Affiliation:
1Department of Biomedical Sciences, Advanced Medical and Dental Institute, Universiti Sains Malaysia, Penang 13200, Malaysia
Email: effa@usm.my
ORCID: https://orcid.org/0000-0003-4188-5506
Affiliation:
2Department of Pathology, Hospital Tuanku Fauziah, Kangar 01000, Malaysia
3Ministry of Health Malaysia, Federal Government Administrative Centre, Putrajaya 62590, Malaysia
Affiliation:
2Department of Pathology, Hospital Tuanku Fauziah, Kangar 01000, Malaysia
3Ministry of Health Malaysia, Federal Government Administrative Centre, Putrajaya 62590, Malaysia
Affiliation:
3Ministry of Health Malaysia, Federal Government Administrative Centre, Putrajaya 62590, Malaysia
4Department of Internal Medicine, Hospital Sultanah Bahiyah, Alor Setar 05460, Malaysia
Affiliation:
3Ministry of Health Malaysia, Federal Government Administrative Centre, Putrajaya 62590, Malaysia
4Department of Internal Medicine, Hospital Sultanah Bahiyah, Alor Setar 05460, Malaysia
5Department of Internal Medicine, Sultan Ahmad Shah Medical Centre, International Islamic University of Malaysia, Kuantan 2500, Malaysia
Affiliation:
6Department of Community Medicine, Advanced Medical and Dental Institute, Universiti Sains Malaysia, Penang 13200, Malaysia
ORCID: https://orcid.org/0000-0003-3355-3026
Explor Immunol. 2025;5:1003197 DOI: https://doi.org/10.37349/ei.2025.1003197
Received: May 16, 2024 Accepted: April 02, 2025 Published: May 21, 2025
Academic Editor: Jean Amiral, Hyphen BioMed, France
Aim: The high levels of anti-SSA/Ro and anti-SSB/La autoantibodies are closely associated with a group of diseases related to connective tissues, also known as connective tissue diseases (CTD). The current study attested to profile the multifactorial association between interleukin IL-6 and IL-10 in sera from the study cohort to underline its putative prognostic and therapeutic characteristics for future application in CTD.
Methods: The study cohort was recruited from government hospitals and screened for autoantibody using Enzyme Immunoassay (EIA) and Immunofluorescence Assay (IFA) while cytokine levels were measured using ELISA.
Results: Our data showed the mean age of female patients is 38.1 years. Higher mean levels of both cytokines were observed in the first year of disease onset and menopause autoimmune-CTD patients. The mean levels of IL-6 and IL-10 were significantly higher in positive anti-Ro/La compared to the control group (p < 0.05). Also, the significant correlation of IL-6 and IL-10 in CTD patients as opposed to healthy control has underlined the putative role of these biologics.
Conclusions: These data suggest the putative manipulation of IL-6 and IL-10 as prognostic and therapeutics molecules in managing CTD, as an alternative to steroid-based medications to control the disease manifestations.
Autoimmune diseases (AIDs) are conditions where immune cells of the host attack its cells and tissues leading to the production of autoantibodies targeting self-proteins [1]. There are more than 80 types of AIDs such as type 1 diabetes (TID), multiple sclerosis (MS), Sjogren’s syndrome (SS), rheumatoid arthritis (RA), graves’ disease (GD) and systemic lupus erythematosus (SLE) [1, 2]. AIDs are the third most common disease in the USA after cancer and cardiovascular diseases [3]. The prevalence of AIDs is about 5–8% in the general population predominantly females (78%) and varies according to ethnicity and geographical area [4]. Asian and non-Caucasian patients are more prone to develop severe SLE with poorer outcome [5]. In our multiracial setting, the prevalence of female SLE was 85.9% compared to that of males (14.1%) and the majority of them were Malays followed by Chinese, Indians, and others [6].
The aetiology of AIDs is not entirely elucidated but pieces of evidence are suggesting that the multifactorial pathogenesis of autoimmunity, which includes genetic, and epigenetics regulation may herald changes into the uncontrolled immune responses, leading to destructive reactions of autoantibodies towards self-proteins binding [7, 8]. Autoantibodies are the immunoglobulins reacting against self-molecules or proteins [9]. Due to its high titre and varying subtypes in AIDs, autoantibody detection against cellular components has been utilized as one of the serologic indicators during autoimmune conditions [10, 11]. A nuclear-speckled pattern of cells is commonly found in several AIDs including SLE, SS, scleroderma and mixed connective tissue disease (MCTD), indicating the presence of anti-nuclear antibodies against the nuclear proteins [12]. While the presence of pathogenic autoantibodies is highly specific in organ-specific AIDs such as thyroiditis and TID, in systemic AIDs like SLE, it is less specific and directed against multiple organs [8]. In SLE and other systemic autoimmune conditions such as subacute cutaneous lupus erythematosus (SCLE), anti-Ro is commonly found autoantibodies while anti-La is mostly found in SS patients [13]. Anti-Ro autoantibodies are usually detected alone in human sera whereas anti-La autoantibodies are often in company with anti-Ro autoantibodies [14]. And because cytokines play a major role in the pathogenesis of AIDs, they may serve as putative biomarkers for local and systemic inflammatory responses [15].
