Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
ORCID: https://orcid.org/0000-0003-4805-1758
Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
ORCID: https://orcid.org/0000-0003-3940-1787
Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
ORCID: https://orcid.org/0000-0002-1803-2620
Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
ORCID: https://orcid.org/0000-0001-9556-6602
Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
ORCID: https://orcid.org/0000-0003-1619-7592
Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
Email: smatsuda@cc.nara-wu.ac.jp
ORCID: https://orcid.org/0000-0003-4274-5345
Explor Med. 2022;3:468–478 DOI: https://doi.org/10.37349/emed.2022.00108
Received: June 20, 2022 Accepted: October 14, 2022 Published: October 27, 2022
Academic Editor: Feng Tian, Shandong Provincial Hospital Affiliated to Shandong University, Shandong Provincial Hospital Affiliated to Shandong First Medical University, China
The article belongs to the special issue The Role of Gut Microbiota and its Metabolites in Gastrointestinal Diseases
Inflammatory bowel disease (IBD) is a multifactorial chronic disease. Patients with IBD have an increased risk of developing colorectal cancer which has become a major health concern. IBD might exert a role of engrams for making the condition of specific inflammation in the gut. Dysregulation of immune cells induced by the command of engrams might be crucial in the pathogenesis of damages in gut epithelium. The anti-proliferative (APRO) family of anti-proliferative proteins characterized by immediate early responsive gene-products that might be involved in the machinery of the carcinogenesis in IBD. Herein, it is suggested that some probiotics with specific bacteria could prevent the development and/or progression of the IBD related tumors. In addition, consideration regarding the application of studying APRO family proteins for the comprehension of IBD related tumors has been presented. It is hypothesized that overexpression of Tob1, a member of APRO family proteins, in the epithelium of IBD could suppress the function of adjacent cytotoxic immune cells possibly via the paracrine signaling.
Inflammatory bowel disease (IBD) is a multifactorial chronic inflammatory disease mainly consisting of Crohn’s disease (CD) and ulcerative colitis (UC), which brings about injury in gut epithelium and/or the relapsing severe inflammation in the gut [1]. Pathologically, IBD is also characterized by a complex and dysfunctional immune response [2]. In fact, pathological lymphatic dysfunctions have been detected in gut epithelium with IBD [3], indicating that errors in the lymphatic regulation may be a common feature of IBD. Patients with IBD have an increased risk of developing colorectal cancer, with poorer survival compared with the general population [4]. A standard test in identifying the lesions of malignant dysplastic and/or neoplasia in IBD is morphological changes of gut epithelium which are challenging to be detected by endoscopic examination. With an increasing population and rising burden of IBD [5], there is an increasing requirement for the better-quality detection of IBD-associated dysplasia and/or cancer.
In animal models, the colitis-associated colorectal cancer represents an important tool to explore the mechanistic basis of cancer-related inflammation, providing an evidence that several inflammatory mediators may play key roles in the perpetuation of colitis and in the initiation of colorectal cancer [6]. In particular, dextran sulphate sodium (DSS)-induced colitis is a reproducible model that morphologically and symptomatically resembles UC in humans. Cyclic administration of DSS in drinking liquid might result in the development of colorectal cancers with the pathological feature that is also similar to those of human colitis-associated neoplasia [7]. In the progression of IBD, an imbalance in the ecology of the intestinal flora might be a key factor [8]. The study of the intestinal flora of patients with IBD related cancer has shown that changes in intestinal flora are significantly involved in the early stage of the disease [9]. For example, a study has found that the content of butyric acid in fecal extracts from patients with IBD has been significantly lower than that in those from a healthy control [10], implying that supplements with certain bacteria might reduce the incidence and/or development of IBD. Therefore, alteration of gut microbiota has been proposed as a promising protocol for the treatment of IBD [11]. Therapeutic techniques that focus on the relationship between the pathogenesis of IBD and/or related carcinogenesis and the management of intestinal flora could be a potential strategy for the treatment of IBD and/or related carcinogenesis [12].
