Affiliation:
1Department of Medicine, Surgery and Dentistry, University of Salerno, Baronissi, 84081 Salerno, Italy
2Department of Pharmacy, University of Salerno, 84084 Fisciano, Salerno, Italy
Email: giulia_izzo@virgilio.it
ORCID: https://orcid.org/0000-0002-4673-9957
Affiliation:
1Department of Medicine, Surgery and Dentistry, University of Salerno, Baronissi, 84081 Salerno, Italy
ORCID: https://orcid.org/0000-0002-4583-5971
Explor Foods Foodomics. 2023;1:178–191 DOI: https://doi.org/10.37349/eff.2023.00014
Received: July 10, 2023 Accepted: August 21, 2023 Published: October 29, 2023
Academic Editor: Marcello Iriti, Milan State University, Italy
The article belongs to the special issue Ketogenic Diet as Medical Nutrition Therapy
Epidemiological studies have reported an association between obesity/metabolic syndrome (MetS) and male reproductive disorders. Endocrine dysfunctions, direct testicular damage, chronic low-grade inflammation, and insulin resistance (IR) are involved in the occurrence of male obesity secondary hypogonadism (MOSH) which in turn alters the metabolic imbalance, creating a sort of vicious circle. Since IR and chronic inflammation state play a pivotal role in MOSH, low-calorie and low-carbohydrate diet protocols may be administered in obese men to improve their metabolic and hormonal profile. The ketogenic diet (KD) has been reported to determine positive effects on body weight, IR, cardio-metabolic risk, hypothalamic-pituitary-testicular (HPT) axis, and prostate with possible improvement of plasma androgens levels, sexual function (SF), and male fertility. This review aims to evaluate the effectiveness of KD on testicular function. Emerging evidence reports that very low-calorie KD (VLCKD) may revert MOSH by restoring HPT axis function and testosterone (T) levels. Moreover, VLCKD could improve SF, prostate health and lower urinary tract symptoms (LUTSs) in overweight/obese male patients. VLCKD may also positively impact spermatogenesis although evidence is still poor. Future studies are warranted to clarify the effectiveness of KD on testicular and prostate gland function.
The worldwide prevalence of obesity and metabolic syndrome (MetS) has dramatically grown in the last decades thus resulting in a pandemic [1, 2]. Obesity is characterized by abnormal or excessive fat mass accumulation, and it should not be considered merely an aesthetic issue but a medical problem potentially impairing quality of life and general health. MetS is a medical condition featured by a cluster of at least three metabolic abnormalities including i) obesity [mainly central obesity, defined as a waist circumference (WC) ≥ 88 cm for women and ≥ 102 cm for men]; ii) increased blood pressure (≥ 130/85 mmHg); iii) insulin resistance (IR)/raised fasting plasma glucose (FPG; ≥ 100 mg/dL); iv) high plasma concentrations of triglyceride (≥ 150 mg/dL), and v) low concentrations of high density lipoprotein (HDL) cholesterol (< 50mg/dL for women and < 40 mg/dL for men) [3]. MetS shows a complex and multifactorial pathophysiology, involving a sedentary lifestyle, high fat/carbohydrate diet, IR and pancreatic β-cell dysfunction, lipid toxicity, altered adipokine production, oxidative stress and glucose toxicity, chronic inflammation, gut microbiota, genetic and environmental factors [3, 4]. Both obesity and MetS may potentially influence many aspects of human physiology and determine multiple comorbidities including endocrine and reproductive disorders [5–7]. Epidemiological studies [8–10] have described an association between obesity/MetS and male hypogonadism, infertility, erectile dysfunction (ED), benign prostatic hyperplasia (BPH) and lower urinary tract symptoms (LUTSs). Indeed, the adipose tissue and the male reproductive system are linked in a mutual and complex relationship. Several factors play a central role in obesity-related andrological diseases, and it has been stated that diet and weight loss (WL) might improve male sexual and reproductive dysfunctions [7, 11]. Since IR and chronic inflammation state play a pivotal role in male obesity secondary hypogonadism (MOSH), low-calorie and low-carbohydrate diet protocols such as ketogenic diet (KD) might have positive effects on male sexual and reproductive health. In light of the impact of obesity/MetS on the male reproductive system, the aim of the present review is to evaluate the effectiveness of KD on testicular function.