In most AIDs, the balance between pro- and anti-inflammatory cytokines determines the extent of inflammation. Pro-inflammatory cytokines contribute to the initiation and propagation of autoimmune inflammation, whereas anti-inflammatory cytokines facilitate the regression of inflammation and promote recovery from an acute phase of the disease [16]. A previous study has shown that certain cytokines exhibited pleiotropic effects, which may exert both pro- and anti-inflammatory effects in the same vicinity [17]. Cytokines are secreted by activated immune cells and divided into two subtypes i.e., type 1 and type 2 subtypes. Type 1 cytokines including interferon-γ (IFN-γ) and IL-2 are produced by Th1 cells responsible for mediating cellular responses. On the other hand, type 2 cytokines such as IL-4, IL-5, IL-6, and IL-10 are produced by Th2 cells and non-T cells such as B cells and monocytes mediate humoral responses [18]. Furuzawa-Carballeda and colleagues (2014) [19] showed that patients with primary SS had a higher number of Th17 and Th19 cells and the cytokine imbalance in local glands and peripheral blood contributed to chronic inflammation in SS [17]. It was shown that peripheral primary monocytes from SS patients produced significantly higher IL-6 and BAFF levels than healthy subjects [20]. Besides, abnormal IL-6 expression may contribute to the development of other AIDs such as RA in which, synovial fluid from the joints of active RA patients showed increased IL-6 levels [21].
Interleukin-6 (IL-6) is a pleiotropic cytokine executing a diverse number of biological functions, including hematopoiesis, blood vessel permeability, inflammations and immune responses [22]. Given the multifaceted roles of IL-6 in adverse inflammatory reactions, the correlation between IL-6 levels and other disease burdens has been studied over the years. As reported by Trovato and colleagues [23], a positive correlation was observed between free serum IL-6 levels and the age of HIV-positive patients developing the AIDs over time during the disease course, suggesting the interplay of IL-6 in autoimmunity. Recently, Taylor and colleagues (2024) [24] have examined that IL-6 inhibition affects joint inflammation and other comorbidities associated with individuals with RA.
On the other hand, IL-10 cytokine is responsible for inhibiting the production of IFN-γ cytokines and regulating chemokine receptor CCR7 [1, 25]. It also triggers B lymphocytes to induce the secretion of autoantibodies or immunoglobulins [26]. Human IL-10 is a homodimer of 37 kDa and each monomer consists of 160 amino acids [27]. In primary SS patients, IL-10 is secreted by T cells mainly at the inflammatory site in salivary glands and by peripheral blood mononuclear cells. IL-10 and IL-6 play a central role in the maturation of plasma cells and activation of immunoglobulin synthesis [28]. A higher level of IL-10 was detected in the serum of primary SS patients compared to the control group [29]. This is associated with high titres of immunoglobulin A (IgA) of RF, anti-Ro, and anti-La specific antibodies corresponding to the severity of lymphocytic infiltration in the salivary gland [17]. In addition, a significant increase in IL-10 was found in the saliva of SS patients compared to that of healthy controls. This was significantly related to the severity of dryness of the mouth and eyes as well as the erythrocyte sedimentation rate [25].
Therefore, this study was conducted to determine the association between the pleiotropic cytokine of IL-6 and anti-inflammatory IL-10 with multifactorial analyses in female patients with anti-Ro and anti-La autoantibodies among cohort study recruited from government healthcare multicentre. We compared the presence of single positive anti-Ro and double positive anti-Ro/La with multiple factors including demographic, differential diagnosis, medication, disease progression, menopause status and levels of both IL-6/IL-10 in these patients to measure the fundamental relationship. It is hoped that data from the study may widen the potential use of IL-6/IL-10 as target biomarkers for advanced treatment development against AIDs, other than RA [14].
Sera from patients who attended the rheumatology clinic suspected of connective tissue diseases (CTD) positive were collected from the Microbiology Unit, Pathology Department, Hospital Tuanku Fauziah, Kangar, Perlis, Malaysia. The medical records were retrieved to match the samples for demographic data analysis and criteria sub-stratification. Designated groups were as the following: anti-SSA/Ro as Group 1 (n = 35), double positive anti-SSA/Ro-SSB/La as Group 2 (n = 35), and negative control sample as Group 3 (n = 36). Inclusion criteria include females aged 18 years old until 80 years old, diagnosed with the presence of anti-Ro or anti-La or both regardless of differential diagnosis. Exclusion criteria include males, age group under 18 years old, and hemolyzed samples. Meanwhile, the negative control group was recruited from blood donors attending the blood bank for blood donations from the same facility and the age range was matched with the study group. All consents and experimental protocols were approved by the Medical Research and Ethics Committee (MREC), Kementerian Kesihatan Malaysia (MREC approval number: (6) KKM/NIHSEC/P17-1363) and Human Research and Ethics Committee (JEPEM), Universiti Sains Malaysia (USM/JEPEM/17110570).
Menopause status and medicines taken were obtained from the medical records unit of the hospitals using the subject datasheet form. The year of disease onset was defined as the time at which an individual was diagnosed.
