Affiliation:
1Research Unit of Biochemistry of Medicinal Plants, Food Sciences and Nutrition, Department of Biochemistry, Faculty of Science, University of Dschang, Dschang P.O. Box 67, Cameroon
2Department of Food Process Engineering, National Institute of Food Technology, Entrepreneurship and Management, Thanjavur 613005, Tamil Nadu, India
Email: stambotene@yahoo.fr
ORCID: https://orcid.org/0000-0002-2454-5983
Affiliation:
2Department of Food Process Engineering, National Institute of Food Technology, Entrepreneurship and Management, Thanjavur 613005, Tamil Nadu, India
Email: venkat@iifpt.edu.in
ORCID: https://orcid.org/0000-0002-5111-8442
Explor Foods Foodomics. 2025;3:101087 DOI: https://doi.org/10.37349/eff.2025.101087
Received: April 07, 2025 Accepted: June 12, 2025 Published: June 30, 2025
Academic Editor: Josep Rubert, Wageningen University, Netherlands
Aim: The germination of maize leads to many physiological changes in the plant. These changes are responsible for the appearance, disappearance, and variation in concentration of numerous compounds, including secondary metabolites. The aim of this study was to compare the secondary metabolite profile of two maize varieties germinated under controlled optimal conditions.
Methods: To achieve this, the Atp-Y variety was soaked for 25.12 h at 25.54˚C in the presence of 0.5238% plant ash, germinated for 144.37 h, and matured for 37.65 h. For the Coca-sr variety, the grains were soaked for 1.608 h at 36.63˚C in the presence of 1.1093% plant ash. Germination and ripening took 144.37 h and 27.07 h, respectively. The compounds were extracted in methanol (HPLC grade) before being injected into a gas chromatography-mass spectrometry (GC-MS) equipped with an Rtx-5MS column for metabolite profiling.
Results: These analyses showed that variety and optimum germination conditions influenced the secondary metabolite profile. This profiling identified 15 and 12 compounds in the Atp-Y and Coca-sr varieties, respectively. Of these compounds, 8 were identified in both varieties. The groups of compounds identified were fatty acids, esters, ketones, phenols, polyols, alcohols, sterols, and unclassified substances. Fatty acids were the most abundant, with proportions of 90.93% and 91.08% Atp-Y and Coca-sr, respectively. Within this group of compounds, (Z,Z)-9,12-octadecadienoic acid was the most abundant (46.58% for Atp-Y and 53.84% for Coca-sr), followed by (E)-9-octadecenoic acid (30.39% for Atp-Y and 25.09% for Coca-sr). 2-Methoxy-4-vinylphenol, a phenolic compound, was identified and quantified at 0.28% only in the Coca-sr variety, while the only polyol, 1,4-anhydro-D-mannitol, was identified in the Atp-Y variety.
Conclusions: In view of these results, we would suggest using the Atp-Y variety to benefit from a wide range of compounds, but also to respect the malting conditions in order to benefit from the different compounds.
The uncontrolled and abusive use of drugs and certain generic drugs is at the root of the many cases of microbial resistance observed [1]. To mitigate this problem, alternatives involving the use of food plant extracts, functional foods have emerged and continue to develop [2–3]. To this end, much work has been done to find, isolate, and characterise active molecules in food plants in order to link their profiles to their biological potential. This is the case for phenolic compounds, flavonoids, carotenoids, and anthocyanins with antioxidant, antimicrobial, antidiabetic, and anticancer potential isolated from cashew nuts by da Silva et al. [4]. Taiwo et al. [5] reported the presence of the sesquiterpene lactone, zoapatanolide A, in methanolic extracts of a food plant commonly consumed in Nigeria. Similarly, Kewuyemi et al. [6] detected the presence of more than 150 compounds in sorghum fermented in the presence of different strains of lactic acid bacteria. These compounds where especially alcohol like glycerol, amides [dodecanamide and (Z)-9-octadecenamide], esters like dodecanoic acid ethyl ester, octanoic acid 2-dimethylaminoethyl ester and hexadecanoic acid 2-hydroxy-1-(hydroxymethyl)ethyl ester, fatty acids methyl esters (FAMEs) like tridecanoic acid methyl ester, methyl hexadecanoate, methyl(E,E)-9,12-octadecadienoate, (Z)-9-octadecenoic acid methyl ester, trans-13-octadecenoic acid methyl ester, cis-13-octadecenoic acid methyl ester, pentadecanoic acid 14-methyl-methyl ester, methyl stearate, octanoic acid 2-dimethylaminoethyl ester and hexacosanoic acid methyl ester, and others compounds classes like furans, phytosterols and phenols. In the same vein, Bolaji et al. [7] identified more than 35 compounds in soaked and fermented maize meals and revealed no influence of the production conditions. These compounds were especially aroma-like, such as acetaldehyde, ethanol, acetic acid, propanol, 2,3-butanedione, hexanal, 2,3-butanediol, heptanol, propanoic acid, and hexadecanoic acid. These observations show a variability of compounds between cereals and demonstrate the metabolic potential of cereals, such as maize.
