Affiliation:
1MM College of Pharmacy, Maharishi Markandeshwar (Deemed to be University), Mullana-Ambala 133207, Haryana, India
ORCID: https://orcid.org/0000-0001-5702-0932
Affiliation:
2Amity Institute of Nanotechnology, Amity University Uttar Pradesh (AUUP), Noida 201313, Uttar Pradesh, India
ORCID: https://orcid.org/0000-0002-5419-6147
Affiliation:
1MM College of Pharmacy, Maharishi Markandeshwar (Deemed to be University), Mullana-Ambala 133207, Haryana, India
ORCID: https://orcid.org/0000-0002-4784-6383
Affiliation:
3Department of Biosciences, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences, Chennai 602105, Tamil Nadu, India
ORCID: https://orcid.org/0000-0001-8867-7603
Affiliation:
4Institute of Pharmaceutical Sciences, Kurukshetra University, Kurukshetra 136119, Haryana, India
ORCID: https://orcid.org/0000-0002-4399-328X
Affiliation:
5Faculty of Pharmacy, Puncak Alam Campus, Universiti Teknologi MARA (UiTM), Shah Alam 40450, Malaysia
ORCID: https://orcid.org/0000-0002-9042-4290
Affiliation:
6Department of Rasashastra and Bhaishajya Kalpana, Faculty of Ayurveda, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005, Uttar Pradesh, India
Email: rohitsharma@bhu.ac.in
ORCID: https://orcid.org/0000-0002-3682-3573
Explor Med. 2023;4:782–812 DOI: https://doi.org/10.37349/emed.2023.00178
Received: May 03, 2023 Accepted: July 24, 2023 Published: October 31, 2023
Academic Editor: Guangwen Cao, Second Military Medical University, China
The prevalence of skin cancer has increased hastily in the recent decade for both kinds of melanoma and non-melanoma skin cancer. Skin cancers mostly encompass keratinocyte cancers: cutaneous squamous cell carcinoma, basal cell carcinoma, and melanoma. This review discusses the recent advancements in the treatment of skin cancer. In addition to chemotherapy, immunotherapy, targeted therapy, and photodynamic therapy (PDT), there are several other therapies for skin cancer. Additionally, PDT use in combination with chemotherapy, radiation, immunotherapy, and surgery is being actively investigated. This review will specifically address the pathophysiology of skin cancer, diagnostic approaches, and current therapies used in the topical treatment of skin cancers and introduce emerging treatment using nanotechnology that may be beneficial for these indications.
The largest organ in the human body is the skin. This skin forms a barrier that protects inner organs from damage. It also acts as a reservoir for fluids, lipids, and vitamin D synthesis [1]. The epidermis (skin’s external surface) along with the dermis (middle layer) are the skin’s two most noticeable layers. Rising rates of skin cancer are being linked to increased exposure to ultraviolet radiation (UVR) among young individuals who also tend to have inadequate sun protection. While sun-exposed parts like the face, neck, and hands are to an extent prone to the development of skin carcinoma, the disease may appear elsewhere in the body.
Cancer may start either in the basal cells or the squamous cells of the skin. Cancers of the skin that are not melanoma are called non-melanoma skin cancers. There are around 3 million incidences of non-melanoma skin cancer detected throughout the year. Skin melanoma is regarded as the 17th most common cause of cancer death in the world. Skin melanoma is also known as cutaneous cancer or malignant melanoma. It is also possible for melanoma to develop in the mucosal membranes (narrow, wet tissue coat which covers the lip surface). In males, skin melanoma ranks 13th while for females, it ranks as the 15th most commonly reported cause of cancer death. In 2020, the incidence of skin melanoma was 150,000 [2]. Compared to other types of skin cancer, skin melanoma has a higher propensity to invade neighboring tissues and metastasize [3]. In males, melanoma frequently appears on the forehead or the area connecting the elbow and pelvis whereas, in females, the bottom extremities are common areas for the development of malignant melanoma. Increased public awareness has led to higher rates of screening tests, and self-examinations along with the detection of both melanoma and non-melanoma skin cancers.
Although the exact cause of basal cell carcinoma (BCC) development is unknown, BCC has been linked to the pilosebaceous unit since tumors often appear in hairy areas [4]. Several people think that pluripotent cells present in the basal surface of the epidermis or follicular arrangement are the source of BCCs. These cells may give rise to hair, sebaceous glands, and apocrine glands at any point in life. Tumors may form in external root casein follicles of hair and more specifically in stem cell follicles of hair in the bulge area, which is found just beneath the duct of the sebaceous gland.
Both sporadic and nevoid BCC rely on the patched (PTCH)/hedgehog intracellular signaling pathway [5]. Throughout fetal development, this pathway affects the differentiation of several tissues. During embryonic development, it still forms a vital part of the regulation of cell division along with fate.
The hedgehog transcription causes the production of an extra-cellular protein that binds to the receptor network on the cell membrane, triggering a series of events that culminates in cell division [4, 5]. Three human homologs to BCC were found, but the sonic hedgehog (SHH) protein is the closest and most functional match [5–8]. Hedgehog receptor complexes in cell membranes rely on a protein called PTCH to bind ligands. Hedgehog signals are sent to target genes in part by smoothened (SMO), another protein found in the receptor complex [9]. When SHH is available, it binds to PTCH, causing the latter to dissociate from SMO and trigger its activation. The SMO signal is carried to the nucleus by orthologous glioma-associated oncogene homolog (GLI) proteins. PTCH suppresses SMO by binding to it when SHH is not present [10]. It is revealed that mutations inside the PTCH gene reduce the protein-binding capacity with SMO, creating an effect similar to that of the presence of SHH. Hedgehog signaling is unhindered because unbound SMO and downstream GLI are always on. Mutations in the SMO gene may also activate this pathway, allowing for unchecked tumor growth signaling [11]. The hedgehog signaling mechanism in cancer therapy is illustrated in Figure 1.
The majority of BCC showed alterations inside PTCH or SMO genes, but the mechanism by which these mistakes cause carcinogenesis is poorly understood [12]. Several researchers believe that disruptions in the hedgehog pathway are necessary for BCC development [13]. Sun-exposed areas are more prone to BCC. The changes of ultraviolet (UV)-specific nucleotide in PTCH as well as tumor suppressor gene tumor protein p53 (TP53) were discovered by Ouhtit et al. [14] to contribute to the beginning of early-stage BCC [15]. In certain cases, concerning BCC, mutations inside the tumor suppressor gene TP53 have been linked to UVR. Another TP53 gene mutation associated with BCC risk is rs78378222, a single-nucleotide polymorphic allele [16]. Fragmentary change (frameshift) in the B-cell leukemia 2 (BCL2) associated X protein (BAX) gene was detected in a small percentage of BCC cases. Reduced levels of BCL2 proteins are characteristic of the infiltrative aggressive BCC class.
Researchers have discovered two different mechanisms by which radiation causes cancer [17]. This primary mechanism is the beginning of extended cell proliferation, which boosts the probability of error in the transcription process, which may lead to cellular transformation. The secondary mechanism is via alteration caused by immediate injury to the DNA during duplication, which may lead to the actuation of proto-oncogenes as well as the deactivation of tumor suppressor genes inside the cells [18].
Constant exposure to UVR suppresses the cutaneous immune system, leading to immunologic insensitivity to cutaneous malignancies, and ultimately the development of BCC [19, 20]. Decreased numbers of thymus cell antigen 1 positive (Thy1+) cells, dendritic epidermal T cells, and Langerhans cells are indicative of its local effect. Immunosuppressive chemicals [such as prostaglandin (PG), interleukin 1 (IL-1), interleukin 10 (IL-10), and tumor necrosis factor-alpha (TNF-α)] and the systemic proliferation of suppressor T cells are also deemed harmful to BCC’s growth.
It has been shown that a group of proteins called mismatch repair (MMR) proteins induce cell death at the G2/M-phase checkpoint in a physiologically relevant way [21]. However, mutated cells can survive since the MMR proteins will fail to detect the DNA damage that they have been pre-conditioned to. In non-melanoma skin cancers, MMR protein levels are elevated relative to the typical skin which confirms MMR abnormal regulation [22]. The most prevalent reason for developing BCC is being out in the sun too much, especially when as a child or young adult. The risk of skin cancer varies depending on where the patient lives. Clinical development of BCC often occurs 20–50 years after UV exposure has occurred [23, 24].
