Affiliation:
1Medicine Department, Cheikh Khalifa International University Hospital, Faculty of Medicine, Mohammed VI University of Health Sciences (UM6SS), Casablanca 20000, Morocco
ORCID: https://orcid.org/0000-0001-7226-9410
Affiliation:
1Medicine Department, Cheikh Khalifa International University Hospital, Faculty of Medicine, Mohammed VI University of Health Sciences (UM6SS), Casablanca 20000, Morocco
Affiliation:
2Biology Department, Faculty of Sciences, Mohammed V University, Rabat 10000, Morocco
Email: meryemmabrouk4@gmail.com
ORCID: https://orcid.org/0000-0002-3631-1519
Affiliation:
2Biology Department, Faculty of Sciences, Mohammed V University, Rabat 10000, Morocco
ORCID: https://orcid.org/0000-0001-8750-9106
Affiliation:
1Medicine Department, Cheikh Khalifa International University Hospital, Faculty of Medicine, Mohammed VI University of Health Sciences (UM6SS), Casablanca 20000, Morocco
Affiliation:
1Medicine Department, Cheikh Khalifa International University Hospital, Faculty of Medicine, Mohammed VI University of Health Sciences (UM6SS), Casablanca 20000, Morocco
Affiliation:
1Medicine Department, Cheikh Khalifa International University Hospital, Faculty of Medicine, Mohammed VI University of Health Sciences (UM6SS), Casablanca 20000, Morocco
Affiliation:
1Medicine Department, Cheikh Khalifa International University Hospital, Faculty of Medicine, Mohammed VI University of Health Sciences (UM6SS), Casablanca 20000, Morocco
ORCID: https://orcid.org/0000-0003-0823-1404
Affiliation:
1Medicine Department, Cheikh Khalifa International University Hospital, Faculty of Medicine, Mohammed VI University of Health Sciences (UM6SS), Casablanca 20000, Morocco
ORCID: https://orcid.org/0000-0003-4604-6111
Affiliation:
1Medicine Department, Cheikh Khalifa International University Hospital, Faculty of Medicine, Mohammed VI University of Health Sciences (UM6SS), Casablanca 20000, Morocco
ORCID: https://orcid.org/0000-0002-0155-8191
Explor Med. 2022;3:583–591 DOI: https://doi.org/10.37349/emed.2022.00115
Received: July 18, 2022 Accepted: November 04, 2022 Published: December 29, 2022
Academic Editor: Akiko Mammoto, Medical College of Wisconsin, USA
The article belongs to the special issue Exploring Aortic Disease
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can engender multi-system inflammatory syndrome. Its main symptoms are cardiovascular and thromboembolic problems that can develop into severe complications. The present case is about a 55-year-old patient who was admitted for critical ischemia of the right lower limb and necrosis of the right forefoot. The patient was infected with coronavirus disease 2019 (COVID-19) one month before her admission. The patient also has cardiovascular risks including type 2 diabetes and hypertension. The performance of ultrasounds revealed a thrombus in the right atrium and the pulmonary artery, and arteriography detected an occlusion of the right popliteal joint for which she had an endovascular recanalization and amputation of the right forefoot. This case highlights that SARS-CoV-2 infection could be considered a serious cardiovascular disease requiring cardiovascular explorations to initiate hospital management and avoid severe complications.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has a mainly respiratory tropism, but it can have a significant impact on the cardiovascular system. SARS-CoV-2 affects the cardiovascular system through two mechanisms of action (Table 1) [1].
Impact of the two major mechanisms of affecting the cardiovascular system by SARS-CoV-2
| Direct effect on the cardiovascular system | Indirect effect on the cardiovascular system |
|---|---|
| - Myocarditis | - Venous thromboembolism |
| - Acute coronary syndrome | - Pulmonary embolism |
| - Heart failure | |
| - Arrhythmia | |
| - Cardiogenic shock |
The direct mechanism of cardiac tropism via the membrane protein angiotensin-converting enzyme 2 (ACE2) highly expressed on the surface of the cardiovascular system allowing the virus to penetrate intracellularly. This impact is associated with a worse prognosis [2].
The indirect mechanism is via the induction of an intense systemic inflammatory response, a cytokine storm, and hypoxia secondary to lung injury.
SARS-CoV-2 is a single-stranded ribonucleic acid (ssRNA) beta-coronavirus that enters target cells via a spiral membrane protein that binds with high affinity to the ACE2 receptor [3].
ACE2 regulates the renin-angiotensin system by metabolizing the vasoconstrictor and proinflammatory angiotensin 2 to the vasodilator peptide angiotensin1-7. Viral binding decreases the expression of ACE2 on the cell surface, which results in an increase in angiotensin 2 inducing vasoconstriction and inflammation. Thus, local viral replication generates an innate immune response which, through macrophage activation, increases the production of interleukin-1β (IL-1β) and IL-6 which activate the endothelium and the expression of cell adhesion molecules such as tissue factor, plasminogen activator inhibitor-1 (PAI-1) and von Willebrand factor resulting in a prothrombotic state [4].
