bile duct syndrome
The supplementary figure for this article is available at: https://www.explorationpub.com/uploads/Article/file/1001302_sup_1.pdf.
SS and KF: Conceptualization, Writing—original draft, Writing—review & editing. HLW and JL: Validation, Writing—review & editing, Supervision. CP: Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not required.
Not required.
Informed consent to publication was obtained from relevant participants.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