Cancer of the basal cell type is more common at higher elevations and lower latitudes. Climate change and sunbathing, both of which have caused increased exposure to UV, may be central to the rising rates of BCC. Tanning beds and other treatments using UVR have also been associated with enhanced threats to normal basal cell activation while condoning increased mutation. Nevertheless, neither shorter UVB radiation (290–320 nm) nor extended UVA radiation (320–400 nm) are responsible for the development of BCC. UVB radiation from the sun is regarded as the leading cause of skin cancers [25].
Mutations may occur if unprotected DNA is exposed to UV light which can break the nucleic acids’ unsaturated chemical bonds. The ozone layer in the atmosphere blocks all UVC radiation. Melanin absorbs UVA rays, which then transmit free radicals to cellular DNA which then affects the gene’s expression. The translocation of cytosine (C)-C to thymine (T)-T, or C to T, is a common kind of mutation caused by UV light. Tumor formation and proliferation may occur from this process of activating oncogenes or deactivating tumor suppressor genes [26]. While it may recover from superficial wounds, the skin cannot repair internal or genetic damage. This harm accumulates throughout a lifetime with each new episode of sun exposure [27]. Evidence suggests that mutations caused by the TP53 gene proved more frequent for BCC. Although exposure to UV is suggested as a reason for the formation of this mutation, this link has been thus far established solely in 9 chromosomes in affected individuals suffering from familial basal cell nevus syndrome (Gorlin syndrome). The tumor suppressor gene repair is impacted by this mutation [28, 29].
In both sporadic and hereditary types of BCC, the hedgehog signalizing passageway is inappropriately stimulated. Gain-of-function mutations occur in the signaling proteins such as SHH, SMO, and GLI cause (PTCH1), while loss-of-function mutations occur in the tumor suppressor protein and PTCH homologous [30]. Life-span-threat of BCC is somewhat higher for those who are constantly immune-compromised, such as those who have had an organ or stem cell transplant or those who are living with HIV/AIDS [31, 32].
Individuals receiving organ transplants should be advised to restrict exposure to the sun and warned that they could have a higher risk of developing skin cancer. There may be a synergistic relationship between immunosuppression and sun exposure during the progression of skin cancer. Between 65% and 75% of immunosuppressed individuals get skin cancer, a percentage which is 10 times higher than that of the overall population. Recipients deployed for organ transplants have an increased likelihood of contracting skin cancer. Among this vulnerable group, the incidence of skin cancer could hit 100 each year [33, 34].
A skin biopsy is frequently carried out for the confirmation of the diagnosis as well as the identification of BCC’s histologic species [35]. Generally speaking, a shave biopsy is all that is needed. An excisional or punch biopsy generally requires evaluation of the shallowness of a pigmented abrasion if it turns out to be malignant melanoma since it might be challenging to differentiate between melanoma and pigmented BCC in such cases [36, 37]. Although BCC may usually be diagnosed with a superficial sample that includes the dermis, the tumor formation could be overlooked. For instance, an ulcerated BCC might re-epithelialize with the common epidermis while cancer persists at a deeper level [38]. Although the biopsy of a BCC could comprise half to a full lesion, sampling of the clinical margins should be halted for a definitive diagnosis to be made. Even though they are not often used, punch biopsies are a quick and easy approach to retrieving a substantial specimen. If a tumor is large or has many different morphologies, several biopsies may be necessary to get a confirmed diagnosis [39].
Histological confirmation is required for the proper and final diagnosis of BCC for example, the eyelid, and this is often obtained by excisional (punch or shave) biopsy. This method of sampling provides details about the BCC’s histological subtype. Cytology, on the other hand, is a quick option that might potentially provide and help confirm a diagnosis during the first visit [40]. Although its sensitivity in detecting BCC of the eyelid remains unclear, the consensus is that the method is accurate. There is doubt on whether its sensitivity is sufficiently high for use in preoperative surgical planning. Cytology traced through excisional biopsy provides 92% susceptibility and 75% prediction accuracy for diagnosing BCC, according to research by Barton et al. [41]. A comparison was made against those from the secondary set of affected individuals undergoing incisional biopsy, etiology assessment, and abscission with subsequent etiology authentication. This detection rate for BCC was 100% in the second group, and their accuracy in making predictions was 96%.
Many are skeptical about using ultrasound technology. Despite its widespread use, ultrasound equipment with high-frequency (20 MHz) and ultra-high-frequency (40–100 MHz) ranges provides only 20% success rate in identifying malignant tumors as opposed to benign ones. Intriguingly, assertions of correct tumor size and depth of invasion are still highly debated [42].
Ophthalmologists may benefit from using laser Doppler as a complementary technique for distinguishing benign from malignant adnexal skin lesions and identifying the tumor border. The cutaneous perfusion rate in the eyelids is much elevated than in different areas of the human body (e.g., forearm). It has also been established that pretarsal skin has a mean perfusion that is 50% higher than that of perceptual skin. The cutaneous perfusion of patients with eyelid BCC was much greater than that of those without the disease [43].
It has been suggested that artificial intelligence (AI) might be used to aid in the diagnosis of skin cancer at an early stage. Skin cancer detection may be difficult, time-consuming, and costly. AI is being utilized to aid in the detection and treatment of skin cancer [44]. Convolutional neural network (CNN) and deep CNN (DCNN) are two examples of AI-based technologies that have been created to identify and categorize skin cancer [44, 45]. Several mobile health applications have already used AI-based algorithms, making this method readily available to the public [46]. One may save both money and time by using AI for skin cancer screening.
AI-based algorithms for skin cancer identification have been the subject of several research looking at their diagnosis accuracy. One research has shown that both AI-approaches (CNN and DCNN) could accurately categorize photos of different skin lesions in terms of their potential danger [47]. Screenings that may be performed during normal primary care visits or even by the patients themselves were the focus of another research that created a suspicious pigmented lesion (SPL) analysis system utilizing DCNNs to detect skin lesions that warrant further examination [48]. However, how and for whom AI-based algorithms should be used in clinical treatment is still debatable.
Although AI is as good as human dermatologists at spotting skin cancer in dermoscopy images, it may miss the diagnosis if the photo is only taken from one angle or if the lighting is poor. Therefore, doctors should not rely only on AI-based algorithms for skin cancer screening; instead, they should utilize them as one tool among several [49].
BCC cases almost need surgical treatment [50]. The surgical procedure will vary according to the kind, depth, and location of the tumor. Local therapy with chemotherapy and immune-modulating medicines may be helpful in certain cases of BCC. To be more specific, these substances may cause a reaction in small superficial BCCs. The Food and Drug Administration (FDA) of the United States has approved topical imiquimod 5% cream for the treatment of non-facial superficial BCCs with a radius lower than 2 cm. It provides common practice for treating lesions once a day, five days a week, over 6–12 weeks [45, 46].
Surface BCC may also be treated with topical fluorouracil, which undergoes approval from the FDA for its application twice daily, 3–6 weeks regimen [51]. Fluorouracil is often given for small peripheral BCCs over the trunk as well as extremities region, even though no recognized restrictions on the administration of fluorouracil based on lesion size or location have been established. Those who are predisposed to developing BCC may benefit from topical administration of imiquimod or fluorouracil, likely for the treatment of precancerous tumors.
Generally, hedgehog pathway inhibitors (HHIs) are applicable for treating local advanced BCC in patients who will not undergo surgery or radiation therapy, or in patients whose infection returned following treatment with any of these modalities, as well as patients with metastatic BCC [49, 50]. In 2012, the FDA approved the primary HHIs, vismodegib (ERIVEDGE®) [52, 53] whereas, in the year 2015, the FDA approved sonidegib (ODOMZO®) [54, 55]. These medicines block the action of SMO, a transmembrane protein essential in signaling transduction along the whole hedgehog pathway [56–60].
It has been suggested that persons with metastatic BCC who are resistant to HHIs may benefit from a combination of itraconazole along with arsenic trioxide. Treatment with intravenous arsenic trioxide for 5 days, every 28 days and oral itraconazole for days 6 through 28 days. Different researchers have suggested that maintenance of dose might be required for effective inhibition of the hedgehog pathway as well as causing the therapeutic response since some patients reported 3 months of steady disease but no shrinkage of tumors [61].