DSS may act as a toxic substance to gut epithelium, and the disruption of gut epithelium results in colonic inflammation, allowing the more exacerbation of existing inflammation [13]. Addition of DSS to drinking liquid, modifying the concentration of DSS and/or the frequency of administration, could get a reproducible model of gut inflammation as well as a useful model of IBD such as UC [14]. In the model of DSS-induced IBD, an inflammatory infiltration of T helper (Th) cells has been found within the site of histopathological epithelial damage [15]. Among Th cells, the Th17 cells characterize a subset of effector T cells distributed in the gut mucosa, which typically secrete inflammatory cytokines such as interleukin 17 (IL-17), IL-21 and IL-23 to damage gut epithelium [16]. It has been shown that the Th17 cells transferred to the immune-deficient mice could induce severe colitis [17]. In addition, the number of Th17 cells and protein expression of inflammatory cytokines such as IL-17, IL-21 and IL-23 are significantly increased in the gut mucosa with the inflammation of IBD [18]. It has lately been shown that reactivation of the neuronal ensembles called “engram” is enough to recover the inflammatory condition under the inflammation with DSS-induced colitis [19], suggesting that DSS-induced colitis might exert the roles of engrams for making the condition of inflammation in IBD (Figure 1). Long-lasting synaptic changes within the neuronal network mediate memory, which may also constitute memory engrams [20]. The neurological and/or immunological “memory engrams” could renovate the initial disease state via the relationship between brain and immune system when reactivated [21]. Memory engrams have been identified in the hippocampus, amygdala, or cortex, which has been hypothesized to be functionally connected to each other as a unified engram complex [22]. Nonetheless, the formation of engrams might be involved in the gut microbiota-brain axis that is a bidirectional network where the gut and central nervous systems are linked for organizing health and/or disease on the host [23]. Interestingly, berberine could modulate the effect of gut microbiota-brain axis via the alteration of microglial action [24]. In addition, it has been reported that berberine ameliorates DSS-induced colitis by suppressing Th17 responses [25]. Moreover, the berberine may also improve metabolic disorders through modulation of the gut microbiota-brain axis [26]. Balancing the Th17/regulatory T cells (Tregs) may be a key factor involved in the inflammation of DSS-induced colitis [27]. Therefore, dysregulation of Th17 cells induced by the administration of DSS might be crucial in the damage of gut epithelium [28].
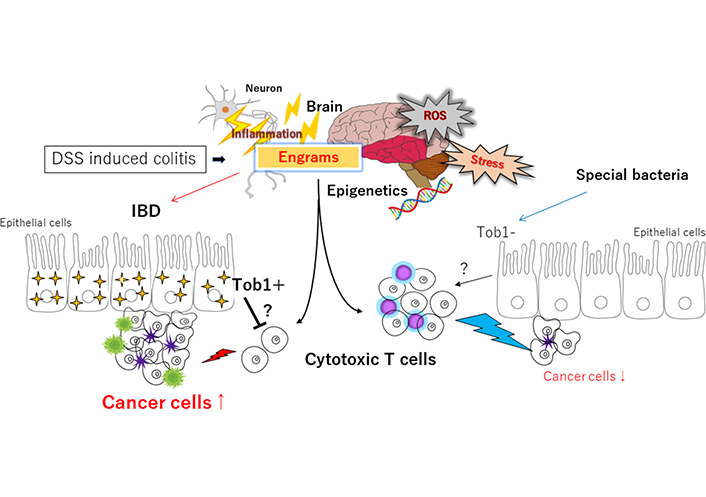
Schematic representation of the hypothetical effects of engrams and/or Tob1 for the tumorigenesis in DSS-induced IBD. Engrams may be created in the condition of repeated inflammation with reactive oxygen species (ROS) and/or various stresses, which could employ active immune cells to damage colon epithelium and/or cancer cells. Whereas Tob1 expression in the epithelium induced by the inflammatory stresses could inhibit the action of immune cells, which may eventually allow the development of tumors. Certain commensal bacteria in the gut can repress the expression of Tob1, which may consequently prevent the tumorigenesis. Note that some critical pathways such as Wnt/beta-catenin signaling have been omitted for clarity. Tob1: transducer of erbB2 1; ?: uncertainty; ↑: expansion, enlargement; ↓: shrink, reduction
Anti-proliferative (APRO) protein family in humans includes six members, namely Tob1, Tob2, B cell translocation gene 1 (BTG1), BTG2/tetradecanoyl phorbol acetate-inducible sequences 21 (TIS21)/pheochromocytoma cell-3 (PC3), BTG3/abundant in neuroepithelium area (ANA) and BTG4/PC3B, which play significant roles in regulating cell proliferation, invasion and apoptosis of many cancers [29]. Gene expression of Tob1 and BTG1 is slightly increased in patients with UC compared to patients with CD [30]. Please note that more UC patients have been found to possess dysplasia and/or adenocarcinoma within the colon [31]. Correspondingly, it has been shown that colorectal cancer occurred more frequently in UC patients rather than in CD patients (29% vs. 17%; P < 0.005) [32]. Tob1 expression is occasionally decreased in inflamed mucosa of IBD patients compared with healthy human subjects, however, severe colitis is observed in Tob1−/− mice compared with control animals [33]. Accumulating evidence has shown that Tob1 functions as a tumor suppressor. However, Tob1 expression may be upregulated during the progression of colon