Testicular function consists both of male gametes (spermatozoa) and sexual hormones (androgens) production. Male hypogonadism is a clinical syndrome characterized by an impairment of testicular function resulting in low serum testosterone (T) levels, eventual impairment of spermatogenesis and a cluster of signs/symptoms due to T deficiency including sexual/reproductive dysfunctions (e.g., reduced sexual desire/activity, decreased spontaneous erections, infertility), reduction of testis volume, gynecomastia, generalized asthenia, mood/cognitive alterations, anemia, deterioration of muscle mass/strength and bone health [7, 11, 12]. Furthermore, male hypogonadism is often associated with metabolic abnormalities such as obesity, IR, MetS and type-2 diabetes mellitus (T2DM) [5–7, 11, 12]. T deficiency and metabolic disorders are linked in a mutual and bidirectional relationship [11]. On one hand, low T levels can increase lipoprotein lipase activity, fatty acid uptake and triglyceride formation [11], adipocyte proliferation [13], pancreatic β-cell [14] and mitochondrial dysfunction [11] thus raising the risk of visceral obesity, T2DM and MetS. On the other hand, overweight and obesity may be associated with endocrine abnormalities resulting in T deficiency and a higher risk of hypogonadism (the so-called “MOSH” or “metabolic hypogonadism”) [5, 9, 12]. Therefore, hypogonadism may contribute to increasing visceral obesity and metabolic abnormalities and, in turn, obesity/MetS may induce hypogonadism, creating a sort of vicious circle.
Male obesity may be associated with hypogonadotropic hypogonadism (HH), characterized by low T levels (< 12.1 nmol/L or < 350 ng/dL), reduced inhibin B and low/normal follicle-stimulating hormone (FSH) and luteinizing hormone (LH) concentrations [7].
Several mechanisms/factors (Figure 1) may negatively impact the hypothalamic-pituitary-testicular (HPT) axis in overweight/obese men [7].
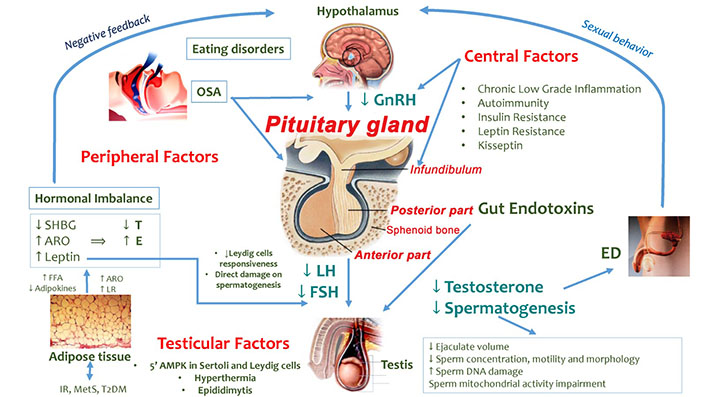
Factors involved in HPT axis dysfunction in overweight/obese men. GnRH: gonadotropin-releasing hormone; SHBG: sex-hormone binding globulin; ARO: aromatase; E: estradiol; ED: erectile dysfunction; FFA: free fatty acids; LR: leptin resistance; OSA: obstructive sleep apnea; 5’AMPK: 5’AMP-activated protein kinase. ↓: decrease; ↑: increase
Note. Adapted from “How much does obesity affect the male reproductive function?,” by Bellastella G, Menafra D, Puliani G, Colao A, Savastano S; Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Group. Int J Obes Suppl. 2019;9:50–64 (https://www.nature.com/articles/s41367-019-0008-2). © 2019, The Author(s), under exclusive licence to Springer Nature Limited.
For instance, central factors enclosing chronic inflammation state, IR and LR may affect the hypothalamic and the pituitary gland’s activity [7, 15]. Experimental and clinical studies have shown that pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α) [16, 17], interleukin-6 (IL-6) and IL-1β [18, 19] may suppress the GnRH and LH secretion. Similarly, C-reactive protein (CRP) may contribute to suppressing the HPT axis and to worsening the IR condition [11, 20]. The latter one in turn may induce MOSH by affecting the hypothalamic kisspeptin neurons and GnRH secretion, as shown in animal models [21]. Furthermore, increased levels of inflammatory mediators represent risk factors for inflammatory processes and pituitary autoimmunity which may take part in the HH pathophysiology [7, 22, 23]. It has also been hypothesized that the leptin excess and LR mechanisms (probably mediated by down-regulation of the leptin receptor) [11] in obese men may result in reduced GnRH, LH and FSH levels and, consequently, impaired testicular function [7].