Cemiplimab (LIBTAYO®), the first immunotherapy, received accelerated approval in February 2021 for infected individuals suffering from metastatic BCC previously treated with an HHI or for whom an HHI is not appropriate [62]. Small superficial BCCs in low-risk areas may be treated with 5-fluorouracil (5-FU) 5% cream [63]. Preventing the methylation of deoxyuridylic acid and inhibiting thymidylate synthase both slow down DNA synthesis and put a stop to cell growth. Around 80% of cure rates had been reached for well-selected (e.g., thin) tumors. The cream is often used 2 times per day for not less than 6 weeks for treatment of superficial BCC [63]. As 5-FU is effective against BCCs which are very compact to view from the naked eye, it shows its applicability in people suffering from basal cell nevus syndrome or preventively treating subclinical tumors. Nonetheless, since few tumors behave in the same way, close observation of patients is essential.
Since it may not reach tumor cells deep enough in the dermis, 5-FU is usually not recommended in different categories of BCC. Itching and crusting are normal and expected; severe itching and discomfort may occur, but scarring is not as much. There is an alarmingly high rate of recurrence [63]. Recombinant DNA technology was used to create the protein known as interferon alfa-2b, which is produced by the interferon gene [59–61]. Effectiveness in treating tiny, nodular, and superficial BCCs has been shown. A high rate of success in treating BCC malignancies (up to 80%) has been achieved. Responses of BCCs to intralesional interferon alpha had been demonstrated to be variable in several preliminary studies [64–66]. Three instances of principal non-recurrent BCC along with 5 instances of principal superficial BCC were treated when 1.5 million IU of interferon alfa-2b were administered intralesionally 3 times weekly for 3 weeks in an exploratory experiment done by Greenway et al. [67].
Imiquimod 5% cream (ALDARA®) had received approval through the FDA for treating non-facial superficial BCC [68]. Several studies have demonstrated that imiquimod when given twice daily for 6–12 weeks, can fully cure superficial BCC. Treatment frequency is often increased from 3 times to 7 times weekly to once or twice daily as tolerated for maintaining mild to moderate irritation of the skin [64, 65]. Patients may control various levels of skin irritation by adjusting the frequency of application [69, 70]. Therapy courses are typically 12 weeks long, however, they do not have to be taken consecutively [71].
Topical imiquimod cream (once a day, 5 times a week, for 6 weeks) was found to be better in comparison to methylaminolevulinate photodynamic treatment in a trial involving 601 patients going through the histological confirmation of superficial BCC, while topical fluorouracil (twice a day, for 4 weeks) found to be non-inferior (two sessions with an interval of 1 week). Patients treated with methylaminolevulinate photodynamic therapy (PDT) had a lower rate of tumor recurrence at 3 and 12 months after therapy than those managed with imiquimod cream (83.4%) or fluorouracil cream (80.1 %) [72].
The selective acetylenic retinoid tazarotene (Tazorac®) might be applicable for the safe treatment of minor BCCs. Inhibiting cell growth and promoting apoptosis in BCC cells is thought to be how tazarotene causes this kind of skin cancer to regression. The majority of resistant tumors displayed keratotic differentiation, and in one case series, 70.8% of BCCs shrank by more than 50% clinically and dermoscopically, and 30.5% cured without outbreak following 3 years. This study treated 154 small, superficial, and nodular BCCs with tazarotene gel at a concentration of 0.1% for 24 weeks (109 patients). Alterations were tracked using dermatoscopy and histological analysis [73].
The application of topical tazarotene for treating BCC has not been approved as yet. Long-term treatment for 5 to 8 months is required. Instances of dry or irritated skin had been reported as a side effect of tazarotene, however, these episodes resolved after the drug was discontinued.
Radiation therapy may be applicable for large wounds along with patients who are not surgical candidates, since BCCs are commonly radiosensitive (e.g., due to allergy from anesthetics, recent anticoagulant treatment, the inclination for the formation of keloids) [74–76]. Recurrence rates for BCC at 2 and 5 years were 2.0% and 4.2% respectively, through the retroactive study of 1,715 histologically compulsive primary cutaneous carcinomas [712 BCCs, 994 squamous cell carcinomas (SCCs), and 9 tumors with definite BCC and SCC properties]. The recurrence rates of SCC were 1.8% in 2 years and 5.8% in 5 years [77]. Patients with aggressive malignancies who underwent surgery may benefit from adjuvant radiation therapy after the procedure [78]. Patients with advanced local BCC undergo absolute remission in about 70% of cases after treatment [79].
Because of its high success rate, radiation was formerly the treatment of choice. However, in view of the effort and expenses involved, it is now used occasionally. Radiotherapy is a realistic alternative for the treatment of recurrent cancers, due to advances in the development of surgical techniques and other therapeutic methods. This option may be saved for more severe or difficult first lesions that need oculoplastic surgery. Moreover, it slows down the need for skin grafting where surgical intervention might leave a big scar.
To treat BCC, PDT has been employed for over 20 years [75, 76]. To photoexcite applied porphyrins on neoplastic and preneoplastic cells, PDT makes use of certain wavelengths of light. The oxygen in the surrounding tissue rapidly soaks up the extra energy, forming singlet oxygen radicals. These free radicals have a rapid cytotoxic effect on the surrounding tissue. In the United States FDA provides only licensed 5-aminolevulinic acid for PDT of actinic keratoses. After sitting for 1 h, the material is exposed to blue light for one minute.
Whether administered orally, intravenously, or topically, PDT localizes into tumor cells before being activated by light exposure (e.g., laser). These treatments are usually only used as a last resort because of their limited efficacy. The cosmetic outcome of PDT is good, but it might cause some unpleasant side effects such as local edema, erythema, blistering, and ulceration [80, 81].
The success rate of PDT in curing superficial BCC was only 50% in research carried out by Calzavara-Pinton et al. [82], whereas it was 83% in curing nodular BCC. For eyelid BCC, PDT had not shown more effectiveness than other standard therapies. PDT with methylaminolevulinate has demonstrated great effectiveness and may be a treatment option for those with BCC in the eyelid, according to research by Puccioni et al. [83].
The FDA had approved vismodegib (ERIVEDGE®) as the first-line treatment for the therapy of advanced BCC. SMO, a crucial transmembrane protein in hedgehog signal transduction in malignant epithelial cells, had undergone downregulation [84]. The FDA granted acceptance based on data from a unique, global, and open-labeled research (n = 104). A total of 96 of the 104 people were usable for analysis. Out of 33 individuals, none of them had a complete response. Twenty-two percent (n = 63) of patients suffering from local advanced BCC had limited response whereas 20% showed a complete response [85]. The mechanism of action of vismodegib is described in Figure 2.
In 2015, the FDA approved sonidegib (ODOMZO®), a second HHI. In infective individuals suffering from local advanced BCC refractory to restorative radiation or surgery, or with metastatic BCC, the approval was focused on the BCC outcomes with LDE225 treatment (BOLT) trial. Participants were randomly assigned to receive either 200 mg or 800 mg of sonidegib orally once a day, allocations made according to disease stage, histological subtype, and geographic region. Three affected individuals in the 200 mg group got a complete reaction, while other 35 got a limited response, with a total response rate of 58%. A fraction of affected individuals experienced tumor reduction for at least 6 months, and this number ranged from 1.9 months to 18.6 months [86]. The mechanism of action of sonidegib is described in Figure 3.
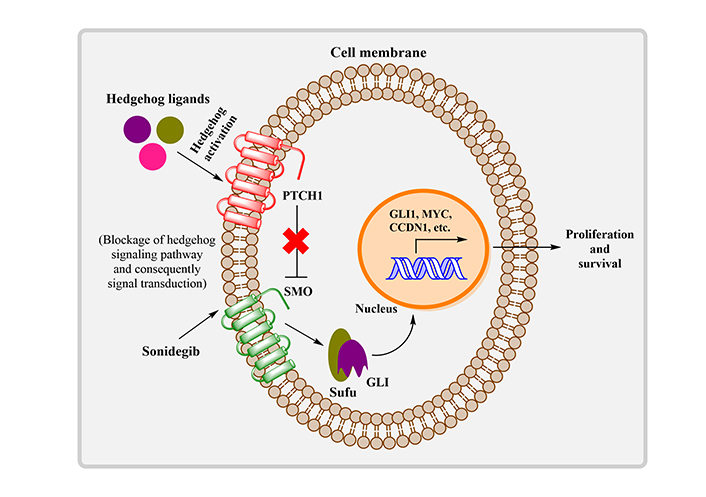
The mechanism of action of sonidegib. MYC: myc proto-oncogene protein; CCDN1: cell cycle regulator G1/S-specific cyclin-D1; Sufu: suppressor of fused
Once the hedgehog pathway was suppressed, the first immunotherapy approved by the FDA for advanced BCC was cemiplimab, an inhibitor of programmed death. The drug received full approval in 2021 for treating individuals suffering from local advanced BCC along with fast-track authorization for individuals suffering from metastatic BCC [87].