Maize is the most widely grown cereal in the world, second only to rice in terms of consumption [8]. It grows on most of the planet and is the main source of food for people in developing countries, particularly in Africa. Maize is consumed for its richness in vitamins, especially B-complex vitamins, carotenoids in coloured varieties, carbohydrates, especially starch (almost 80%), simple sugars such as glucose and fructose, protein (almost 7% of dry matter) and minerals such as calcium, sodium, phosphorus and magnesium [9–12]. In addition to these nutrients, maize is also a significant source of numerous antinutrients such as phytates, trypsin inhibitors, and haemagglutinins, which limit the digestibility of various nutrients [13]. During maize consumption, treatments such as fermentation, dehulling, roasting, boiling, and malting are used to reduce the anti-nutrient content while improving nutrient digestibility and the development of new molecules [6, 14, 15]. Several studies have reported the development of numerous compounds of biological interest during these treatments and the improvement of organoleptic properties [11, 16].
Malting is one of the most common grain transformation processes used in maize processing. It involves the typical key stages of germination, steeping, and maturation [17]. The many physiological changes the plant undergoes during this process are the basis for the synthesis of new small molecules or macromolecules and the improvement of the flavour profile, highly valued by the brewing industry. These transformations include the formation of biogenic amines from the decarboxylation of amino acids by decarboxylases [18], simple sugars following the action of amylases [13], fatty acids such as palmitic, oleic and stearic acids, esters, aldehydes, alcohols, phenolic compounds, hydrocarbons, numerous volatile compounds and ketones [19, 20]. The study and profiling of all these compounds in a cereal such as maize after malting would therefore provide an additional database for researchers and also for breweries on the varieties and malting techniques to be applied in order to benefit from them. The use of appropriate tools such as GC-MS (gas chromatography-mass spectrometry) with a very large library for the identification of new compounds formed during this profiling process would facilitate the process of understanding the formation and development of these bioactive compounds. In addition, the high sensitivity and voltage stability of this instrument (70 eV) allow for the acquisition of distinct peaks [21].
Previous studies conducted on these two genetically and phenotypically different varieties (Atp-Y and Coca-sr) revealed a difference in composition and variant malting conditions [9, 10, 12–14, 17]. For the first time, we are going to use a metabolomics approach to characterise these two new germinated maize meals obtained under optimal conditions.
This work began in November 2023 at the Research Unit of Biochemistry of Medicinal Plants, Food Science and Nutrition of the University of Dschang in Cameroon and ended in June 2024 at the National Institute of Food Technology, Entrepreneurship and Management, Thanjavur (NIFTEM-T) in India, with the determination of the secondary metabolite profile, and did not require any ethical approval because it did not involve human or animal subjects. The research focuses on profiling metabolic compounds from two germinated maize meals.
The experimental methodology used in this work was both descriptive and qualitative. Not only was the compound profile of the different samples carried out, but also the proportions of compounds in each extract were determined.
The material used in this work was chemical, physical, and biological in nature. The chemical material consisted of methanol (HPLC grade) and Na2SO4 (99.99% of purity) supplied by HiMedia Laboratories Pvt. Ltd (Maharashtra, India). Test tubes and vials were supplied by SIGMA-ALDRICH Corporation (Bangalore, India). The electric super-stirrer used for sample homogenisation and the stirring plate were supplied by Borg Scientific (model LS3). The analytical instrumentation consisted of a GC-MS from Agilent Technologies (Shanghai 200131, the People’s Republic of China).
The biological material consisted of two maize varieties (Atp-Y and Coca-sr) collected at the Polyvalent Station of the Research Institute of Agriculture for Development of Dschang (Menoua Division, West Cameroon Region). Plant ash was prepared from unripe banana-plantain peels collected in the town of Bafoussam (Mifi Division, West Cameroon Region).
The method described by Tambo et al. [11] was used. In November 2023, 1,000 g of unripe banana-plantain peel was collected from households in Toungang village in Bafoussam. The peels were dehydrated by solar drying for 7 days with daily exposure of 8 h. At the end of this process, they were incinerated in a muffle furnace (CI-6S, REMI ELEKTROTEKNIK, India) previously calibrated at 505˚C for 5 h. The ash obtained was reduced to powder using a mixer (POLYMIX KINETICA, Japan) before being preserved in plastic bags and stored in a desiccator.
The optimum malting conditions for the two maize varieties, as determined by Tambo et al. [11], were applied in this study. Maize grains of the Atp-Y variety were soaked for 25.12 h at 25.54˚C in the presence of 0.5238% vegetable ash. After this step, the seeds were spread on muslin cloth in an incubator (model TT 9052, Techmel and Techmel, USA) calibrated at 30˚C for 144.37 h. During this stage, they were watered once every two days (15 mL/100 g of seeds). After germination, the grains were placed in black polyethylene bags for 37.65 h to allow the enzymes to mature. For the Coca-sr variety, the beans were soaked for 1.608 h at 36.63˚C in the presence of 1.1093% vegetable ash. Germination and post-germination took 144.37 h and 27.07 h, respectively. These germination processes followed the optimal conditions defined by Tambo et al. (2022) [11] when optimising the amylolytic activity of these two maize varieties. The malting process was completed by drying the grains in a ventilated oven (Venticell) at 45˚C for 48 h. The dried grains were sterilised and crushed in a mixer before being placed in polyethylene bags in a desiccator.