Samples were screened for CTD using an automation Enzyme Immunoassay (EIA), Immunocap100 (Thermo Scientific, USA). Any presence of anti-nuclear antibody (ANA) patterns was confirmed by indirect Immunofluorescence Assay (IFA), MBL (Japan). Speckled IFA pattern was tested for extractable nuclear antigen (ENA) marker specific to anti-Ro and anti-La autoantibodies by EIA using Immunocap100 (Thermo Scientific, USA). Low titration of these autoantibodies may indicate the incidence of autoreactive B and T cell activities. This method is commonly used to confirm the presence of anti-nuclear antibodies associated with CTDs in patient’s blood samples. Sera from patients will be incubated on a glass slide covered with a monolayer of malignant human epithelial cell lines. The slides are washed and any bounded autoantibodies to the cell nuclei will be visualized using the detection antibody, conjugated to a fluorescence tag. The slides are ready for viewing using a fluorescence microscope (This slide is performed for staining at a dilution of 1:800 at a magnification of 40×).
Micro bicinchoninic acid (BCA) protein assay kit by Thermo Scientific™ (USA) was used to quantify the total protein. Sera samples were diluted at a ratio of 1:1,000 (1 μL sera: 999 μL PBS). Samples were pipetted into the 96-microplate wells in triplicates. 150 μL of working reagent was added to each well and the plate was mixed thoroughly on a plate shaker for 30 seconds and incubated at 37°C for 2 hours. After incubation, the plate was cooled to room temperature and the absorbance was measured at 562 nm on a spectrophotometer (Thermo Scientific). The standard curve was used to determine the protein concentration of each sera sample.
IL-6 and IL-10 were measured using pre-coated ELISA plates against these analytes according to the manufacturer’s protocol (Elabscience, China). A standard curve graph was prepared by plotting the average Blank-corrected 450 nm reading for each reference standard versus IL-6/IL-10 concentration in pg/mL. The standard curve was used to determine the IL-6/IL-10 concentration of each sample.
Data from experimental analyses were presented as a mean of triplicates with standard error mean (mean ± SEM) and standard deviation (SD). The data were statistically analysed using IBM SPSS statistics for Windows software (Version 19.0. Armonk, NY: IBM Corp). Analyses for multifactorial between cytokine levels and patients diagnosed with autoimmune-CTD were performed by multiple logistic regression. Pearson correlation test was performed to analyse the relationship between pro- and anti-inflammatory cytokines and anti-Ro, anti-La and anti-Ro/La autoantibodies. Comparison between control and positive groups was tested for significance using the one-way analysis of variance ANOVA test and p value of less than 0.05 (p < 0.05) is considered significant.
We first identified the demographic distribution of single anti-Ro and double anti-Ro/La autoantibodies in the cohort study. Samples from patients were analysed for demographic parameters. From the data, the mean age of a female with a single positive anti-Ro and a double positive anti-Ro/La is 38.1 years old (SD ± 15.01) (n = 70). Malay women represented the highest frequency of both single and double positive cases in the region at 72.6%, followed by Chinese at 17.1%, Indian at 7.5%, and other races at 2.8% respectively.
ANA reporting commonly involves two components i.e., quantitative and qualitative of ANA. Table 1 has tabulated the quantitative measurement of ANA using antibody titre. Four titration ratios were used to categorize the level of autoantibodies detection in patient’s samples i.e., 1:100, 1:400, 1:800, and > 1:800 dilution titration ratio. It was shown that on 31.4% of samples from single positive anti-Ro, ANA was optimally detected at the lowest titration ratio of 1:100, while 25.7% detected at a titration ratio of 1:800, 22.9% detected at a ratio > 1:800, followed by 20.0% samples detected with ANA at 1:400 titration ratio. Meanwhile, in double positive anti-Ro/La, 37.2% of samples were detected with ANA at the highest dilution titration i.e., > 1:800 titration ratio. This was then followed in 34.3% of samples with ANA detected at a 1:800 ratio, 17.1% of samples detected with ANA at a 1:100 ratio, and followed by 11.4% of samples with ANA detected at a 1:400 titration ratio.
Analysis of titration of ANA IFA staining in single positive anti-SSA/Ro (Group 1) and double positive anti-SSA/Ro anti-SSB/La (Group 2)
| Titration | Group 1 (n = 35) | Group 2 (n = 35) |
|---|---|---|
| n (%) | n (%) | |
| 1:100 | 11 (31.4) | 6 (17.1) |
| 1:400 | 7 (20.0) | 4 (11.4) |
| 1:800 | 9 (25.7) | 12 (34.3) |
| > 1:800 | 8 (22.9) | 13 (37.2) |
ANA: anti-nuclear antibody; IFA: Immunofluorescence Assay
In the cohort study, we analysed the titration of ANA IFA staining in single positive anti-SSA/Ro and double positive anti-SSA/Ro anti-SSB/La for nuclear-speckled pattern detection (Figure 1). A course-speckled pattern is a common indication of autoantibodies to anti-U1RNP and anti-Sm proteins, while a fine-speckled pattern refers to the presence of anti-Ro and anti-La autoantibodies [9].