cancer, which is considerably correlated with tumor size and a prognostic indicator such as survival rate in the colon cancer. In addition, Tob1 deficiency appears to lead to the reduced tumorigenesis in DSS-treated cancer, suggesting that Tob1 is an adverse prognostic factor, and that Tob1 is a therapeutic target for colon cancer [34]. On the other hand, BTG1 protein in colon cancer tissues is expressed at low levels, which is also associated with the clinic-pathological features [35]. Low expression of BTG1 might be involved in the progression of pancreatic ductal adenocarcinoma, suggesting that BTG1 might be a prognostic marker of survival rate in the cancer [36]. Similarly, Tob1 is downregulated within the tissues of pancreatic cancer, and the Tob1 overexpression inhibits the proliferation of pancreatic cancer cells [37]. Tob1 may suppress the proliferation of pancreatic cancer cells by regulating several genes on calcium pathway [37]. Additionally, downregulated expression of Tob1 has been found in malicious gastric cancer, suggesting that low expression of Tob1 may be an independent indicator of poor prognosis in gastric cancers [38]. Consistently, downregulation of Tob1 is associated with shorter survival of gastric cancer patients, suggesting that Tob1 may be considered a potential marker for predicting the outcomes of patients with gastric cancer [39]. To summarize the above, an anti-proliferative gene product Tob1 in APRO family tends to be induced by inflammatory stimulus of IBD. While Tob1 could suppress the proliferation and/or carcinogenesis in a cell, Tob1 overexpression would eventually seem to promote the tumorigenesis in IBD. Here, it is supposed that increased tumorigenesis might be conceivably brought on via the inhibition of antitumor immunity by Tob1 (Figure 1).
How does overexpression of Tob1 could inhibit the antitumor immunity? It has been reported the role of APRO family members is involved in the regulation of immune response by Th17 cells. For example, Tob1 weakens the IL-2 production in Th17 cells, and Tob1 also blocks the expression of cell cycle genes, suggesting that Tob1 suppresses the proliferation of Th17 cells through several pathways including signal transduction, transcription, and post-transcriptional regulation [40]. Micro RNA such as miR-590 could enhance the pathogenic inflammation with Th17 cells through inhibiting the expression of Tob1 [41, 42]. While Th17 cells are associated with autoimmunity, they also exist in the places around various solid tumors favoring cancer cells-killing [43]. Therefore, it is plausible that Tob1 might be involved in the IBD related carcinogenesis via the modulation of cytotoxic T cells (Figure 1). In this context, it has been shown that a pharmacological compound such as lithium chloride, an inducer for the expression of Tob1 [34], could inhibit the inflammation of virus-induced infection by downregulating cytokine-related genes [44]. In addition, lithium chloride may modulate the inflammation through regulating the capacity of dendritic cells to promote the differentiation of Th17 cells [45].
Gut microbiota could play an imperative role in human health and in various diseases. While Bacteroides and Pseudomonas could induce colitis [46], certain bacteria might encourage the development of a good gut microbiota that helps shrink the carcinogenesis. For the purpose, it has been struggled to look for specific bacteria which could repress the tumorigenesis of IBD. Here, a result of preliminary experiment with certain probiotics has been demonstrated for the understanding of the relationship between APRO family proteins and IBD related carcinogenesis (unpublished data, and personal communication). A mouse model for creating the colorectal cancer of IBD has been employed with using azoxymethane (AOM) and DSS as shown elsewhere [47]. The AOM/DSS treated ICR mice had been fed with 3 species of specific bacteria or without the specific bacteria for more than 12 weeks after the last DSS administration (Figure 2A). Then, the intestinal tracts between cecum and anus were excised after euthanization. All formed tumors were near the location of anus. Consistent to the previous reports, treatment with AOM and DSS without the specific bacteria had led to 100% incidence of colorectal tumors in mice. No tumors had been detected in the non-treated mice (data not shown). Proteins were extracted from the excised colon tissues, and analyzed by western blots with indicated antibodies. Please note that no protein samples contain any fragment of macroscopic and/or microscopic tumors. As shown in Figure 2B, every sample with colorectal tumor exhibited the expression of Tob1, but not Tob2 (Figure 2B).