Study 1620, a phase II, nonrandomized, and open-label investigation, provided the evidence needed to provide clearance for locally advanced BCC. Cemiplimab was administered to patients (n = 112) with locally advanced or metastatic BCC, whose BCC had advanced while on HHI therapy or those who were not eligible for HHI therapy, had severe toxicity, or who were at the end of the intended course of treatment. Both persons with locally advanced BCC [objective response rate of 29% (95% confidence interval: 19–40%)] and those with metastatic BCC [objective response rate of 21% (95% confidence interval: 8–41%)] had high rates of response. Seventy-nine percent of advanced local BCC responders and all metastatic BCC responders had a noticeable duration of response (DOR) for 6 months. The median DOR was not achieved in any treatment group [85].
Polymeric micelles have become increasingly important as a drug carrier in recent years for nanoscale drug delivery systems. The majority of skin diseases would benefit from topical drug applications. The properties of the skin barrier have a major influence on the concentration of effective drugs. Although polymeric micelles, a novel drug carrier, are better at delivering drugs through the skin than conventional dosage forms such as cream, ointment, and gel, topical treatment with these products is ineffective due to low drug permeation into the targeted skin layers. Polymeric micelles have many advantages as nanocarriers, including improved drug solubilization in the skin, increased hydrophobic drug separation in the stratum corneum, drug delivery to hair follicles and keratinocytes inside epidermis sheets as well as provision of sustained and slow drug release [88]. It appears that the micelles describe an efficient carrier for targeted drug delivery though the barrier of the skin is altered for dermatological conditions such as acne, psoriasis, and burns [89]. The different applications given by polymeric micelles for the management of skin cancer [90] have been established in Table 1.
Different polymeric micelles in skin cancer management
| No. | Polymeric micelles | Diseases |
|---|---|---|
| 1 | Doxorubicin | Lymphoma skin carcinoma |
| 2 | Phthalocyanine | Dermis phototoxicity |
| 3 | Dexamethasone | Lymphoma skin carcinoma |
| 4 | Celecoxib | Non-lymphoma skin carcinoma |
| 5 | Docetaxel | B16F10 melanoma |
| 6 | Sunitinib | Melanoma skin cancer |
| 7 | Redaporfin | Pigmented melanoma |
| 8 | Zinc(II) phthalocyanine | Metastatic melanoma |
The major goal of active targeting is the enhancement of drug movement at the main location through particular cooperation such as the binding of antibodies and antigens, or local beard paths which include heating and ultra-sonication. As a result, it is possible to actively engineer the ligand coupling of the carrier or the aggregation of the pH-perceptive moiety, enabling the biological characteristics of tumor tissues such as the over-expression of cell surface cancer-related antigens that are present in normal tissues, the low levels of tumor-specific antigens, and the tumor’s relative higher acidity (pH 7.0) as compared to normal tissue’s (pH 7.4). This drug is only combined in the tumor sites using this technique, which also allows for cellular uptake of the drug via endocytosis [91].
With the availability of enhanced permeability and retention (EPR) effect, the polymeric micelle can passively target the solid tumor. Studies in the fields of pharmacology, biochemistry, and pathology reveal that solid tumors frequently exhibit pathophysiological traits such as immature lymphatic capillaries, partial vasculature architecture, hypervascular, and evacuation of vascular permeability aspects that mimic discharge [92]. Numerous characteristics of the tumor vasculature, such as increased asymmetry, abnormal basement membrane production, an abundance of proliferating endothelial cells, and a lack of pericytes, lead to rapid vascularization, which is necessary to supply oxygen and nutrients to flourishing tumors. These features also increase the permeability of macromolecules via blood vessels. Additionally, because the lymphatic drainage system cannot function properly due to the premature lymphatic capillaries, macromolecules also engaged through an extended period in the tumor interstitium [93].
As a result, several routes proved that this EPR effect leads to a passive aggregation of nanoparticles along with macromolecules inside the solid tumor, raising the value of the therapeutic index and lowering adverse ill-effects. Additionally, the recent discovery states the proper orifice size inside vasculature in the range between 200 and 600 nm in the size of human tumors, allowing for passive tumor targeting [94]. Numerous factors, including the release of PGs, primary fibroblasts, bradykinin, nitric oxide (NO), the development factor in tumor tissues, and the overexpression of genes like vascular endothelial development factor or vascular penetrability factor cause the tumor microvasculature to be more permeable. According to the research, the permeability of the tumor’s blood arteries varies depending on the type of tumor, where in the body it is located, and how far along it is in the development process. The physicochemical characteristics of the polymer also influence how much extravasation of polymeric nanoparticulate occurs [95].
Hydrogels are hydrophilic polymers having a three-dimensional (3D) lattice that can store large amounts of water. Different types of natural or biocompatible synthetic polymers may be utilized for their development [90–93, 95–98]. Due to their distinctive qualities, such as ease of handling, higher water content, tunable mechanical strength, and manageable curing and swelling kinetics. Drug delivery systems frequently use hydrogels, tissue engineering, and wound healing over the past few eras [99–104]. In comparison to other conventional drug delivery therapies, the use of hydrogel-based formulations as an anti-proliferative delivery strategy in melanoma skin tumors offers many benefits [103]. The side effects of this hydrogel formulation were less severe than those of synthetic chemotherapy. By lengthening the half-life of the drug, enabling controlled drug release, and subsequently reducing non-targeted exposure, chemotherapy and gene therapy have both been demonstrated to be more successful when delivered using hydrogel-based drug delivery systems. Researchers have many options with hydrogels to improve cancer drug delivery systems [105]. Various polymers/hydrogel systems employed in the treatment of skin cancer are detailed in Table 2.
Description of various polymers/hydrogel systems utilized for the treatment of skin cancer
| No. | Polymers (hydrogels) | Cell/cell lines | Type of cancer | Results | References |
|---|---|---|---|---|---|
| 1. | Chitosan hydrogel | A mouse model of CD8+ T cells | Skin cancer | Similar results from immunization as that of dendritic cell vaccination in cancer treatment | [106] |
| 2. | Polyethylene glycol (PEG) hydrogels | Human cell lines produced from metastatic melanoma (A375) and the radial growth phase (WM35) | Skin cancer | PLX4032 (vemurafenib)’s cytotoxicity decreased on more flexible substrates via metastatic A375 cells, which resulted in decreased proliferation rather than an increase in death | [107] |
| 3. | PEG-peptide hydrogels | Radial growth phase human melanoma cells (WM35) and metastatic cells (A375) | Skin cancer | Drug sensitivity in cells was reduced by 3D spherical models compared to 2D ones | [108] |
| 4. | Lanthanum-doped chitosan (La-CS) hydrogels | Mouse melanoma cells (B-16) and skin fibroblast cells (L929) | Skin cancer | B-16 melanoma cell proliferation is anticipated, while L929 skin fibroblast cell toxicity is decreasing | [109] |
| 5. | Sericin/dextran composite-hydrogels | Human liver cells (HL7702) and mouse myoblast cells (C2C12) | Skin cancer | Substantial conquest of cancer growth via hydrogel loaded with doxorubicin | [110] |
| 6. | Paclitaxel-encapsulated cell-penetrating-peptide-modified transfer-somes-embedded hydrogel (PTX-CTs/Gel) | Mouse melanoma cells (B16F10) | Skin cancer | A decrease in cancerous cell proliferation combined with systemic chemo through Taxol® | [111] |
Due to a magnetic field’s capacity to functionalize and steer them, magnetic nanoparticles (MNPs) are characterized as cutting-edge tools in the medicinal field. Magnetic elements like nickel, cobalt, and iron, and their oxides are frequently found in MNPs [112]. Hyperthermia, tissue repair, magnetic drug targeting, cell and tissue targeting, improved resolution magnetic resonance imaging (MRI), and transfection are examples of potential MNP biomedical applications. Hyperthermia, which is regarded as a non-invasive cancer treatment methodology, is one of the most promising uses of MNPs and is made possible by the usage of magnetic field-induced excitation of MNPs. Additionally, it has been determined that magnetic iron nanoparticles are an auspicious tool for site-specific drug delivery, and therapeutic and diagnostic tools [113].