Secondary metabolites were extracted using methanol. For this, 1 g of each sample was weighed and placed in glass tubes to which 5 mL of 99.99% pure methanol (HPLC grade) was added. The mixture was vortexed for 5 min using a Borg Scientific electric super stirrer (model LS3). The vortexed mixture was then placed on a calibrated rotary shaker plate (Borg Scientific, model LS3) and agitated at a speed of 245 rpm for 12 h. At the end of this phase, the supernatant was collected and passed through a membrane filter (Whatman No.3 paper) containing 5 g of Na2SO4 (HPLC grade) previously soaked in methanol. The Na2SO4 would capture any water molecules present in the extract. The filtrate was collected directly into vials (SIGMA-ALDRICH Corporation, Bangalore, India), which were then transferred directly to the GC-MS analyser.
The identification of secondary metabolites in the extracts of the two flours was carried out using a high-resolution GC-MS system. The Agilent 8890 GC system (Agilent Technologies, Shanghai, China) was coupled to an Agilent 5877B GC/MSD mass spectrometry system and equipped with an Agilent 7693A automatic injector (autosampler). The column was an Rtx-5MS column (5% diphenyl/95% dimethylpolysiloxane, 30 m × 0.25 mm ID × 0.25 mm df). The oven temperature ranged from 50 to 250˚C at a rate of 10˚C/min. Temperature isotherms were observed every 1 min, and the final time was 40.50 min. The injector temperature was set at 280˚C, and the mass spectrometer temperature was 290˚C. The hexane and sample injection rate was 1.000 mL/min, while the helium transport rate was 25 cm/s at 250˚C. Once these parameters had been verified, the solvent (hexane) was injected to check for any impurities in the column. The solvent was eluted from the column after 40 min, and then 1 µL of each sample (ratio 10:1) was self-injected in split mode at 60 psi at a temperature of 250˚C for 1 min. The mass peak of each molecule was detected using a mass spectrometer pre-calibrated at 200˚C for the electron source and 290˚C for the temperature interface. A full scan at a voltage of 70 eV over a range of 50–550 amu was used to acquire all mass spectra. Identification of the different peaks, determination of retention times, and the percentage of each compound in the different samples were possible using the NIST version 2020 library connected to the GC-MS post-run analysis Chrom Compare T1 software (ChromSpace) installed on an HP desktop (Agilent Technologies). A signal-to-noise ratio of 100 was used, and identification along the signal was performed with a minimum match of 50% before assigning a name to a peak or signal. Analyses were repeated 2 times, and the results are the average of these analyses.