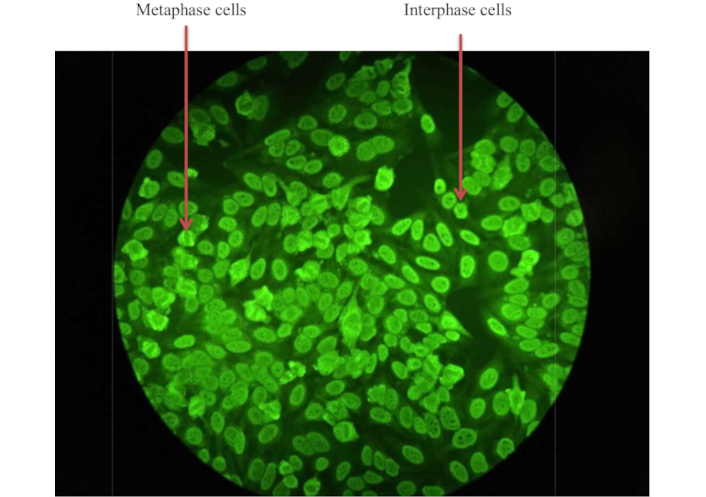
A representative of an indirect Immunofluorescence Assay (IFA) staining of a patient’s serum for antinuclear antibodies (ANA). The green fluorescence indicates the presence of ANA binding to nuclear antigens within the cells. The nuclear-speckled pattern was detected in a sample from the cohort at a dilution of 1:800 at a magnification of 40×. The metaphase cell showed no staining of the condensed chromosomal region, while the figure showed a fine to discrete speckled pattern of the interphase cell in a uniform distribution. The course-speckled pattern is a common indication of autoantibody binding of anti-U1RNP and anti-Sm proteins while the fine pattern represented the anti-Ro and anti-La binding
For any positive ANA, antibody titration needs to be performed to determine the quantification of ANA that has been detected in the samples. In this study, four titration ranges were used to determine the presence of ANA. Up to 31.4% of subjects with single positive anti-SSA/Ro are optimally detected with ANA at the lowest antibody titre while 17.1% with double positive anti-SSA/Ro anti-SSB/La are optimally detected at the same titre. The study cohort showed that most individuals with a single positive anti-SSA/Ro can be detected at lower antibody titre, indicating higher levels of ANA formed during the disease occurrence.
Next, we analysed the clinical characteristics of these patients from the same cohort. All patients were stratified into clinical attributes as shown in Table 2. These attributes were analysed to determine the correlation of the factors contributing to the incidence of autoantibody formation. The single-positive anti-Ro autoantibodies (Group 1) and double-positive anti-Ro/La autoantibodies (Group 2) were tabulated in Table 2 with the attributes analysed. Differential diagnosis for individuals with anti-Ro and/or anti-Ro/La autoantibodies includes SLE, SS, MCTD, RA, and other types of AIDs. It was shown that most patients were treated with steroid-based drug therapy to minimize signs and symptoms.
Clinical characteristics of patients in Group 1 (single positive) and Group 2 (double positive group)
| Variables | Group 1 (n = 29) (%) | Group 2 (n = 25) (%) |
|---|---|---|
| Age (year) | 40.7 (17.7)a | 34.0 (27.0, 55.5)b |
| Age group | ||
| Under 25 | 8 (27.6) | 6 (24.0) |
| 26–45 | 9 (31.0) | 11 (44.0) |
| 46 and older | 12 (41.4) | 8 (32.0) |
| Disease progression | ||
| ≤ 1 year | 5 (17.2) | 10 (40.0) |
| 2–4 years | 7 (24.1) | 8 (32.0) |
| ≥ 5 years | 6 (20.7) | 5 (20.0) |
| Missing | 11 (37.9) | 2 (8.0) |
| Diagnosis | ||
| SLE | 4 (13.8) | 14 (56.0) |
| SS | 1 (3.4) | 2 (8.0) |
| MCTD | 4 (13.8) | 1 (4.0) |
| Other AID | 9 (31.0) | 6 (24.0) |
| Others | 11 (37.9) | 2 (8.0) |
| Menopause status | ||
| Yes | 9 (31.0) | 7 (28.0) |
| No | 20 (69.0) | 18 (72.0) |
| Medication | ||
| On steroid | 16 (55.2) | 22 (88.0) |
a Mean (standard deviation); b Median (interquartile range); SLE: systemic lupus erythematosus; SS: Sjogren’s syndrome; MCTD: mixed connective tissue disease; AID: autoimmune disease. Data not available: Confirmed diagnosis date from 6 subjects from Group 1 and 10 subjects from Group 2 are not available
Table 2 shows the characteristics of patients in both groups. Group 1 i.e., single positive group consisted of 29 patients with a mean age of 40.7 (SD ± 17.7) years old. The minimum and maximum age were 18 and 80 years old, and the majority (41.4%) were in the age group 46 and older. Most of the patients (82.8%) were of Malay ethnicity. In regard to disease progression, about 24% of the patients have been diagnosed between two to four years. Approximately 37.9% of the sample had missing information on disease onset. The majority of the group were diagnosed as others, followed by 31% with other AIDs, 13.8% with SLE and MCTD, respectively, and 3.4% with SS. Of all patients, only 31% had menopause and 55.2% took steroid medication.