The present findings, first of all, might provide a mechanistic evidence that probiotics with specific bacteria could prevent the development and/or progression of IBD related cancers. Similarly, it has been reported that Clostridium butyricum as a probiotic promotes IL-10-producing macrophages, which could enforce the inhibitory effect on the inflammation in mouse intestine [48]. In addition, Saccharomyces boulardii (S. boulardii) may effectively reduce the carcinogenesis in an AOM/DSS-induced mice model [49]. Furthermore, the regulation of intestinal microbiota by S. boulardii could adjust the balance of Th17/Treg cytokines [50]. Second, these data might suggest an appliance of APRO family proteins for the better-quality detection in IBD-associated cancers. For example, some samples of endoscopic mucosal resection of adenomatous polyps in the colon should be analyzed with Tob1 antibodies and/or DNA-primers for the early detection of cancers. At present, characterization of colonic neoplasia lesions in IBD remains challenging [51]. Therefore, checking the expression of Tob1 within a level of protein or RNA might improve the rate of neoplasia detection.
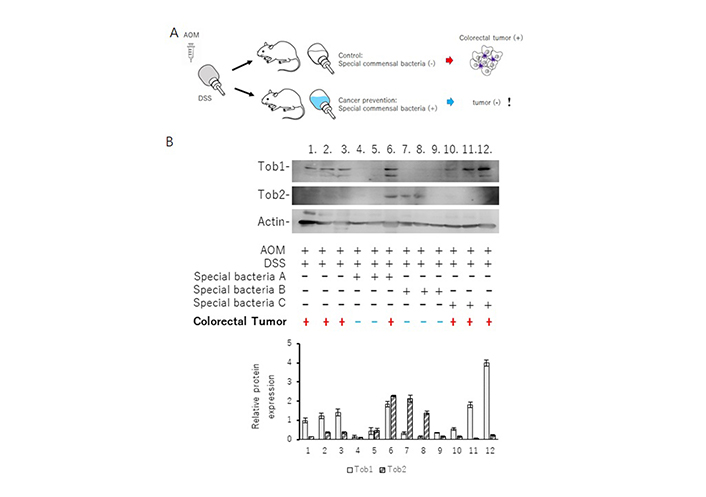
ICR mice were treated with AOM/DSS to establish a mice model of IBD related colorectal tumorigenesis. Twelve treated mice were randomly divided into four groups with or without three types of special bacteria (A, B, and C) exposure in drinking. The mice were sacrificed after the end of the 14th week. Examination of the colon showed visible colorectal tumors at the mice of number 1, 2, 3, 6, 10, 11, 12. Tumors were mainly located in the distal portion of the colon. Protein isolation from the proximal portion of the colon and western blots were performed with the indicated antibodies as described elsewhere. Quantitation of Tob1 and Tob2 relative protein levels has been shown at lower panel. Data are means ± standard error of mean (n = 3 in each group). !: indication of an amazing result; black +: used; black –: not used; red +: tumor existed; blue –: tumor not existed
It has long been considered that a chronic inflammation is associated with increased incidence of malignancy. Pro-inflammatory cytokines released from Th17 cells, which are important for the adaptive immune response in IBD, have been also implicated in the pathogenesis of IBD related carcinogenesis. The Th17 cells are a heterogenous population of effector cytotoxic T cells characterized by the secretion of several cytokines including IL-17 [52]. It has been shown that an anti-IL-17 antibody treatment of mice exposed to DSS has reduced the development of colorectal tumors [53]. A role of Th17 cells in tumor development has been suggested to lead to decreased tissue inflammation and/or to increased resistance for tumor growth [54]. IL-22 and IL-23 expression, which is also related to the activation of Th17 cells, has been shown to be increased in human colitis and/or IBD related colon cancer [55]. In