Biomechanical stress generated by acute inflammation, vasoconstriction, platelet activation, and endothelial dysfunction can destabilize atherosclerotic plaque [5].
An increasing number of aortic, carotid, and peripheral lesions in the setting of SARS-CoV-2 have been reported [6–8]. The multi-system inflammatory syndrome can be present with several cardiac manifestations including several types of arrhythmias and atrioventricular block, but junctional tachycardia in SARS-CoV-2 is rare. The absence of deep vein thrombosis in SARS-CoV-2, suggests the presence of thrombosis of the right atrium or the trunk of the pulmonary artery rather than an embolism [9, 10].
A case of a 55-year-old woman is reported [non-smoker, body weight: 75 kg, height: 1.70 m, body mass index (BMI): 25.95, glycated hemoglobin (HbA1c): 9%] with cardiovascular risk factors: type 2 diabetes, insulin and arterial hypertension [medical treatment: type 2 diabetes, 25 international unit (IU) in the morning and 25 IU in the evening; for hypertension, Detensiel 5 mg/day (maximum tolerated dose)].
Medical history: infection with coronavirus disease 2019 (COVID-19) 1 month ago (COVID-19 pneumopathy treated according to the Moroccan protocol, including antipyretics and analgesics, azithromycin, zinc, multivitamins, and preventive anticoagulation). We provided the patient’s hematological and biochemical parameters (Table 2).
Hematological and biochemical parameters
| Hb | White blood cell | Platelets | Na+ | K+ | C-reactive protein (CRP) | Procalcitonin | Creatinin | Urea | Troponin | D-dimer |
|---|---|---|---|---|---|---|---|---|---|---|
| 114 g/L | 10.7 × 109/L | 406 × 109/L | 136 mmol/L | 3.3 mmol/L | 40 mg/L | 0.055 ng/mL | 4.9 mg/L | 0.19 g/L | 0.019 ng/mL | 170 ng/mL |
Admitted for critical ischemia of the right lower limb with necrosis of the right forefoot (Figure 1). The transthoracic echocardiography objectified a thrombus in the right atrium and the pulmonary artery (Figure 2). The doppler ultrasound of the supra-aortic trunks came back without anomalies. The thoracic computed tomography angiography objectified a thrombus of the right atrium and the pulmonary artery. On day 2 of her hospitalization, the patient presented a junctional tachycardia with favorable evolution. The arteriography objectified an occlusion of the right popliteal joint (Figure 3) for which she had an endovascular re-canalization, stenting (Figure 4) and amputation of the right forefoot with good healing of the amputation stump (Figure 5) and output at day 7.
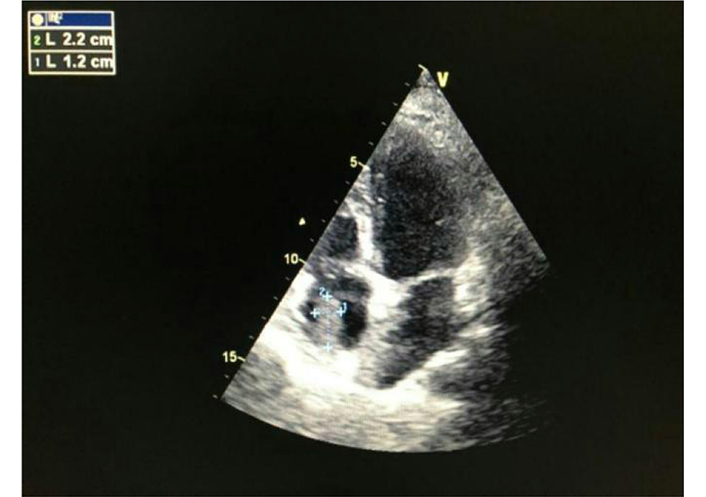
Transthoracic echocardiography objectifying a thrombus of the right atrium (echography from General Electric, Paris, France)
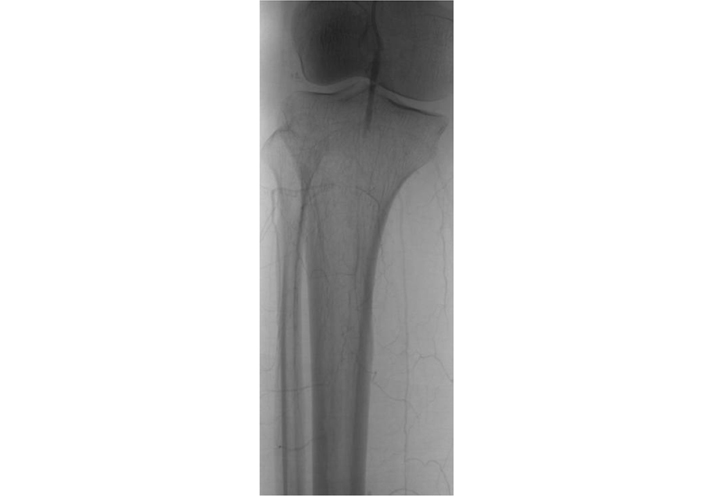
Arteriography showing occlusion of the sub-articular popliteal artery (INNOVA gloss amplifier from general electric, Paris, France)
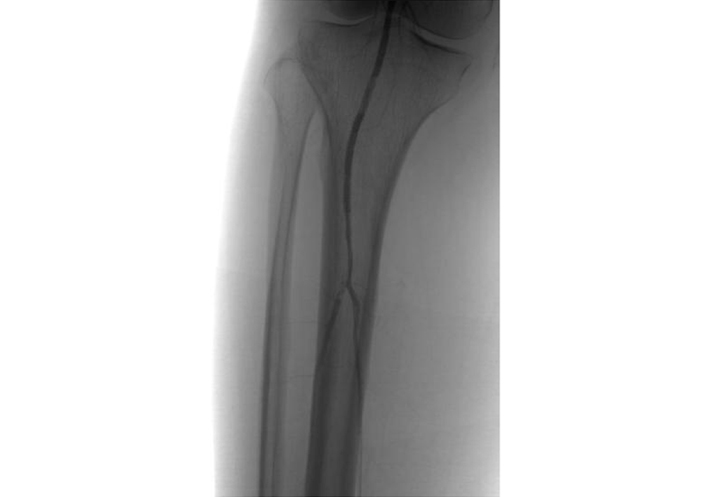
Recanalization of the sub-articular popliteal artery (INNOVA gloss amplifier from general electric, Paris, France)