The metabolites profile of the two maize varieties (Atp-Y and Coca-sr) produced under optimal conditions was carried out using gas chromatography coupled to mass spectrometry in order to detect the influence of malting conditions and variety on the phytochemical composition. Figure 1 shows that the Coca-sr variety had fewer peaks than the Atp-Y variety. Liu et al. [22] also reported variation in the compound profile of passion fruit. Similarly, Kewuyemi et al. [6] found variation in the number of compound peaks present in ting extracts subjected to different fermentation conditions. The large number of peaks in the Atp-Y variety indicates a genetic predisposition of this variety to provide these compounds, as well as malting conditions that would have favoured their appearance. The number of peaks observed is much lower than that of Kewuyemi et al. [6], which can be explained by the use of an additional detector by these authors and by the combination of extraction solvents that allows a better extraction of the compounds. This is also the case for the work of Bolaji et al. [7] and Zhogoleva et al. [23], who identified 35 compounds in fermented maize and 120 different compounds in pigeon pea, respectively.
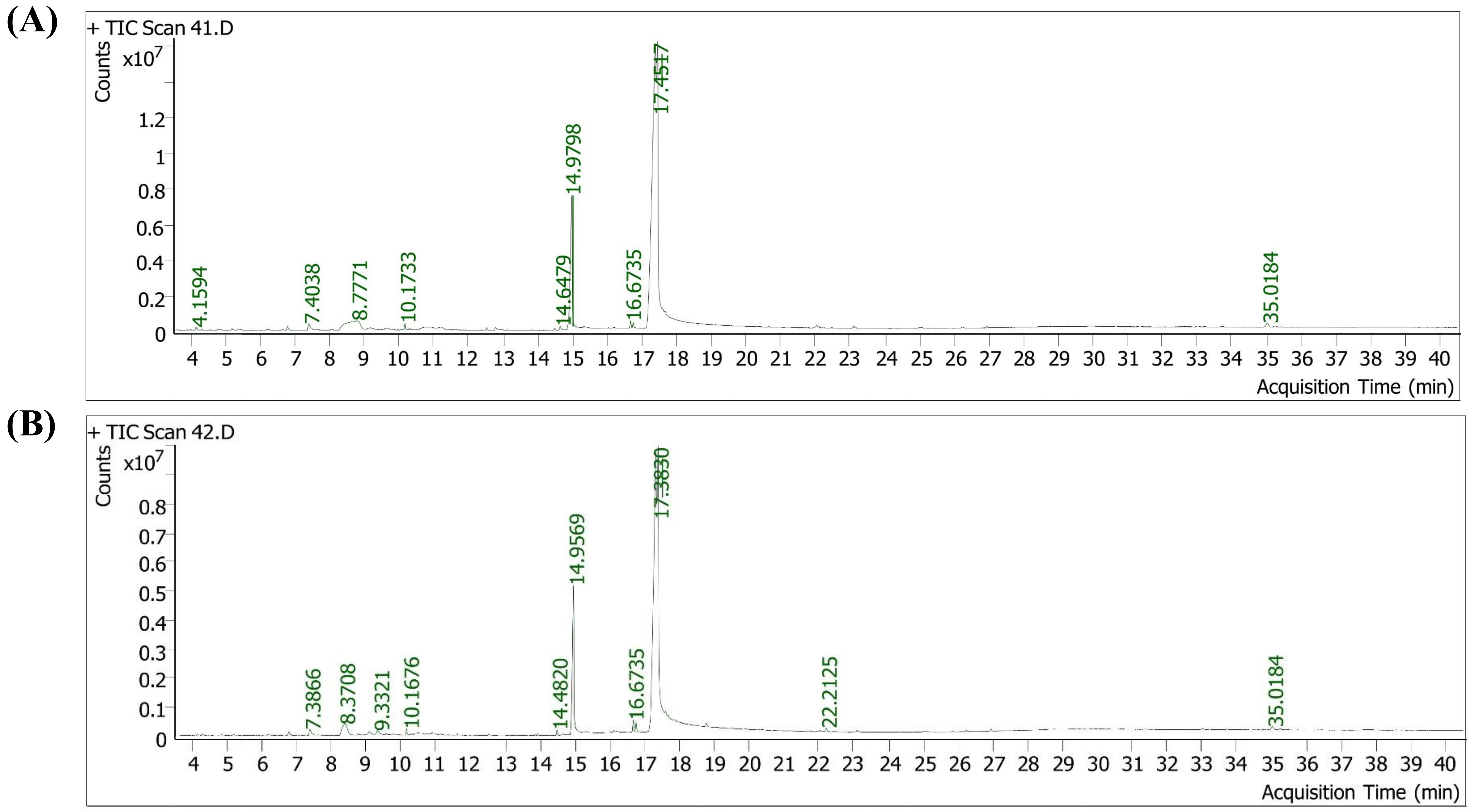
Chromatographs of the secondary metabolite profile of two varieties of germinated maize. (A) Coca-sr;(B) Atp-Y
Tables 1 and 2 show the secondary metabolite profile of the two germinated maize flours and a summary of the metabolites profile. It can be seen from Table 1 that the compounds were separated based on the nature of the functional groups and therefore their interactions with the stationary phase. The correlation factors between the peaks and the names of the identified compounds ranged from 61.50% to 98.10%, confirming the results presented. These tables show that 19 compounds belonging to the class of fatty acids, sugars, esters, alcohols, ketones, phenolic compounds, sterols, and other groups of compounds were identified, of which 15 in the Atp-Y variety and 12 in the Coca-sr variety. The number of compounds extracted and listed in these two maize varieties is lower than the 29 compounds identified in bambara seeds by Adetokunboh et al. [24]. These different classes of compounds are responsible for numerous biological properties in the body, such as antioxidant, anticancer, antimicrobial, anti-diabetic, anti-obesity, colour, odour, and flavour roles [7, 25–27]. Of the identified compounds, 8 were found in both maize varieties. Adetokunboh et al. [24] also found 8 identical compounds of different classes in caramelised and roasted bambara seeds. These included ethyl(2,4,4-trimethylpentyl)phosphinofluoridate, dodecanoic acid, n-hexadecanoic acid, methyl(Z,Z)-9,12-octadecadienoate, methyl 11-octadecenoate, (Z,Z)-9,12-octadecadienoic acid, (E)-9-octadecenoic acid, and gamma-sitosterol.