Meanwhile, in double positive group (Group 2), there were 25 patients with a median age of 34 years. The minimum and maximum age of patients in this group were 18 and 69 years old, and the majority (44%) were in the age group 26–45 years. Most of the patients (88%) were of Malay ethnicity. The majority (40%) had the disease less than one year and 56% had a diagnosis of SLE. Only 28% of the patients had menopause and up to 88% were taking steroid medication. In the current study, disease onset is defined as years from symptom onset to diagnosis time. It was recorded that in single positive patients, disease onset commonly occurred within two to four years in 24.1% of the studied cohort. While in double positive patients, disease onset commonly occurred at less than a year in 40.0% of the studied cohort. Interestingly, around 20.7% of single positive and 20.0% of double positive patients were recorded to have their diagnosis after 5 years of disease onset. This may suggest the differences in the degree of disease progression in patients with a single and double autoantibody among the cohort study.
CTDs are one of the most common AIDs in developed countries and is associated with high levels of anti-SSA/Ro and anti-SSB/La autoantibodies [3]. To outline the fundamental correlation between IL-6 and IL-10 levels and formation of these autoantibodies in differential diagnosis of CTD, we measured these cytokines and mapped to clinical characteristics in patients with single positive anti-SSA/Ro and double positive anti-SSA/Ro anti-SSB/La.
Our data showed that both cytokines were highest among patients with single positive anti-Ro who were diagnosed in less than a year (Table 3). Meanwhile, IL-6 was highest in patients with double positive anti-Ro/La who were diagnosed within 4 years. However, there was no significant difference in IL-10 levels in patients with double positive anti-Ro/La regardless of disease onset. It is interesting to note double positive patients diagnosed in less than a year and after 5 years have lower serum IL-6 levels.
Analysis between IL-6 and IL-10 cytokine levels and disease onset in single positive anti-SSA/Ro (Group 1) and double positive anti-SSA/Ro anti-SSB/La (Group 2)
| Cytokine | Year* | Group 1 | Group 2 | ||
|---|---|---|---|---|---|
| (Mean in pg/mL ± SD) | n (29) | (Mean in pg/mL ± SD) | n (25) | ||
| IL-6 | ≤ 1 | (97.5 ± 140.0) | 5 | (27.5 ± 15.3) | 10 |
| 2–4 | (38.5 ± 20.0) | 7 | (121.5 ± 201.6) | 8 | |
| ≥ 5 | (33.9 ± 27.9) | 6 | (40.2 ± 20.4) | 5 | |
| NA | (46.6 ± 50.5) | 11 | (46.4 ± 10.1) | 2 | |
| IL-10 | ≤ 1 | (56.5 ± 46.4) | 5 | (34.5 ± 30.5) | 10 |
| 2–4 | (37.0 ± 28.5) | 7 | (33.0 ± 41.4) | 8 | |
| ≥ 5 | (35.5 ± 25.1) | 6 | (29.4 ± 22.4) | 5 | |
| NA | (36.6 ± 32.6) | 11 | (30.8 ± 27.3) | 2 | |
* Year of disease onset; SD: standard deviation; NA: not available. Data not available: History of disease onset for 6 subjects from Group 1 and 10 subjects from Group 2 are not available
Next, we measured the association between pro-inflammatory cytokine IL-6 and anti-inflammatory cytokine IL-10 with medication in the study cohort. As shown in Table 4, there was no significant association between cytokine levels and medication prescribed in CTD vs control group (p > 0.05). In these patients, high levels of IL-6 have been recorded in the study cohort as opposed to the control group showed 58.76 vs 27.41 pg/mL (p > 0.05). On the other hand, IL-10 was recorded to be slightly higher as compared to the control group showed 38.86 pg/mL vs 22.04 pg/mL (p-value > 0.05). However, there was a significant difference in medication between CTD and the control group (p < 0.001) (Table 4). Data from medical records showed that 38 of the CTD patients diagnosed were managed with steroid-based therapy such as corticosteroids and prednisolone. While the 16 subjects were treated with other than steroids. The most frequent steroids used for treatment are prednisolone or methylprednisolone in combination with antimalarial drugs such as hydroxychloroquine (HCQ) or chloroquine (CQ).
Analysis of medication associated with the levels of IL-6 and IL-10 cytokines in patients with autoimmune connective tissue diseases (CTD)
| Variables | CTD (n = 54)(pg/mL) | Control (n =35)(pg/mL) | Crude OR(95% CI) | p-valueb | Adjusted OR(95% CI) | p-valuec | |
|---|---|---|---|---|---|---|---|
| IL-6a | 58.76 (103.83)a | 27.41 (28.93) | 1.011 (0.997, 1.026) | 0.110 | 1.005 (0.979, 1.032) | 0.693 | |
| IL-10a | 38.86 (32.19)a | 22.04 (20.33) | 1.011 (0.997, 1.025) | 0.133 | 1.002 (0.972, 1.033) | 0.888 | |
| Medication | Steroid | 38 | 1 | 0.002 (0.000, 0.021) | < 0.001* | 0.002 (0.000, 0.026) | < 0.001* |
| Non-steroid | 16 | 34 | 1.00 | - | 1.00 | - | |
a Mean (SD); b Simple linear regression; c Wald statistic (Multiple logistic regression); * Significant value since p < 0.05. OR: odds ratio; CI: confidence interval; CTD: connective tissue disease. Data not available: Medication record for 16 subjects of CTD are not available
To correlate the influence of estrogen hormone in autoantibody formation, we measured the cytokine levels among menopause and non-menopause patients in the current cohort as tabulated in Figure 2. Figure 2 showed that the mean levels for both cytokines were high in menopause patients diagnosed at a very early stage i.e., within a year as compared to non-menopause patients with the same disease onset. Interestingly, IL-6 continued to spike in menopause patients diagnosed within 2 to 4 years compared to non-menopaused patients of the same onset (138.3 ± 250.7 vs 55.0 ± 65.1) (Figure 2a). Meanwhile, IL-10 was recorded to reduce in the same group of patients (35.5 ± 34.2) (Figure 2b) and gradually increased in menopause patients who were diagnosed for 5 years and more (46.6 ± 23.5).