For further explanation, the re-canalization concerned the posterior tibial artery and the right popliteal artery with a stent. There has been an anterograde puncture of the right femoral artery and the placement of a 6-F introducer. The angiography showed that the right popliteal artery is occluded.
To perform a recanalization of the right popliteal artery, the following decisions were taken: a systemic heparinization of 30 mg unfractionated heparin (UFH), a catheterization of the right popliteal artery with a 0.035/260 cm guide and a 5F vertebral probe were performed. Moreover, an angioplasty with 2 mm/120 mm, 2.5 mm/120 mm, and 3 mm/120 mm balloons of the popliteal artery and the peroneal and posterior tibial arteries were performed. An angiographic control was carried out as follows: the dissection of the subarticular popliteus, and stenosis at the origin of the peroneal artery. Finally, a stent of 3 mm/38 mm was placed.
The final angiographic control came out satisfying which leads us to say that it was a successful recanalization of the popliteal artery and posterior tibial artery.
The patient underwent a drug treatment in the hospital based on heparin therapy and severe acute pancreatitis (SAP), avlocardyl antibiotherapy, and acetylsalicylic acid clopidogrel.
As for the diagnostics and treatment provision, the patient was seen in consultation for necrosis of the forefoot having motivated an arterial aorta and myocardial infarction (MI) on which the diagnosis of arterial lesions was made. As part of the workup for the extension of the atheromatous disease, a cardio opinion with the endotracheal tube (ETT) was requested and this revealed a thrombus in the right atrium and acute pancreatitis (AP) during her hospitalization, the patient presented a junctional tachycardia. Anticoagulant therapy was started on admission and endovascular treatment was performed in addition to other medications to control junctional tachycardia.
The severity of the course of critical ischemia in patients with peripheral arterial occlusive diseases is well established in the literature, mainly the poor survival and high mortality rate [11, 12]. Several hypotheses have been raised to explain the pathophysiology of COVID-19 associated with cardiovascular events, including ACE2 receptor-mediated endothelial injury, microvascular dysfunction and thrombosis, and IL-6-mediated cytokine storm [13, 14]. Overall, the recommendations aim to avoid elective surgeries, but patients will still need urgent interventions such as critical limb ischemia with a high risk of infection and limb loss [15]. Another important factor that may mean that patients with arteriopathy obliterans of the lower extremity are more susceptible to infection for SARS-CoV-2 is the age of patients with medical comorbidities such as high blood pressure and diabetes linked to poor prognosis in patients infected with SARS-CoV-2 [16, 17]. Thrombotic complications appear to be an important problem in patients with SARS-CoV-2 who are at risk of developing disseminated intravascular coagulation [18]. Increased levels of D-dimer, fibrin degradation product, and prolonged prothrombin time have been associated with poor prognosis in patients affected by the novel SARS-CoV-2 [19–22].