Late in the 1970s, the idea of adopting MNPs for targeting tumor cells inside a person’s body to treat cancer first emerged. MNPs can be utilized to heat the tumor and kill it since tumor cells are more susceptible to temperature rise than normal cells. The cancer cells will be damaged by the introduction of MNPs inside tumors, which tend to release energy in the form of heat under exposure to a developing magnetic field [114]. The effective part of MNPs in skin cancer treatment is described in Figure 4.
Phototherapy continued as a reliable and frequently chosen treatment option for many dermatoses contempt the development of various efficient systemic medicinal and biological agents for dermatology. The major aspects which subsidize the conquest of disease activity are immune suppression, cell cycle arrest, and altered expressions of cytokines [115]. Psoriasis and other skin conditions can be treated safely and effectively with phototherapy [116]. Dr. Niels Finsen, the 1903 Nobel Laureate for the field of Medicine of Physiology, performed the first UV therapy and showed UV’s beneficial impact on lupus vulgaris, a type of tuberculosis of the skin [117]. Currently, extracorporeal photochemotherapy (photopheresis), narrowband UVB (311–313 nm), broadband UVB (290–320 nm), UVA 1 (340–400 nm), 308 nm excimer laser, and UVA plus a psoralen (PUVA) are used in phototherapy [115]. Essential elements of PDT include photosensitizers. Reduced dark cytotoxicity, tumor-specific aggregation, enhanced photocytotoxicity, reduced skin photosensitivity, and ease of application are all characteristics of an ideal photosensitizer [118]. The detailed applications of phototherapies for skin cancer therapy along with their mechanism are described in Table 3.
Applications of phototherapies for the management of skin cancer along with their mechanism of action
| No. | Source of light (phototherapy) | Target | Treatment of skin cancer | Mechanism of action | References |
|---|---|---|---|---|---|
| 1 | PUVA | Psoralen and DNA | Skin mastocytosis and cutaneous T-cell lymphoma | Apoptosis, cell cycle arrest, reactive oxygen species (ROS) production, and DNA replication inhibition | [119] |
| 2 | UVA1 (340–400 nm) | Chromophores | Atopic dermatitis, localized scleroderma | T-cell apoptosis and tissue remodeling | [120] |
| 3 | PDT | Photosensitizers | Superficial skin cancer | Apoptosis and ROS production | [121] |
| 4 | Ablation [CO2 laser (10,800 nm); erbium:yttrium aluminum garnet laser (Er:YAG laser, 3,850 nm)] | Water in and outside the cells | Superficial skin cancer | Evaporation | [122] |
| 5 | Non-ablation (dye laser) | Hemoglobin (Hb) | Telangiectasia, vascular lesions, as well as hemangioma | Photoselective thermolysis | [123] |
| 6 | Extra-corporeal photopheresis | Chromophore | Erythrodermic cutaneous T-cell lymphoma | T-cells depletion | [124] |
There is effective as well as appealing methods for treating skin cancer using nanoparticle-based medication delivery. This method boosts therapeutic effectiveness and efficiency by delivering biomolecules to specific locations with little or no side effects [125, 126]. Topical treatments based on nanoparticles are yet to receive commercial approval. For use in dermatology, several technologies using nanoparticles have been developed, manufactured, and controlled [127]. Metallic nanoparticles, which are composed of gold, silver, and metallic oxides, are commercially available and are being researched for use in the dermal delivery of active pharmaceutical ingredients. The particles in the metallic nanoparticles are aggregated on the surface and do not have any adverse effects on the epidermis [128]. These nanoparticle-based formulations have not yet received FDA approval in topical/dermal skin cancer therapy, even though nanoparticle-based therapy is receiving significant awareness and interest for the therapy of skin cancer and different disorders [129]. Clinical trials for NB-001, a brand-new nano-based topical antiviral emulsion, are being conducted for cold sores and herpes labialis which was developed by NanoBio® Corporation [130]. Furthermore, paclitaxel formulations based on nanoparticles are currently being tested in clinical trials to determine their safety, acceptability, and effectiveness in the treatment of cutaneous melanomas under non-melanoma skin cancer [130].
Vinca alkaloids, taxanes, dacarbazine, temozolomide, cisplatin, and nitrosoureas are some of the medications used in skin cancer chemotherapy. Cisplatin is widely used to treat melanoma skin cancer [131]. Actinic keratosis and BCC are two skin cancer disorders that are intensively and widely treated with a different medication called 5-FU. 5-FU has a strong hydrophilic character and may penetrate the subcutaneous layer to reach tumor tissues and approach the target [132].
Dacarbazine is an active medication with a short half-life and low solubility that was used to treat skin cancer. The FDA provides approval for dacarbazine to be used as a single agent for skin cancer therapy due to its anticancer properties. Dacarbazine was a great choice for melanoma skin cancer chemotherapy. To be delivered topically in melanoma skin cancer therapy, dacarbazine is enclosed inside lipid nanoparticles. The FDA also recommended carboplatin as therapy for melanoma skin cancer. It belongs to the class of second-generation drugs with platinum compounds. Through intratumoral injection, carboplatin undergoes loading through polycaprolactone nanoparticles using chitosan-glycerophosphate gel [133]. In 2017, Su et al. [134] created the paclitaxel-loaded copolymer nanoparticles as well as discovered the anticancer impact of carboplatin during in vitro and in vivo testing. Additionally, drugs used to treat melanoma skin cancer are cisplatin, nitrosoureas, vinca alkaloids, taxanes, and temozolomide [135]. Temozolomide was created as solid lipid nanoparticles, and these were used in the administration of temozolomide. For targeting human melanoma cells via an in vitro setting, temozolomide was also delivered via polyamide amine (PAMAM) dendrimer [136]. Ferrous oxide nanoparticles are regarded to be superparamagnetic materials because they acquire a significant amount of magnetic incentive through an exterior magnetic field, making them promising for biomedical applications [137]. They can be used as an MRI contrast because they provide a lot of contrast per unit, requiring just a small number of particles for imaging and lowering toxicity [138]. These particles can transform the energy of an external magnetic field into heat that can be used to treat cancers since tumor cells are more susceptible to high temperatures than healthy human cells [139]. Their utility can be further increased by adding different functional groups to their surface, which improves their biocompatibility and biodegradability. Additionally, polymers that can be added to the surface, such as dextran, cellulose, polylactide-co-glycolide (PLGA), or PEG improve their biodegradability and biocompatibility [140].
The main system that cancer affects is the immune system. Antigen presenting cells (APCs), which include dendritic cells (DCs), make tumor-associated antigens (TAAs) available to T cells in an unstoppable antitumor response. When the receptors were triggered by peptides and major histocompatibility complex (MHC) molecules, the T cells became active. After that, these effector cells pass through the tumor and eventually penetrate the tumor tissue [141]. The entire process will result in the production of the cells necessary for the growth of skin cancer. To overcome these challenges, cytokines were used as the initial immune therapy indication in melanoma skin cancer treatment, which improved patient survival in the long run. This strategy was effective in getting support for its goals of fostering cell proliferation and immune system activation. A high dose was required for the application procedure because cytokines have a limited half-life. Patients experience numerous unneeded side effects as a result of the high amount being administered [142].
The goal of cancer vaccines is to provide potential means, by enhancing the immunological memory of TAAs, to produce enduring reactions to the formation of cancer. DNA- or RNA-based nucleic acid cancer vaccines are also possible. This strategy requires that the DNA or RNA be taken up by the APCs and translated to result in the synthesis of the antigen. Clinical trials revealed no meaningful clinical outcomes despite the intriguing results of the nucleic acid vaccine in melanoma animal models, often due to obstacles to nuclear delivery and immunogenicity. Another innovative method of cancer prevention is TAA peptide vaccination [143].