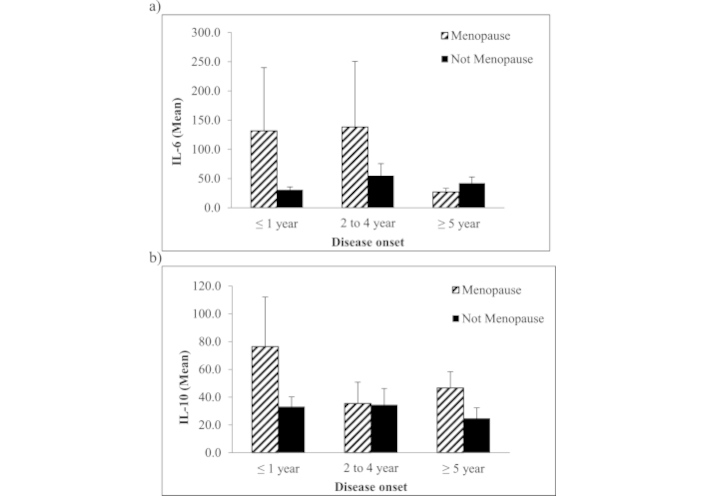
Mean in pg/mL of (a) IL-6 and (b) IL-10 cytokines in patients with autoimmune-CTD at different disease onset versus menopause status. The high mean levels of IL-6 and IL-10 are significantly observed on the first year of disease onset and in menopause autoimmune-CTD patients. Patients who haven’t reached menopause are shown to have low levels of IL-6 compared to menopausal patients. Interestingly, the higher mean IL-10 levels are observed in menopause patients on their first year of disease onset while reduced in menopause patients who suffer longer than that. In contrast, those who have not menopause significantly reduced their expression regardless of disease onset. Error bar represents the standard error mean (SEM)
The correlation of IL-6 and IL-10 with Group 1 (single positive anti-Ro) and Group 2 (double positive anti-Ro/La) is tabulated in Table 5. From the data, it was indicated that IL-6 was significantly associated with both Group 1 and Group 2 with p-value of 0.045 and 0.023 respectively. On the other hand, current findings showed that IL-10 was significantly associated with Group 1 only (p < 0.05) while not significantly correlated with Group 2 (p > 0.05).
Correlation of IL-6 and IL-10 with anti-Ro and anti-La autoantibodies in single positive anti-SSA/Ro (Group 1) and double positive anti-SSA/Ro anti-SSB/La (Group 2)
| Groups | IL-6 | IL-10 | ||
|---|---|---|---|---|
| r | p-value | r | p-value | |
| Group 1 (n = 35) | –0.195 | 0.045* | –0.327 | 0.001* |
| Group 2 (n = 35) | –0.220 | 0.023* | –0.120 | 0.219 |
* Significant value since p < 0.05
Lastly, we measured the mean concentration of these cytokines in Group 1 and Group 2 versus control healthy donor (Figure 3). The mean levels of IL-6 and IL-10 in Group 1 and Group 2 were significantly higher than the healthy controls (p < 0.05). The mean levels of IL-6 in Group 1 were (59.71 ± 13.75 pg/mL), while Group 2 were (70.56 ± 19.54 pg/mL) as compared to healthy control (20.48 ± 2.53 pg/mL). Meanwhile, the mean levels of IL-10 in Group 1 were (45.52 ± 7.287 pg/mL) and Group 2 were (32.96 ± 4.79 pg/mL) and healthy controls were (17.09 ± 2.01 pg/mL). Based on the ANOVA test, the differences in mean levels obtained among cohort study were statistically significant (p < 0.05).
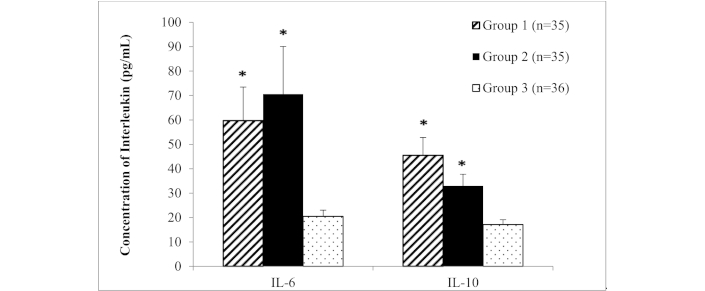
Mean concentration of IL-6 and IL-10 in female positive anti-Ro (Group 1) or positive anti-Ro and La (Group 2) versus negative control (healthy donors) (Group 3). ANOVA test; * Indicate significance level p < 0.05 as compared to control group. Error bar represents the standard error mean (SEM)
Our work was conducted to attest to the significant association between pro- and anti-inflammatory cytokines, IL-6 and IL-10 respectively in patients with single anti-Ro and double anti-Ro/La autoantibodies from cohort study. The cohort was selected from subjects diagnosed with CTDs at different disease onset. We analyzed factors associated with both cytokines to underline the important role of these cytokines in either the development of autoantibodies, disease progression or manifestations.