Secondary metabolites profile of both germinated maize flours
| N | RT (min) | Compound name | Formula | Library MW | Relative area (%) | Match factor | |
|---|---|---|---|---|---|---|---|
| Atp-Y | Coca-sr | ||||||
| 1 | 4.1594 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | C6H8O4 | 144.00 | ND | 0.22 | 93.40 |
| 2 | 6.7915 | 1-(2-Hydroxy-5-methylphenyl)ethanone | C9H10O2 | 150.10 | 0.31 | ND | 90.60 |
| 3 | 6.7916 | 2-Methoxy-4-vinylphenol | C9H10O2 | 150.10 | ND | 0.28 | 93.70 |
| 4 | 7.3866 | Ethyl(2,4,4-trimethylpentyl)phosphinofluoridate | C10H22FO2P | 224.10 | 0.73 | 0.78 | 81.30 |
| 5 | 8.3708 | 1,4-Anhydro-D-mannitol | C6H12O5 | 164.10 | 3.49 | ND | 79.10 |
| 6 | 8.7771 | 2-Ethyl-2-(hydroxymethyl)propan-1,3-diol | C6H14O3 | 134.10 | ND | 6.23 | 78.10 |
| 7 | 9.0918 | Nonanoic acid | C9H18O2 | 158.10 | 0.32 | ND | 73.60 |
| 8 | 9.3321 | 2-Methylpropyl 3-oxobutanoate | C8H14O3 | 158.10 | 0.59 | ND | 66.40 |
| 9 | 10.1676 | Dodecanoic acid | C12H24O2 | 200.20 | 0.29 | 0.26 | 94.60 |
| 10 | 14.4820 | Methyl hexadecanoate | C17H34O2 | 270.30 | 0.29 | ND | 94.00 |
| 11 | 14.6479 | 2-Amino-4,5-dimethoxyacetophenone | C10H13NO3 | 195.10 | ND | 0.34 | 80.70 |
| 12 | 14.9569 | n-Hexadecanoic acid | C16H32O2 | 256.20 | 13.35 | 11.89 | 98.10 |
| 13 | 16.6735 | Methyl(Z,Z)-9,12-octadecadienoate | C19H34O2 | 294.30 | 0.68 | 0.33 | 95.80 |
| 14 | 16.7536 | Methyl 11-octadecenoate | C19H36O2 | 296.30 | 0.57 | 0.28 | 95.60 |
| 15 | 17.3144 | (Z,Z)-9,12-octadecadienoic acid | C18H32O2 | 280.20 | 46.58 | 53.84 | 97.60 |
| 16 | 17.3830 | (E)-9-octadecenoic acid | C18H34O2 | 282.30 | 30.39 | 25.09 | 81.50 |
| 17 | 17.6005 | Ethyl linoleate | C20H36O2 | 308.30 | 1.67 | ND | 61.50 |
| 18 | 22.2125 | 1-Eicosanol | C20H42O | 298.30 | 0.27 | ND | 78.20 |
| 19 | 35.0184 | Gamma-sitosterol | C29H50O | 414.40 | 0.47 | 0.24 | 81.10 |
RT: retention time; MW: molecular weight; ND: not detected
Summary of the secondary metabolites profile in both maize varieties
| Classification | Atp-Y | Coca-sr |
|---|---|---|
| Total number of compounds (n) | 15 | 12 |
| Total number of identical compounds (n) | 8 | |
| Different classes of compounds (%) | ||
| Fatty acids | 90.93 | 91.08 |
| Esters | 3.80 | 0.95 |
| Ketones | 0.31 | 0.56 |
| Phenol | 0.00 | 0.28 |
| Polyols | 3.49 | 0.00 |
| Alcohols | 0.27 | 6.23 |
| Sterols | 0.47 | 0.24 |
| Miscellaneous compounds | 0.73 | 0.78 |
Fatty acids were the most abundant compounds in both maize varieties. They were found at 90.93% for Atp-Y and 91.08% for Coca-sr (Table 2). These observations are in contrast to those of Kewuyemi et al. [6], who reported the abundance of various or unclassified compounds in fermented ting seeds. In the same vein, Adetokunboh et al. [24] reported a high content of aldehyde compounds in caramelised bambara seeds. Furthermore, the abundance of fatty acids as opposed to aldehydes, sugars, and ketones is in contrast to the data from numerous studies [24, 28]. However, they are consistent with the work of Bolaji et al. [7], who found fatty acids to be the major compounds in fermented maize. Fatty acids are responsible for many biological and nutritional properties in organisms. Long-chain fatty acids have antimicrobial and anticancer properties, while saturated fatty acids (SFA) are responsible for regulating blood cholesterol levels, transmitting nerve impulses, and are precursors of numerous vitamins and hormones [29–31]. The richness of fatty acids in the flour of the Coca-sr variety can be explained by its greater lipolytic activity and shorter post-germination time. Increased post-germination time leads to the conversion of fats into ketone compounds, aldehydes, and other derivatives [23]. A total of 5 different fatty acids (Table 1) were identified in the two maize varieties. The most abundant was (Z,Z)-9,12-octadecadienoic acid (46.58% for Atp-Y and 53.84% for Coca-sr), followed by (E)-9-octadecenoic acid (30.39% for Atp-Y and 25.09% for Coca-sr), then n-hexadecanoic acid (13.35% for Atp-Y and 11.89% for Coca-sr), then dodecanoic acid (0.29% for Atp-Y and 0.26% for Coca-sr), and finally nonanoic acid (0.32% for Atp-Y and not detected for Coca-sr). These observations are similar to those of Kewuyemi et al. [6] and Ahmed et al. [32], who obtained palmitic and linoleic acids as the main fatty acids in fermented ting. The n-hexadecanoic acid is the main compound responsible for flavour in cereals, so its high content in the Atp-Y variety would make it more fragrant [33]. The higher proportion of (Z,Z)-9,12-octadecadienoic acid in the Coca-sr variety is thought to be related to the conversion of some of these acids to methyl esters in the Atp-Y variety. It is also thought to be related to the overexpression of the gene responsible for the synthesis of this fatty acid in this variety. The presence of a low proportion of SFA in these two varieties is consistent with the work of numerous authors who have reported that, during germination, the activities of the lipases that release unsaturated fatty acids require a basic pH, which is the case with the addition of plant ash in this work [6, 34]. The low proportions of SFA in these germinated grains can make them good for consumption because these classes of fatty acids can initiate many diseases, like atherogenesis. Linoleic acid is an essential fatty acid that is a precursor to many other so-called essential acids such as EicosaPentaEnoic acid (EPA) and DocosaHexaEnoic acid (DHA), which are responsible for many biological properties such as coagulation, brain development, thermoregulation, neuroprotection, antioxidant