The concept of gene therapy was developed in 1966, and to put it together, nucleic acids were utilized to replace a damaged or missing gene. Plasmid DNA (pDNA), RNA interference (RNAi), and antisense oligonucleotides (AONs) are three different groups of genetic materials that can be used in gene therapy to cure cancer. Depending on the targeted material from the aforementioned categories, these methods will allow the blow-in or give away of a specific gene. Additionally, manufactured nanoparticles have improved the local application of genetic material transport and transfection. RNAi molecules were also used intratumorally in a variety of ways with liposomes to further the development of effective therapies for melanoma skin cancer management [144].
In a recent study, charged nanoparticles coupled with vaccines eliminated tumors or prolonged life in malignant mice [145]. The researchers said the nanoparticle may be mass-produced, maintained at room temperature, and delivered by general practitioners to treat various tumors. Mice with melanoma tumors were injected with lab-made charged nanoparticles. The nanoparticles helped the adenovirus vaccine-triggered melanoma-fighting cells find the tumor and defeat its defenses (as shown in Figure 5). Five of nine mice given the nanoparticle-adenovirus vaccination combination were cancer-free, while the other four lasted more than 100 days—3 times longer than those given simply the vaccine and 5 times longer than those given nothing. Cytotoxic T lymphocyte cells, which fight cancer, are activated by the adenovirus vaccines. Tumors release chemical signals to appear harmless and avoid discovery.
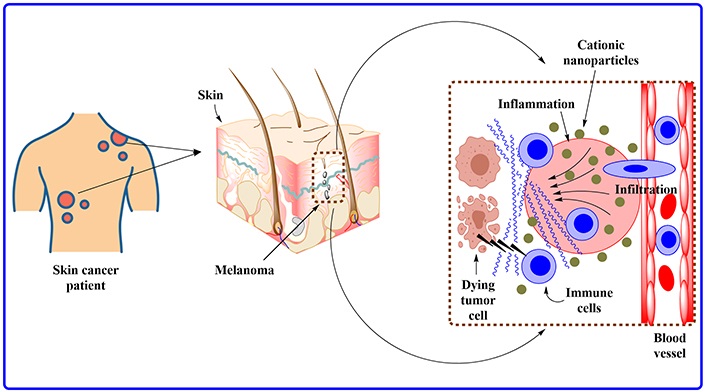
The image depicts a melanoma tumor complete with its supply of blood and lymph fluid. This zoomed view demonstrates how the charged nanoparticles recruit the body’s immune system to track down and destroy the tumor
Drug delivery devices called nanogels can be used to treat a variety of illnesses. Numerous studies have been conducted on the development of nanogels for medication delivery, especially in cancer theranostics [146]. Ethosomal nanogels may provide promise for skin cancer therapy. This controlled formulation displayed Z-average values of 125.67 nm, apparent zeta potential values of –17.1 mV, and average flux values of 54.72 and 59.83 µg/cm2 per hour for drug-loaded ethosome, respectively. The ethosome that was loaded with rhodamine B also reached a depth of 183.82 µm. The analysis of nanogels texture revealed an index in the range of 225.45 g/s for viscosity, 209.34 g for firmness, 189.48 g for cohesion, and 59.45 g/s for consistency. This improved ethosome nanogels significantly reduced the growth of the B16F10 murine tumor cell line (P < 0.05) [147]. Sahu et al. [148] investigated the toxicity and penetration potential of a biocompatible chitosan nanogel encapsulating capecitabine employing an ionic interaction mechanism with pH-triggered transdermal targeting. Using Pluronic F127 as an ion gelation catalyst and Transcutol® as a surface decorating agent to improve non-ionic penetration, the chitosan-capecitabine nanogel (CPNL) was created. The CPNL exhibits fine form and nano size range when studied by transmission electron microscopy (TEM), scanning electron microscopy (SEM), and dynamic light scattering (DLS) analysis with cationic charge and slightly acidic pH assessed by zeta potential and pH analysis. It displayed drug-release properties that were pH-dependent and mimicked the microenvironment of skin cancer. On the human keratinocyte cell line (HaCaT), the CPNL’s apoptotic index and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay exhibited the best cell retention and toxicity after 24 h of treatment. The ex vivo examination of skin penetration revealed notable diffusion and penetration capabilities.
The in vitro anticancer potential of PLGA nanogels loaded with 5-FU (FPNGs) and glazed with nerolidol sesquiterpene was investigated by Rathore et al. [149] in 2021. Both normal PLGA nanogels and FPNGs were created using the emulsification-solvent evaporation process. Nerolidol (2%) sesquiterpene was used as a surface coating to increase the effectiveness of nanogels’ permeation inside the stratum corneum. This size range of the nanogel formulation FPNGs is 220 ± 0.25% nm, as determined through the DLS method. At various pH ranges, the entrapment efficiency was calculated to be around 42% for the release network in a sustained manner over 24 h. Using the HaCaT cell line, confocal microscopy examination revealed the profile of cell uptake and localization. The MTT experiment showed that nanogels were compatible with cells, and the apoptosis assay, which showed an apoptotic index of 0.87, confirmed this experiment. They found that FPNGs are a powerful nanogel system for treating skin cancer that may be used to boost the chemo-therapeutic potency of bioactives with sustained and controlled release at the targeted site [149].
Biocompatible and biodegradable chitin was mixed with the anticancer drug curcumin to form curcumin-loaded chitin nanogels (CCNGs). Curcumin and chitin are both insoluble in water. The produced CCNGs do, however, disperse quite well and steadily in water. DLS, SEM, and Fourier transform infrared (FTIR) analyses of the CCNGs revealed particles in the spherical size range of 70–80 nm. When compared to neutral pH, the CCNGs released more at an acidic pH. Human dermal fibroblast cells (HDF) and A375 (human melanoma) cell lines were used to test the cytotoxicity of the nanogels. According to the findings, CCNGs are less harmful to HDF than melanoma at concentrations between 0.1 mg/mL and 1.0 mg/mL. The apoptotic effect of CCNGs was investigated using a flow-cytometric assay, and the findings indicate that at higher concentrations of the cytotoxic range, CCNGs showed comparable apoptosis to the control curcumin, however, the control chitin nanogels had little impact on apoptosis. In comparison to the steady-state transdermal flow of the control curcumin solution, the CCNGs demonstrated a 4-fold growth. The epidermal horny layer appeared to be loosening in the histological analyses in specimens of porcine skin treated with the produced components, allowing permeation without any visible evidence of irritation. These findings imply that by successfully penetrating the skin, the synthesized CCNGs have unique benefits for melanoma therapy which is the main prevalent and dangerous category of skin cancer [150].
According to Soni et al. [151], cross-linked swell-able polymer networks produce nanogels, which are 3D hydrogel materials with a high capacity to hold water. They do not dissolve into the aqueous media. Nanogels’ size range is from 20 nm to 200 nm and combines the properties of nanoparticles and hydrogels [146, 147]. Nanogels’ gel property makes them able to expand when in contact with bodily fluids [152, 153]. Because of this, they are adaptable in near passage to the target location and increase the circulation of treatments along with drugs. Additionally, the design of nano gels helps in drug release in particular anatomical circumstances [154]. They can easily pass through biological barriers and membranes since they are nanoparticles. They help get medications over the blood-brain barrier. They can avoid the reticuloendothelial system as well. Because of this, nanogels exhibit great stability, biodegradability, and biocompatibility [155]. For the prospective treatment of various illnesses, including but not limited to cardiovascular ailments, tumors, and other malignancies, nanogels are adaptable and suited to be produced in diverse dosage forms. These are just a few of the many distinctive characteristics that nanogels possess [156]. Nanogels can be used in theranostics and medical imaging, and they are effective in medication cocktail therapy. This functionality is crucial for lowering tablet loads and enhancing the effectiveness of various illness conditions’ treatments. In 2018, Tran et al. [157] developed nano-gel formulations containing paclitaxel and 5-FU for combination cancer therapy using PEG methyl ether (mPEG) and chitosan. Using therapeutic agents in combination has greatly improved the efficacy of cancer treatment, however, improving the efficiency of medicine delivery still has problems. According to a study by Liu et al. [158] published in 2022, doxorubicin and olaparib were co-loaded on disulfide bond cross-linked polypeptide nanogels for the treatment of breast cancer in mice models. This drug undergoes immediate release for targeting cancer through nanogel when a high glutathione environment is stimulated in cancer cells. In addition, when compared to free drugs and single-drug-loaded nanogels, dual-drug co-loaded nanogels exhibit the highest anticancer activity and have good biological safety. Doxorubicin and olaparib can therefore be delivered simultaneously using polypeptide nanogels, which has promising potential for usage as a cancer treatment. The most dangerous kind of skin cancer is melanoma. Chemotherapeutic drugs cannot be used topically due to the skin’s physiological makeup, low selectivity, and poor efficacy [148].