No information is currently available on the duration of inflammation and thrombosis that may occur after recovery from SARS-CoV-2. By analyzing the literature, only a few cases describe a delayed manifestation of obliterating arteriopathy of the lower limbs in SARS-CoV-2 [23]. The endovascular approach is first recommended by various vascular societies when invasive revascularization procedures are required [24]. A recent analysis reveals that during the pandemic in Madrid 44% of revascularizations were performed by full endovascular methods, while 39% of them were performed by full open revascularization [25].
The endovascular approach minimizes the need for multidisciplinary contributions. Moreover, its evolution is generally favorable in terms of morbidity [26]. In addition, revascularization is performed under local anesthesia, reducing the heaviness of general anesthesia in the operating room for patients with several morbidities. The risk of thromboembolic complications is higher than that of serious illnesses not associated with SARS-CoV-2 infection [9, 27–29]. Some studies have reported an incidence of pulmonary embolism disproportionately compared to that of deep vein thrombosis [30]. This finding led to the hypothesis that in some cases, the pulmonary disorders observed may be due to arterial occlusions by thrombi formed in situ, rather than by pulmonary embolisms following deep vein thrombosis case of our patient who never had venous thrombosis [30]. The presence of intracardiac thrombus has been rarely described in SARS-CoV-2 patients [9–31]. The presence of a thrombus in the right atrium raises several questions: how long has this thrombus existed in the right atrium? is it due to a coagulation dysfunction observed in SARS-CoV-2? is it due to stasis or hemodynamic instability?
From the start of the pandemic, autopsy reports showed that the blood vessels of patients with COVID-19 were clogged. Indeed, in several series of seven COVID-19 autopsies, thrombosis was a prominent feature in multiple organs, in some cases despite full anticoagulation and regardless of the timing of the disease course, suggesting that thrombosis plays a role very early in the COVID-19 process [32]. The finding of megakaryocytes and platelet-rich thrombi in the lungs, heart, and kidneys suggests a role in thrombosis. On the other hand, it has been demonstrated, more recently, that tissue factor (TF) activity from SARS-CoV-2-infected cells activates thrombin, which signals to protease-activated receptors (PARs) on platelets. Blockade of molecules in this pathway may interfere with platelet activation and the coagulation characteristic of COVID-19 [33].
It is necessary to emphasize the critical role of transesophageal echocardiography in the management of the patient by providing reliable and sure information. Currently, transesophageal echocardiography is highly endorsed by experts as an invaluable tool for the management of critically ill patients with COVID-19 [34].
The treatment of patients who have had thrombosis of the right atrium in SARS-CoV-2 and given the condition of the patients, in particular our patient, is based on anticoagulation at a curative dose with the hope of having a complete dissolution of the thrombi [35]. Ventricular rhythmic dysfunction is observed in 50% of patients [36], and numerous arrhythmias have been documented, including atrial fibrillation, premature ventricular contractions, and prolongation of the QT interval, cases of varying degrees of atrial block ventricles have been described [37, 38].
The mechanism of junctional tachycardia is likely secondary to inflammation of the atrioventricular node [39] which is similar to the inflammation and conduction dysfunction seen in myocarditis, rheumatic fever, and Lyme disease [40].
In conclusion, cardiovascular and thromboembolic manifestations in patients infected with SARS-CoV-2 are multiple. Thrombosis of the right atrium and in situ thrombosis of the pulmonary artery are underdiagnosed. This makes it important to perform cardiovascular investigations in patients infected with SARS-CoV-2, especially those hospitalized in intensive care. Arterial complications of the lower limbs resulting in critical ischemia should be treated by an endovascular approach. SARS-CoV-2 is considered as a vascular disease and it is expected that it should be considered as such in order not to under-diagnose the cardiovascular and thromboembolic complications.
ACE2: angiotensin-converting enzyme 2
COVID-19: coronavirus disease 2019
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
The authors thank the medical staff of the Cheikh Khalifa hospital as well as the patient who participated in this study.
SM, YZ and YT : Conceptualization. AL, MM, MC, ZH, MA, CE, HB and YT: Investigation, Validation. YZ and YT: Supervision, Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
The authors declare no competing financial interests.
The recruitment of patients was approved by the Ethics Committee of Cheikh Khalifa Hospital (CEFCK/PR/2021/PR02) and complies with the Declaration of Helsinki.
All participants gave their written informed consent.
The patient has given consent for images or other clinical information relating to his case to be reported in a medical publication.
Not applicable.
Not applicable.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3013
Download: 24
Times Cited: 0
Lova Prasadareddy Kajuluri ... Kathleen G Morgan
John V. Tyberg
Anne-Maree Kelly
Korin Karabulut ... James Morgan
Preetha Rajasekaran ... Bashi V. Velayudhan
Dharmsheel Shrivastav ... Vimal Mehta