and antimicrobial properties but they can also promote necrosis, inflammation and artherosclerosis [35, 36]. According to this profile, we can confirm that the oil from this meal has a low atherogenicity index (AI) and thrombogenic index (TI), high hypocholesterolemic/hypercholesterolemic ratio (h/H), health-promoting index (HPI), fish/flesh lipid quality (FLQ) and nutritive value index [37]. Consumption of these two maize varieties should therefore be recommended in order to benefit from these properties. The presence of elaidic acid, derived from oleic acid, in these extracts allows us to hypothesise a transduction modification specific to these two maize varieties. Furthermore, the presence of this acid would be less beneficial for consumers of these maize varieties, as it is more easily stored due to the absence of enzymes capable of facilitating its digestion in the gastrointestinal tract, which facilitates its excretion. The presence of this fatty acid is therefore thought to be responsible for the onset of coronary heart disease and the risk of stroke. These results, therefore, suggest that we should limit culinary treatments to the Atp-Y variety and reduce its consumption. The high palmitic acid content in Atp-Y is thought to be the result of the overexpression of the gene responsible for its synthesis in this variety, as well as the low pH during germination in this variety, which facilitates the activities of lipases specific to it [6]. This fatty acid is an important source of energy and a major component of biological membranes. Some authors have demonstrated its antimicrobial potential [37]. Dodecanoic and nonanoic acids, also found in higher proportions in the Atp-Y variety, are thought to be responsible for the strong odour of this maize variety. Their presence is thought to give this variety of germinated maize numerous flavour characteristics, such as sweetness, acidity and fruitiness [33].
Esters are the second most abundant class of compounds identified in these two maize varieties (Table 2). Kewuyemi et al. [6] also listed these groups of compounds as the second most abundant class in fermented maize. Esters result from the combination of fats and alcohols either during fermentation or during germination in enzymatic reactions catalysed by lyases. The proportions obtained in the two varieties (3.80% for Atp-Y and 0.95% for Coca-sr) indicate a high esterase activity in the Atp-Y variety (Table 2). The high content in the Atp-Y variety also confirms its strong taste and odour characteristics. Five esterified compounds were identified in the two germ flours. These were 2-methylpropyl 3-oxobutanoate, methyl hexadecanoate, methyl(Z,Z)-9,12-octadecadienoate, methyl 11-octadecenoate, and finally ethyl linoleate. Overall, there was a decrease in the concentration of these compounds in the Coca-sr variety, which is thought to be related to the high activity of esterases, leading to the conversion of these esters into fatty acids. Furthermore, of the 5 compounds, only the compound methyl 11-octadecenoate, was identified in the Coca-sr variety. Previous work has reported the neuroprotective potential of methyl 11-octadecenoate and methyl(Z,Z)-9,12-octadecadienoate [38], antioxidant and inhibitor of carbohydrate digestive enzyme activity of ethyl linoleate [39, 40], antibacterial in methyl hexadecanoate and odourant in 2-methylpropyl 3-oxobutanoate [33]. The latter is thought to result from the degradation of other, more complex molecules during enzymatic reactions. Adetokunboh et al. [24] also reported that the presence of esters in bambara seeds was responsible for improving the flavour of malts obtained from this plant. These analyses also highlight the benefits of using the Atp-Y variety in formulations. It should also be noted that the percentage of esters in the two varieties is much lower than the 23% found by Kewuyemi et al. [6] in tasting samples.
Ketones are the third class of compounds found in the seeds of both maize varieties. The proportions of this class of compounds are 0.31% and 0.56% for Atp-Y and Coca-sr, respectively (Table 2). Ketones are compounds resulting from the conversion of simple sugars during fermentation or from the breakdown of fatty acids. The high content of these compounds in the Coca-sr variety contrasts with the work of Kewuyemi et al. [6], who reported that the proportions of this group of compounds increased with fermentation time. In this study, lactic fermentation, which could occur during maturation and is responsible for the synthesis of ketone compounds, should be more active in the Atp-Y variety due to the longer malting time. It could also be explained by the conversion of certain short-chain fatty acids, such as nonadecanoic acid, which is not present in this maize variety (Coca-sr), into ketone compounds [41]. The high activity of lipases and phospholipases present in Coca-sr would result in a high release of fatty acids, which are the precursors of ketones. Along with aldehydes, ketones help to enhance the flavour of foods and also have biological properties. The presence of ketone compounds also reflects, to some extent, the oxidation of dietary fats. Indeed, the high concentration of vegetable salt (a source of transition metals such as Cu2+ and Fe2+) during the malting process of Coca-sr kernels would have led to a strong oxidation of the fats present. The ketone content obtained is generally lower than the 10% reported by Kewuyemi et al. [6] during spontaneous malting fermentation. This suggests that ketone synthesis is