Population-based analysis showed that race distribution in the studied region may affect the incidence frequency as compared to other races, with a mean age of 38.1 years old. The influence of genetic or race factors on the frequency of autoantibodies has been observed in a few studies [5, 10, 27]. It was demonstrated that Hispanic, African American and Asian individuals are two to three times more prone to develop SLE when compared to Caucasians [5]. Plus, ANA prevalence in the United States population was higher in African Americans compared to Caucasians [10]. The average age of Caucasian SLE patients was 38.6 years old with females predominant [30]. A previous study showed that autoimmune CTD is observed mostly in women of their forties concomitant with the changes in hormonal levels [31]. Meanwhile, analysis of the medical history showed that approximately 97.4% of patients in the study cohort were managed with steroid-based therapy. The common types include prednisolone or methylprednisolone, in combination with antimalarial drugs of HCQ. According to Low and colleagues (2021) [32], treatments for non-communicable diseases may require a long-term duration of prescribed drugs, therefore accessibility and affordability remain the key factors for service providers. In 2019, Park and colleagues [33] reported that HCQ effectively alleviates autoimmunity via Nurr1 receptors. The safety of HCQ has been systematically reviewed by Ruiz-Irastorza and colleagues [34] who reported that HQ prescription displays a wide spectrum of benefits among prescribed patients. The use of HCQ as an off-label treatment option in treating COVID-19 patients has been considered recently [35, 36].
Globally, the average menopausal age is 51 years old while the mean menopausal age among the study population is (49.9 ± 4.27 years old) [37]. To outline the interrelationship between cytokines and disease progression, we further analysed the levels of IL-6 and IL-10 with menopause status among the study cohort. As shown in Figure 2, the current findings showed that both cytokines increased among menopause patients in the early stage of disease development as compared to non-menopause patients. IL-6 continued to spike as the disease progressed along the four-year course. Interestingly, IL-6 levels reduced dramatically as menopause patients progressed into the fifth year of disease onset, with IL-10 remaining low over time. The current findings may suggest the influence of estrogen and progesterone on IL-6/IL-10 cytokine production in patients with positive anti-Ro/La antibodies. Based on current data, it was suggested that reduced estrogen levels exposed women to developing anti-Ro/La autoantibodies which directly correspond to increased risk of developing AIDs. This finding was partially in line with a previous study that reported post-menopausal women are producing higher IL-6 levels following mental stress than men thus providing clinical implications related to inflammatory conditions such as RA and asthma [38].
In addition, the intracellular ERβ expression is significantly lower in CD4+ and CD8+ T cells from female patients with active SLE disease as compared to healthy donors and patients with low active disease [39]. The clinical expression of SLE is mainly associated with sex, independently of age [40]. Younger women have more typical features of SLE such as malar rash, Raynaud’s phenomenon and the presence of anti-Ro/La autoantibodies whereas older women (> 55 years old) mainly presented with discoid lesions and serositis [40]. It was suggested that low expression of the anti-inflammatory effect of ERβ and high serum levels of anti-ERα antibodies are possibly contributing to SLE pathogenesis in female SLE patients [41]. Notably, ERs have been demonstrated in various immune cells involving T and B lymphocyte subsets as well as peripheral NK cells [42]. IL-6 expression is significantly decreased upon stimulation of TLRs (7 and 9) in “KIKO” mice (ERα DNA-binding mutant) compared to wild-type mice. These data showed that TLR-induced cytokines are modulated by ERα and require binding to estrogen response elements for optimal inflammatory response. ERα may bind to transcription factors such as NFκB to regulate transcription of IL-6 cytokine production and cell survival [43].
Our study showed that IL-6 was significantly correlated with both single-positive anti-Ro and double-positive anti-Ro/La autoantibodies groups. This is in agreement with a previous study that reported a higher synthesis of IL-6 from monocytes of idiopathic inflammatory myopathies (IIM) patients, which is highly associated with anti-Ro52 autoantibodies [12, 44, 45]. The role of IL-6 at the early stage of disease development, providing putative manipulation towards prognosis and diagnosis of disease progression. It has been demonstrated that pro-inflammatory IL-6 is involved in the autocrine route which maintains B cell hyperactivity, which leads to autoantibody over-production, and its co-stimulatory factors such as TLR-4, IL-1, and IFN resulting in an increased autoantibody production [45]. In addition, the overexpression of IL-6 superfamily receptor molecule glycoprotein (Gp) 130 on B cells of SLE patients in comparison with healthy control subjects indicated the definitive role of IL-6 on connective tissue disease progression [46]. This protein serves as signal transduction subunits for all IL-6-related cytokines thus contributing to the activation of plasma cells and subsequently antibody overproduction.