Liposomes are effective delivery systems for medicinal medicines because of their hydrophobic lipid composition. Nevertheless, creating the ideal liposome complexity is greatly increased when medicinal medications are delivered effectively to treat melanoma. Targeting multiple pathways to have a cooperative and possibly synergistic effect, lengthening circulation time in the body using PEGylation or other modifications, using a targeted delivery method by conjugating the therapeutic agent to an antibody or peptide, and preventing immune response or toxicity while still protecting the drug or nucleic acids that are encapsulated are all significant considerations. By concurrently targeting many cancer cells, liposomes may represent the next therapeutic advance for melanoma [159]. Compared to more conventional therapy methods like parenteral and oral administration, skin therapies using liposomes are less hazardous. Furthermore, the addition of edge activators to the liposomes results in a less rigid and more flexible bilayer structure, enhancing skin permeability [160]. A team of researchers investigated a brand-new treatment option for SCC. For a cell line in skin cancer that was epidermal growth factor receptor (EGFR)-positive, cetuximab immuno-liposomes significantly increased cellular uptake. Additionally, in comparison with 5-FU solution injection, iontophoresis of liposomes and immune liposomes decreased 5-FU penetration through the skin, which may have a lessening influence on systemic effects. Additionally, when immune liposomes were applied by iontophoresis in comparison to liposomes, significant difference in results were reported [161].
In a study, the effectiveness of co-delivering curcumin and anti-signal transducer and activator of transcription-3 (STAT3) small interfering RNA (siRNA) using cationic liposomes to treat skin cancer was investigated [162]. The curcumin was encapsulated in 1,2-Dioleoyl-3-trimethylammonium propane (DOTAP)-based cationic liposomes and then coupled with STAT3 siRNA. This nano complex’s zeta potential, encapsulation efficiency, and typical particle size were all measured. Cell viability studies in B16F10 murine melanoma cells showed that the co-delivery of curcumin and STAT3 siRNA dramatically reduced the proliferation of cancer cells compared to either liposomal curcumin or STAT3 siRNA alone. The curcumin-loaded liposomes were able to penetrate the skin up to a depth of 160 μm following iontophoretic (0.47 mA/cm2) application. The in vivo effectiveness studies made use of the mouse model for melanoma skin cancer. In comparison to either liposomal curcumin or STAT3 siRNA alone, co-administration of the curcumin and STAT3 siRNA using liposomes significantly slowed the progression of the tumor as indicated by tumor volume and tumor weight. Additionally, the iontophoretic injection of the curcumin-loaded liposome-siRNA combination demonstrated comparable efficiency in preventing tumor growth and suppressing STAT3 protein [162]. PDT is a unique therapy that has the benefits of being able to target tumor cells while sparing normal cells from destruction and not being limited by the size or location of skin lesions. PDT has proven to have several benefits, including low morbidity, minimal functional impairment, greater cosmetic outcomes, and the ability to use it repeatedly several times in one procedure [163]. In practical practice, PDT is an effective treatment for skin malignancies other than melanoma. PDT is being used by more and more clinicians to treat skin cancer patients, particularly older patients. Since 5-aminoulevulinic acid (5-ALA) penetration through the skin is so ineffective on its own, it is crucial to increase delivery efficacy for 5-ALA-mediated PDT, which has been used to treat cancer. According to reports, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) liposomes are effective 5-ALA delivery vehicles and could one day help in 5-ALA-mediated PDT [164].
According to reports [164], the best topical formulation for BCC patients is one that actively targets tumor cells and delivers an antitumoral drug to the tumor site in a prolonged and regulated manner. All evaluated topical preparations had high bloodstream compatibility. The drug’s permeability tests through the synthetic membrane Strat-M® showed that when liposomes were introduced into the G2 (consisting of Pluronic F-108 and water) gel as opposed to being free, the drug’s permeability was increased. The novel complex systems that were created pass the apoptosis tests with flying colors, their levels of tolerance being in the following order: L4 (5-FU liposome-loaded transdermal formulations) > C1 (oil-in-water emulsion) = G2 > G1 [full-interpenetrating alginic acid sodium salt (AG)-hyaluronic acid (HA) network]. The inclusion of AS1411 aptamer-functionalized liposomes with the C1 cream and G1 gel displays a cytotoxic impact that is more comparable to that of free 5-FU, hence it is advised that they be used as efficient drug delivery vehicles. Antitumor therapy’s therapeutic effectiveness is boosted as a result. Finally, it can be agreed that the G1 gel, which is based on biocompatible AG and HA has demonstrated positive biosafety effects, is the optimal topical formulation from all angles, and may be used as a new therapeutic technique for the treatment of BCC. Skin cancer can effectively be treated by cationic liposome-mediated iontophoretic co-delivery of curcumin and anti-STAT3 siRNA. DOTAP-based cationic liposomes were used to encapsulate the curcumin, which was subsequently combined with STAT3 siRNA. This nanocomplex was unique due to its zeta potential, encapsulation efficiency, and average particle size. Cell viability studies in B16F10 murine melanoma cells showed that co-delivery of curcumin and STAT3 siRNA dramatically reduced the proliferation of cancer cells compared to either liposomal curcumin or STAT3 siRNA alone. In order to effectively treat skin diseases, siRNA and small molecules can be delivered topically using cationic liposomes. Liposomal doxorubicin (Doxil®) has a specific pharmacokinetic profile and tissue distribution, which is reflected in the profile of its toxic effects on the skin. These skin reactions are very different from the ones linked to doxorubicin when it is not liposome-encapsulated. The numerous skin responses linked to the administration of Doxil® are highlighted and shown. Clinicians should be familiar with this range of mucocutaneous adverse effects as this medication becomes more generally known and given.
Based on searching the website: https://clinicaltrials.gov/, using various search terms such as skin cancer, BCC, SCC, melanoma, and non-melanoma, the results were chosen.
In clinical trial NCT02218164, researchers studied the effect of the combination of two types of anticancer therapy for SCC patients [165]. Researchers administered a combination of 5-FU/capecitabine as oral pills and PEGylated interferon alpha-2b (injection). Both these drugs are currently approved by the FDA for other types of cancer. The outcome of the study showed that combination drugs were able to cure the patients from carcinoma. Other clinical trials have been tabulated in Table 4.
Ongoing clinical trials under study involving nanomaterials for skin cancer
| Trial No. | Nanomaterial under study | Condition | Phase | Status | References |
|---|---|---|---|---|---|
| NCT00004104 | Liposome/interferon alfa-2b and melanoma vaccine | Melanoma | Phase 2 | Completed | [166] |
| NCT00081042 | Albumin-nanoparticle/paclitaxel (ABI-007) | Melanoma | Phase 2 | Completed | [167] |
| NCT00145041 | Liposome/vincristine | Melanoma | Phase 1 | Completed | [168] |
| NCT00626405 | Albumin nanoparticle/paclitaxel | Melanoma | Phase 2 | Completed | [169] |
| NCT01300533 | Nanoparticle/docetaxel (BIND-014) | Skin cancer | Phase 1 | Completed | [170] |
| NCT02158520 | Albumin nanoparticle/paclitaxel (Abraxane®) | Melanoma | Phase 2 | Completed | [171] |
| NCT03101358 | Topical nanoparticle ointment/paclitaxel (SOR007) | Cutaneous metastases from non-melanoma cancer | Phase 1 and 2 | Completed | [172] |
The term nanotoxicity describes the potential adverse effects that nanoparticles may have on organisms. Although nanotechnology has potential medical applications, it is important to consider the potential risks of using nanoparticles, particularly if they are going to be used to treat skin cancer. The size, shape, charge, and other characteristics of nanoparticles are among the main standards analyzed when assessing nanotoxicity for skin malignancies. Different nanoparticles have different physicochemical characteristics that can affect how dangerous they are. For instance, compared to carbon-based nanoparticles, metallic nanoparticles, such as silver or titanium dioxide, may have different hazardous consequences [173]. The normal size range for nanoparticles is 1 to 100 nm. The behavior and interaction of nanoparticles with biological systems can be influenced by their size and shape. Certain nanoparticles may have a greater ability to enter cells or more readily penetrate the skin, which could impact how dangerous they are.