fermentation-dependent. In total, three ketone compounds were detected, including one [1-(2-hydroxy-5-methylphenyl)ethenone] in the Atp-Y variety and two (2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one and 2-amino-4,5-dimethoxyacetophenone) in the Coca-sr variety (Table 1). The presence of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one in the cultivar indicates the onset of a Maillard reaction in this cultivar as reported by Adetokunboh et al. [24]. Although this compound has a ketone group, it belongs to the class of flavonoids with antimicrobial, anti-inflammatory, antimutagenic, and antioxidant properties [42]. The presence of this flavinic compound in the Coca-sr variety would explain the high antioxidant potential demonstrated in previous work [11]. The identification of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one, which could be described as new, would also be related to the plant’s genetics. The absence of the gene responsible for the synthesis of this compound or its transformation into other molecules, such as carotenoids, would explain the non-detection in the extract of the Atp-Y variety. Liu et al. [43] also reported the strong odour capacity of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one. Other authors [44, 45] have shown that the auto-oxidation of linoleic acids is responsible for the appearance of this molecule and its isomers. In addition to malting conditions, Sidhu et al. [46] reported that the presence of flavonoids in plants is related to other factors such as climatic conditions, soil moisture, exposure temperature, ripening, and exposure time. 2-Amino-4,5-dimethoxyacetophenone is another class of flavonone found in the Coca-sr variety that has been shown to have numerous properties, including antiviral activity against the SARS-CoV-2 virus [47]. Other studies have reported the potential of this molecule to inhibit the proliferation of breast cancer cells by activating the aryl hydrocarbon receptor [48]. This molecule is also thought to be derived from protein degradation (conversion of amino acids into ketone bodies), based on the high proteolytic activity demonstrated in the Coca-sr variety [11]. The compound 1-(2-hydroxy-5-methylphenyl)ethanone, identified only in the extract of the Atp-Y variety, is thought to confer type 2 anti-diabetic and hyperlipidaemic properties. It is also thought to have antimicrobial properties due to its hydrocarbon chain [49, 50].
Phenolic compounds are a group of cyclic molecules that carry hydroxyl groups in the para or ortho position on their surface, giving them a certain agility to release their proton during reduction reactions. The biological properties of many plants are due to the presence of this class of molecules [51]. One phenolic compound in particular, 2-methoxy-4-vinylphenol (0.28%), was detected in the Coca-sr variety, while none was present in the Atp-Y variety. Phenolic compounds are present in high proportions in plants at the beginning of ripening and decrease with time, giving way to molecules such as carotenoids. The longer ripening time of the Atp-Y variety would therefore be responsible for the disappearance of this compound. The non-expression of the genes responsible for the synthesis of this class of molecules could also explain this result. The antibacterial and antioxidant potential of benzo[a]pyrene and its ability to block the development of cancer cells (hyperphosphorylation of retinal blastoma) have been demonstrated by Rubab et al. [52]. In addition to these authors, Lee et al. [53] attributed the inhibition of microbial DNA synthesis, the destruction of microbial cytoplasmic activities, and their energy production processes to the action of 2-methoxy-4-vinylphenol. An ability to enhance flavour was also demonstrated by Jeong et al. [54]. Given the presence of this molecule, supplementation with sprouted maize flour of the Coca-sr variety would therefore be recommended for people suffering from the above-mentioned conditions. However, the low diversity of phenolic compound profiles in these two maize varieties suggests a combination of extraction solvents.
Polyols are carbohydrate derivatives produced by reducing the ketone or aldehyde function. The best known are sorbitol, mannitol, and xylitol. They are used in many formulations as texturing and cooling agents. 1,4-anhydro-D-mannitol is the only polyol identified in these extracts, more specifically in the Atp-Y variety (Table 1). It was found at a level of 3.49% (Table 2). D-mannitol, the 1,4-anhydro tautomer of galactitol, is thought to result from the reduction of the aldehydic function of mannose, which is probably only present in the starch structure of the Atp-Y variety. The release of this compound thus confirms the strong amylolytic activity found in this cultivar in previous work [11]. The reduction of mannose to 1,4-anhydro-D-mannitol, in the Atp-Y variety, is thought to be the result of metabolic activity by the fermenting microorganisms. Fermentative microorganisms develop very rapidly in malt and are responsible for the spontaneous fermentation of malted maize. This fermentation leads to the formation of many alcoholic compounds, including polyols. In addition to its role as a sugar alcohol used in the formulation of many medicines, polyol is highly recommended for diabetics as it is poorly absorbed from the gut. It has also been hypothesised that it may reduce the incidence of glaucoma. These data provide ample evidence that Atp-Y maize would be beneficial for diabetics, cancer patients, and the obese.