Our current study on IL-10 showed that it was significantly correlated only in patients with anti-Ro autoantibodies and is in agreement with a report from Mozo and colleagues (2014) [47] that positive SLE patients have significantly higher serum levels of IL-10 than their negative control. The study has shown that the presence of anti-Ro in anti-RibP-positive SLE patients is found to be related to an increase in IL-10 and IFNα levels in sera [47]. IL-10 is a potent growth and differentiation factor for B lymphocyte proliferation and activation via their antigen receptor or CD40 to secrete large amounts of IgG, IgA, and IgM antibodies [48]. There is a significant association between IL-10 imbalance in patients with anti-Ro52 autoantibodies [49] and the hypermethylation of the IL-10 gene is responsible for the low mRNA expression in Bechet’s disease [50], which may suggest that hyperproduction of IL-10 is associated with the formation of autoantibodies.
In the current study, the mean levels of these cytokines also indicated significant differences among cohort study. We have found that both cytokines were significantly higher in patients as compared to healthy controls. IL-6 was found to be higher than IL-10 in patients with double positive anti-Ro/La autoantibodies, as compared to single anti-Ro autoantibodies patients. Previous reports on serum levels of circulating IL-6 showed that IL-6 is significantly higher in SLE patients (40.66 ± 22.8 pg/mL) compared to healthy control (2.32 ± 3.41 pg/mL) of the Eastern Saudi Arabia region, lupus nephritis vs lupus non-nephritis and in SLE patients in India [51, 52]. Interestingly, the significant correlation of IL-6 with the development of Hashimoto’s thyroiditis among HIV patients over a period may indicate that IL-6 is pivotal for the incidence of autoantibody formation [23].
In addition, our results were also in line with a previous study that the mean serum IL-10 levels were significantly higher in SLE Egyptian patients and correlated with the SLEDAI scores (p = 0.016) [53]. Interestingly, current data reported that IL-10 levels were higher in single anti-Ro autoantibodies than double positive anti-Ro/La autoantibodies, and vice versa for IL-6 sera levels. This may indicate further investigation of the pathogenesis of this differential diagnosis is needed. Persistence inflammation promotes a detrimental role of IL-6, contributing to adverse immune reactions, eventually altering the immune response towards self-proteins. In turn, negative feedback from IL-10 begins to emerge at the beginning of disease progression, as reported by the current study. As the disease progresses due to overwhelming and altered immune response by IL-6 in patients with anti-Ro/La autoantibodies, IL-10 levels are gradually decreased among cohort study. This may be due to the altered epigenetic expression of T-regulatory cells in patients, because of systemic inflammatory response by IL-6. As a result, increased expression of IL-6 during persistent inflammation will induce T-regulatory cell migration away from inflammatory sites, reducing the inhibitory capacity of these cells [54].
In conclusion, it is interesting to note that the differential expression of both cytokines may pave the way for developing biological therapy in AIDs. Current data suggest the possible IL-6 inhibitors/IL-10 stimulants tandem may provide beneficial advancement for the implementation of a treat-to-target strategy in treating AIDs. Overall, our current study may provide additional evidence to the literature on the putative roles of IL-6/IL-10 tandem as biomarkers in autoimmune-associated diseases. Screening and development of small molecules for IL-6 inhibitors and IL-10 stimulants for therapeutic purposes may be put forward for the next step in validating the potential biological therapy of these cytokines for autoimmune CTD patients, other than the established treatment for RA.
AIDs: autoimmune diseases
ANA: anti-nuclear antibody
CQ: chloroquine
CTD: connective tissue diseases
EIA: Enzyme Immunoassay
HCQ: hydroxychloroquine
IFA: Immunofluorescence Assay
IFN-γ: interferon-γ
IgA: immunoglobulin A
MCTD: mixed connective tissue disease
MS: multiple sclerosis
RA: rheumatoid arthritis
SLE: systemic lupus erythematosus
SS: Sjogren’s syndrome
TID: type 1 diabetes
Special thanks to the Ministry of Health for their support and approval. Thanks to the Microbiology Unit, Pathology Department Hospital Putrajaya and Hospital Tuanku Fauziah for sample collections. Special thanks to Mrs. Sangitha, Mr Faizal, and Mrs Atifah from Hospital Putrajaya and Madam Nor Suhaila from Hospital Tuanku Fauziah for their assistance throughout the project.
NESZ: Investigation, Methodology, Data curation, Formal analysis, Writing—original draft, Writing—review & editing. AAZ: Investigation, Methodology, Data curation, Formal analysis, Resources. BB, CLT, and NAZ: Project administration, Resources. EM: Data curation, Formal analysis.
All authors declared no conflict of interest.
All experimental protocols were approved by the Medical Research and Ethics Committee (MREC), Kementerian Kesihatan Malaysia (MREC approval number: (6) KKM/NIHSEC/P17-1363) and Human Research and Ethics Committee (JEPEM), Universiti Sains Malaysia (USM/JEPEM/17110570).
Informed consent to participate in the study was obtained from all participants.
Consent for publication has been obtained together with ethical approval of the study from MOH (MREC No: (6) KKM/NIHSEC/P17-1363) and USM JEPEM (USM/JEPEM/17110570).
Data concerning the participants are not publicly available due to ethical restrictions. Requests for accessing the datasets should be directed to [Nor Effa S. Zulkafli, effa@usm.my].
This study was funded by the Fundamental Research Grant Scheme (FRGS), Ministry of Higher Education of Malaysia [FRGS/1/2019/SKK08/USM/03/14]. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2163
Download: 20
Times Cited: 0