The body’s natural defense against hazardous substances is the skin. Potential skin penetration by nanoparticles could result in regional or systemic consequences. Assessing the toxicity of nanoparticles requires knowledge of how well they can penetrate the skin and reach deeper layers. Nanoparticles can be engineered with specific surface coatings to improve their stability and enhance their interaction with target cells. However, the surface properties of nanoparticles can also influence their toxicity and the presence of certain coatings may mitigate or exacerbate their harmful effects. This has been reported [174] that the interaction between nanoparticles and skin cells can induce various biological responses. This can include oxidative stress, inflammation, DNA damage, or disruption of cellular functions. Such responses may contribute to the development or progression of skin cancers [175]. It is important to keep in mind that there is still much to learn about the nanotoxicity of nanoparticles for skin malignancies, and scientific knowledge is constantly changing. The potential hazards and benefits of using nanomaterials in a variety of applications, including the treatment of cancer, are currently being intensively investigated by scientists and regulatory organizations. Before any new nanoparticle-based medicines have authorization for clinical use, thorough safety assessments are usually carried out [176].
Lists patents related to the diagnosis and treatment of skin cancer utilizing nanotechnology as shown in Table 5.
Patents related to the diagnosis and treatment of skin cancer utilizing nanotechnology
| Patent No. | Description | References |
|---|---|---|
| US11419937B2 | Laser skin treatment may ablate malignant cells and damaged tissue in burn sufferers. Laser skin therapy has many cosmetic uses, including hair removal/reduction, dyschromia treatment, shrinking skin after liposuction, acne treatment, chemical or physical abrasion of unwanted skin markings, surgical nose reduction, and face- and neck-lifts, and another aesthetic skin remodeling. | [177] |
| WO2015031189A1 | Treatment of scar/malignant tissue using photoactive compounds and light, including laser light. Enhancing skin tissue therapy using photoactive plasmonic nanoparticles and light employing ultrasonic delivery devices. | [178] |
| CN108543074B | The invention discloses an exosome-coated nano drug-carrying system for tumor treatment and a preparation method. The system is obtained by loading a nano-material with antitumor drugs, such as chemotherapy, immunotherapy, and tumor microenvironment reforming drugs, by endocytosis of cells and then discharged by the cells. The invention improves the composition, structure, and like of the essential external component biomembrane enveloping the nano drug-carrying system to enable the biological processing of nanoparticles. The innovation wraps the nano drug-carrying system in an exosome, which preserves its composition and structure and has high stability and tumor targeting in blood circulation. | [179] |
| CN108478598B | The invention describes a water-soluble fullerene nanomaterial, manufacture, and use. The innovation has strong water solubility and affinity with organisms, can block tumor blood arteries, and swiftly cut off tumor tissue feeding. | [180] |
| KR101945112B1 | The patch composition contains gold nanorods. Due to enlarged skin pores, medication or functional cosmetic absorbency might triple when skin temperature increases by 10°C. Thus, by utilizing an LED light to enhance skin temperature, the patch composition of the present invention may create a light-emitting effect of the gold nanorods, improving skin distribution of beneficial chemicals. | [181] |
| KR20160003488A | The innovation defines photodynamic cancer cell therapy using a HA-carbon nanomaterial composite. The invention also includes a PDT formulation. The HA-carbon nanomaterial composite outperforms a PDT medication in treating pathogenic cells like cancer cells. | [182] |
| CA2917407C | Albumin-containing nanoparticle/antibody complexes and related techniques and materials for the treatment of skin cancer. | [183] |
| US10765741B2 | Methods and materials for treating skin cancer (such as melanoma) using Abraxane® and an anti-vascular endothelial growth factor (VEGF) polypeptide antibody (such as Avastin®) were described in the patent. | [184] |
Next, the use of controlled release and efficient loading capacity for combinational therapeutic encapsulation will be discussed. The inclusion of antioxidants has been shown to increase the stability of sun-protective compounds in applications aimed at preventing skin cancer. In the area of skin cancer, this combined approach is equally crucial. Immune-stimulating drugs used with chemotherapeutics have the potential to regulate and increase the efficacy of both treatments while reducing their toxicities. Antitumor effectiveness is high, according to recent research, when immunotherapy is used with chemotherapy in nanoparticle [185–188]. The combination of chemotherapy and immunotherapy enhances the body’s natural defenses against cancer by using a kill signal generated by the localized elimination of tumor cells. Additionally, nanoparticles that are sensitive to lasers, pH changes, and surface contact show promise as a technique to more precisely target tumors, even in metastasis, while minimizing damage to healthy tissue.
Although preclinical research on nanoparticle has grown significantly, its use in clinical settings is still restricted, and further study on patient response to nanoparticle administration is necessary before it can be widely used [189]. Manufacturing nanoparticles at a commercial scale is challenging as well since nanoparticle repeatability and stability in clinical application need careful consideration. Despite these obstacles, nanoparticle therapeutic delivery for skin cancer prevention and therapy shows promising results in reducing skin cancer incidence and increasing skin cancer survivorship.
In conclusion, the treatment of skin cancer with nanomaterials has shown considerable potential. The difficulty of delivering treatment to cancer cells while preserving healthy cells could be achieved. This is attributable to the specific physicochemical features of nanomaterials, which enable selective targeting of cancer cells. Liposomes, polymeric nanoparticles, dendrimers, and gold nanoparticles are a few of the nanomaterials that have been studied for the treatment of skin cancer. Although the use of nanomaterials to treat skin cancer is still in its infancy, there are substantial potential advantages. Nanomaterials have the potential to lessen the adverse effects of current treatments while increasing their effectiveness. Additionally, numerous medications may be delivered concurrently using nanomaterials, resolving the issue of drug resistance. However, before nanomaterials can be extensively employed to treat skin cancer, there are several issues that need to be resolved. Nanomaterials’ potential for toxicity is one of the challenges faced. Nanomaterials have been proven to be biocompatible and safe in animal models, but ongoing research is required to determine the effect these materials will have in the long term.
The intricacy of the tumor microenvironment presents another difficulty. Numerous cells, including cancer cells, immune cells, and stromal cells, make up the tumor microenvironment. The effectiveness of medicines based on nanomaterials may be impacted by the interactions between these cells. Therefore, additional studies are required to unravel nanomaterial and tumor microenvironment interaction. Despite these challenges, perspectives on the future use of nanomaterial for the treatment of skin cancer remain optimistic due to their potential advantages, including revolutionizing cancer therapy.
3D: three-dimensional
5-ALA: 5-aminoulevulinic acid
5-FU: 5-fluorouracil
AI: artificial intelligence
BCC: basal cell carcinoma
C: cytosine
CCNGs: curcumin-loaded chitin nanogels
CNN: convolutional neural network
CPNL: chitosan-capecitabine nanogel
DCNN: deep convolutional neural network
DLS: dynamic light scattering
FDA: Food and Drug Administration
FPNGs: polylactide-co-glycolide nanogels loaded with 5-fluorouracil
GLI: glioma-associated oncogene homolog
HA: hyaluronic acid
HHI: hedgehog pathway inhibitor
MMR: mismatch repair
MNPs: magnetic nanoparticles
PDT: photodynamic therapy
PEG: polyethylene glycol
PLGA: polylactide-co-glycolide
PTCH: patched
SCCs: squamous cell carcinomas
SHH: sonic hedgehog
siRNA: small interfering RNA
SMO: smoothened
STAT3: signal transducer and activator of transcription-3
T: thymine
TAAs: tumor-associated antigens
TP53: tumor protein p53
UV: ultraviolet
UVR: ultraviolet radiation
Authors would like to offer special thanks to MM College of Pharmacy, Amity institute of Nanotechnology for allowing carrying out this work and other research projects.
RG, SH, and KW: Writing—original draft, Writing—review & editing, Visualization. HC: Supervision, Investigation, Conceptualization, Writing—review & editing. RP: Formal analysis, Resources, Visualization. ML and RS: Writing—review & editing, Supervision, Methodology. All the authors have equally contributed to conceiving this paper and participated in its revisions. All authors read and approved the final manuscript.
The authors have no relevant conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