Two alcohols, 1-eicosanol and 2-ethyl-2-(hydroxymethyl)propan-1,3-diol, were detected in Atp-Y and Coca-sr maize, respectively (Table 1). The proportions were 0.27% for 1-eicosanol and 6.23% for 2-ethyl-2-(hydroxymethyl)propan-1,3-diol, respectively. The high alcohol content in the Coca-sr variety would indicate strong alcoholic fermentation activity. The presence of 1-eicosanol in the Atp-Y variety is thought to result from the hydrogenation of arachidic acid, which was not detected in extracts from this variety. This alcohol is widely used in cosmetics as an emollient. Pirbalouti et al. [55] also detected this compound in different proportions in coriander oil that had undergone different types of drying. The absence of this compound in the Coca-sr variety may be due to the conversion of linoleic acid ethyl ester to (Z,Z)-9,12-octadecadienoic acid, rather than arachidonic acid, whose hydrogenation leads to 1-eicosanol. 2-Ethyl-2-(hydroxymethyl)propan-1,3-diol is the alcohol most commonly found in germinated and fermented maize, resulting from hydrogenation reactions of short-chain fatty acids [7]. The presence of this alcohol in the Coca-sr variety is thought to be the result of the hydrogenation of 2-methylpropyl 3-oxobutanoate, the decomposition of methyl hexadecanoate, and modification of the chemical structure of nonanoic acid. The presence of 2-ethyl-2-(hydroxymethyl)propan-1,3-diol makes Coca-sr a raw material in the production of alkyl resins, ion exchange resins, and other compounds with high adhesive capacity. It also has properties that prevent the formation of ice crystals in the tissues of organisms, suggesting that Coca-sr germ flour can be used to freeze meat and fish without destroying the tissues. The bitter and alcoholic taste of this maize variety is thought to be due to the presence of this compound [33].
Phytosterols are a class of compounds with multiple carbon rings and one or more alcohol functions, giving them antioxidant properties [56]. They limit the intestinal absorption of cholesterol, thereby reducing the incidence of a number of diseases, particularly cardiovascular disease [6]. In addition to these properties, they are the main precursors of steroid hormones and also help in the detection of modified or blended oils [56]. Gamma-sitosterol is the main and only phytosterol detected in these two varieties of germinated maize. Its content was 0.47% and 0.24% in Atp-Y and Coca-sr, respectively. The high content in the Atp-Y variety can be explained by the overexpression of the gene responsible for its synthesis, as well as by the high activity of the first phytosterol synthesising enzyme (sterol C24-methyltransferase), which was overexpressed at high ripening times. Similarly, the long ripening time of the Atp-Y variety is thought to be at the origin of the conversion of molecules such as fatty acids into phytosterols and later into plant growth hormones. In addition, the high soaking temperature and the higher salt concentration during germination of the Coca-sr variety would have facilitated the elimination of this phytosterol by solubilisation, thermal inactivation, or inhibition of its synthesis by the various ions present in high concentrations in the plant salt. Its potential to limit the proliferation of prostate cancer cells and the occurrence of prostatitis at very low concentrations means that we can recommend the consumption of the Atp-Y variety of sprouted maize to men [57].
Ethyl(2,4,4-trimethylpentyl)phosphinofluoridate is a highly hazardous chemical compound used in the manufacture of a wide range of military equipment, such as Syntex. It is highly irritating and can cause severe visual impairment. The presence of this compound in both varieties is thought to be due to the various insecticides and herbicides applied to these plants during their growth. The levels were 0.73% and 0.78% in the Atp-Y and Coca-sr varieties, respectively. The long soaking time of the Atp-Y variety would have led to the leaching of this compound, while the long ripening time would have been responsible for the degradation of part of it by the microorganisms inherent to the plant that proliferated during the various stages. In addition, the initiation of anabolism with the long maturation time in the Atp-Y variety would have led to the use of part of this chemical contaminant as a carbon source for the growing plant and also as an energy source for the microorganisms [58]. Reducing the level of this compound in the Atp-Y variety is of particular interest for the health of future consumers. Malini et al. [33] also reported this compound in a millet-based fermented drink.
At the end of this work, which aimed to highlight the metabolites profile of two varieties of germinated maize under their respective optimum conditions, it emerged that this composition was mainly dependent on the variety. There were significant qualitative and quantitative differences between the varieties. The Atp-Y variety had the highest number of secondary metabolites (15), of which fatty acids were the most abundant class. Eight identical compounds belonging to different metabolite groups were found in both varieties, with the exception of polyols and phenols, which were found in Atp-Y and Coca-sr, respectively. Of all the compounds identified, (Z,Z)-9,12-octadecadienoic acid was the most abundant in both varieties. This difference in composition shows the interest in consuming each variety and its health benefits, but above all, the importance of respecting the different germination conditions of the two varieties in order to benefit from their metabolites. These results provide initial information on the contribution of germination and variety to the secondary metabolite profile of cereals and open the door to the extraction and application of these metabolites in a wide range of fields.
The compounds profile differed according to the variety;
The Atp-Y variety presented the most volatile compounds;
Similar classes of compounds were found in both varieties, with a high amount of linoleic acid.
Biological application of maize extracts;
Extent work to more maize varieties and applied another treatment.
GC-MS: gas chromatography-mass spectrometry
SFA: saturated fatty acids
The authors take this opportunity to express their sincere gratitude to Prof. Klang Mathilde Julie and Dr. Suka Thangaraju for the help provided during the research.
STT: Conceptualization, Formal analysis, Funding acquisition, Methodology, Software, Data curation, Writing—original draft. VN: Conceptualization, Funding acquisition, Supervision, Writing—original draft, Writing—review & editing.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Not applicable.
Not applicable.
Not applicable.
Data will be made available on request.
Indian Government financed this study through the program “C V RAMAN Fellowship” (DST/INT/CVRF/2022). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