addition, Th17 cells could play a key role of tumor immunity in immune checkpoint inhibitor-targeted immunotherapy by blocking the programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) pathway [56]. Previously, it has been shown that the relationship between APRO molecules and carcinogenesis in a cell may be reminiscent of the relationship between cytotoxic T cells and cancer cells in a body called “immune check points” [57] (Figure 3). It has been shown that PD-1/PD-L1 mediated signaling in gut epithelial cells is one of the major processes that regulate local inflammatory responses, which could be a potential therapeutic tactics for the treatment of human IBD related cancers [58]. Additionally, PD-1/PD-L1 could inhibit the phosphoinositide-3 kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway [59]. Regarding the relationship between Tob1 and PI3K/AKT/mTOR signaling, it has been found that Tob1 antagonizes the PI3K/AKT/mTOR survival signaling and induces cancer cell apoptosis [60]. Tob1 could induce autophagy in gastric cancer cells via decreasing the activation of the PI3K/AKT/mTOR signaling [61]. Interestingly, overexpression of Tob1 in gastric cancer cells can induce autophagy by secreting exosomes [62]. Extracellular vesicles including the exosomes and/or secretomes have expanded attention as possible carriers of disease biomarkers [63]. Previously, we have also shown that secretome, APRO family proteins, microRNAs, and the machinery of RNA interference (RNAi) might exert their effects via the paracrine signaling for the regulation of the self-renewal proliferation of cancer stem cells [64]. Consequently, it might be hypothesized that overexpression of Tob1 inside the colon epithelium in DSS-induced colitis could suppress the function of adjacent cytotoxic T cells via the paracrine signaling with secretome (Figure 3), which might be one of the reasons why tumorigenesis was exactly found in every Tob1 positive individual in Figure 2.
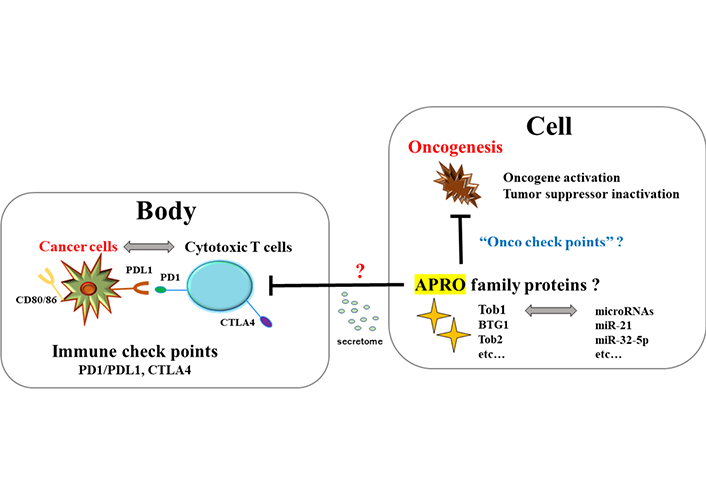
The relationship between tumor suppressor APRO family proteins and oncogenesis in a cell seems to look like the relationship between cytotoxic T cells and cancer cells in a body. It could be hypothesized that the former might be regulated by “Onco check points” with microRNAs for targeting APRO family proteins, whereas the latter is regulated by “immune checkpoints” molecules such as PD-1/PD-L1 and CTLA4. Cells expressing Tob1 might be able to regulate the cytotoxic T cells infiltrating to the adjacent places of Tob1 positive epithelial cells possibly via the paracrine signaling with secretomes. CTLA4: cytotoxic T-lymphocyte-associated protein 4; Onco: oncogenesis; ?: uncertainty
Note. Adapted from “Roles of gut dysbiosis, anti-proliferative proteins, and post-transcriptional regulation in carcinogenesis,” by Sawamura H, Taniguchi K, Ikeda Y, Tsuji A, Kitagishi Y, Matsuda S. J Transl Genet Genom. 2022;6:157–68 (http://dx.doi.org/10.20517/jtgg.2021.57). CC BY.
Cancer has been regarded as a localized disease with a role of the tumor microenvironment, suggesting that cancer should be regarded as an environmental disease [65]. In particular, systemic inflammatory status influenced by physiological changes might affect the tissue microenvironment. Hence, genomic factors could modify disease phenotypes via gene-by-environment interactions [65]. Evidences might also indicate that diet, nutrition, lifestyle, and the other environmental factors could have pathogenic roles via the influences on genome, epigenome, proteome, and metabolome of tumor cells as well as of immune cells [66]. Vigorous research might provide insights into dynamic interactions between gut microbiota, tumor, and immunity during the carcinogenesis processes. In general, carcinogenesis might pursue a multistep progression of normal epithelial cells in changing to inflammation, dysplasia, and carcinoma [67]. Among them, chronic inflammation related to the establishment of engrams might be an important factor for the proceeding carcinogenesis in IBD patients. Therefore, clearing the bad memory of “engrams” might be favorable for the prevention and/or treatment of the carcinogenesis in IBD. In the experiment of dextran sulfate sodium induced colitis, the authors have exerted the chemo-genetic procedure—the designer receptor exclusively activated by designer drugs (DREADD) system for the inhibition of engrams-activity [19]. However, it seems to be impossible to utilize this system in the clinical cancer treatment of humans. Now, is it possible to clear the memory of engrams without any brain damages? We believe this is the key point for the therapeutic interventions. Changes in the conformation of gut microbiota might be accepted by the sympathetic vagal afferent nerve transmitting to the central nervous system CNS via the microglial action, which in turn could produce and/or modulate the responses of engrams. In fact, probiotics have a therapeutic effect on the regulation of chronic inflammation and/or on the reduction of IBD related carcinogenesis [68]. Here, it has been shown the involvement of probiotics and/or Tob1 in the IBD related carcinogenesis. In general, Tob1 has been regarded as a tumor suppressor diminishing the malignant phenotype of cancer cells and/or inhibiting the progression of cell cycle for proliferation. For example, it has been reported that overexpression of Tob1 increases the rate of apoptosis in breast cancer cells, which could improve the radio-sensitivity in cancer therapy [69]. Among breast cancer patients, patients with lower expression of Tob1 had a poorer prognosis [70]. Consistently, many studies reported that reduced expression of Tob1 contributed to accelerated carcinogenesis and/or tumor growth [71]. Besides, abnormal Tob1 expression has been described in various malignant tumors. Other reports have revealed that overexpression of Tob1 participates in the regulation of invasion and/or metastasis in some cancers [72, 73]. Does Tob1 play different roles in different molecular subtypes of cancers? We believe there might be an unsolved or unknown paradigm in this field. The APRO family anti-proliferative proteins might be confidently involved in the regulation of carcinogenesis-machinery to some extents depending on the intracellular and/or extracellular conditions. Given the crucial roles of APRO family proteins, upcoming novel therapeutics should include the modulation of APRO proteins. What is the most attractive member in APRO proteins suitable for the cancer therapy? We believe that is Tob1. Among the APRO proteins, Tob1 uniquely has CAG repeats in its coding sequences [72]. Although the detailed role of the CAG repeat remains unknown, it has been suggested that small interfering RNAs (siRNAs) based on the CAG repeat expansions effectively destroy cancer cells through RNAi [73]. Summarizing the above, next generation medicine for the treatment of colon cancer should contain strategies for altering the environment of colon epithelial cells with certain gut microbiota via the alteration of Tob1 expression as a key target, which could develop prevention and/or therapeutic strategies to finally reduce the cancer burden.
AOM: azoxymethane
APRO: anti-proliferative
BTG1: B cell translocation gene 1
CD: Crohn’s disease
DSS: dextran sulphate sodium
IBD: inflammatory bowel disease
IL-17: interleukin 17
mTOR: mammalian target of rapamycin
PD-1: programmed cell death protein 1
PD-L1: programmed cell death ligand 1
PI3K: phosphoinositide-3 kinase
Th: T helper
Tob1: transducer of erbB2 1
UC: ulcerative colitis
YI and SM contributed conception of the study. YI, KT, SY, HS, AT and SM wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that they have no conflicts of interest.
All animal care and experimental procedures in this study were approved by the Animal Care Ethics and Use Committee of Nara Women’s University (No. 21-01).
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Giuseppe Merra ... Marco Marchetti
Maciej Chichlowski ... Neeraj Pandey
Kevin V. Houston ... David A. Johnson
Lindsey B. Cundra ... David A. Johnson
Byung Soo Yoo ... David A. Johnson
Shymaa M. Al-Jabri ... Awatif A. Al-Judaibi