Peripheral factors including hormonal imbalance related to high leptin and low SHBG levels, ED, OSA and gut toxicity may affect the HPT axis. In particular, the high leptin levels in obese men may impair T production both by direct inhibition of the Leydig cells and a reduced Leydig cell responsiveness to LH stimulation [7, 24]. Moreover, high leptin levels may also directly affect spermatogenesis [25]. Obesity, IR and pro-inflammatory cytokines (e.g., TNF-α and IL-1β) [26] may reduce the hepatic production of SHBG, impacting serum T levels [7]. T circulates in the blood into three fractions including free T (2% of the fraction), SHBG-bound T (30–40%) and albumin-bound T (54–68%) [12]. T bound to SHBG is believed to be not biologically active while free T and albumin-bound T are considered as bioavailable fractions and, consequently, they are metabolically active [12]. In obese men, the SHBG reduction induces an increased availability of free T which is converted into E by the enzyme ARO (particularly abundant in the adipose tissue) [7]. The rise of serum E levels determines the suppression of hypothalamic-pituitary function (negative feedback) with consequent HH. This hormonal imbalance may also concur to worsen the vasculogenic ED in obese men with consequences on coital frequency, T levels, and fertility [7]. Furthermore, the coexistence of OSA, which is characterized by frequent proximal airway obstruction, hypopnea, apnea and oxygen desaturation, may result in desynchronization of circadian rhythmicity, reversible HPT axis dysfunction with suppression of nocturnal LH pulsatility and T rise, and reduced total and free T levels [7]. Also, eating disorder habits can induce further alterations of the internal clock and a possible desynchronization of the circannual T rhythm related to HPT axis function impairment [7]. Obesity and a high fat/calorie diet may also induce a damage in the normal intestinal mucosal barrier causing the passage of gut bacteria from the bowel lumen into the systemic circulation and, consequently, low-grade chronic inflammation which negatively impacts testicular function [7].
Finally, conditions developing into the scrotum (testicular factors) such as hyperthermia and epididymitis may locally affect testicular function. For instance, the abdominal, suprapubic and medial thigh fat, enveloping the scrotum, may increase the intrascrotal temperature with possible direct damage on testicular cells [7].
Moreover, obesity may impact on 5’AMPK which is present both in Leydig and Sertoli cells and is involved in gonadal steroidogenesis, somatic gonadal cell proliferation/survival and spermatozoa maturation [7].
Obesity may have detrimental effects on male fertility and sperm functions [7, 27]. Several studies have evaluated the impact of male obesity on normal sperm physiological parameters (e.g., concentration, motility, morphology) reporting controversial results. Obesity has been related to reduced ejaculate volume [28], lower sperm count/concentration [29, 30], impaired sperm morphology [30] and reduced motility [31]. Obesity may also impact other sperm parameters determining higher sperm DNA damage and mitochondrial activity impairment [7]. Furthermore, MetS has been associated with seminal inflammatory cytokines and reproductive dysfunction in a case-controlled study [32].
The molecular basis of sperm quality alterations in obese males is still unclear. Besides hormonal imbalance, high leptin and adipokine levels, low-grade inflammation, and incorrect nutritional habits (e.g., high fat/energy diet, high carbohydrate intake), many other factors may impact obese men’s fertility. For instance, overweight and obesity may affect sperm quality through a direct action on Sertoli cell function and/or the increase of testicular temperature due to hip/abdominal fat tissue accumulation and high-fat content in the scrotal area [7]. Moreover, obesity may lower some fatty acid levels in spermatozoa (e.g., docosahexaenoic acid, palmitic acid) causing increased DNA fragmentation and a reduction in total sperm count, sperm vitality and motility [7]. Similarly, obesity-related oxidative stress may cause sperm membrane peroxidation, DNA fragmentation and sperm apoptosis [7, 27]. Furthermore, the accumulation of liposoluble endocrine disruptors and toxic substances in the adipose tissue could enhance the hurtful effects of these exogenous compounds [7]. Also, obesity/MetS-related ED and ejaculatory dysfunction (e.g., retrograde ejaculation) may have a negative effect on sexual behaviors and fertility [27]. Finally, it has been also reported that obesity may influence in vitro fertilization outcomes and affect offspring health [7].
Lifestyle changes including physical activity and diet are crucial for obesity/MetS treatment and might positively impact sex hormones, sexual health, and reproduction. Indeed, Esposito et al. [33] have shown an improvement in erectile function in men at risk of intensive lifestyle changes. Moreover, body WL by diet or bariatric surgery seems to revert MOSH by inducing total and free T increase and SHBG normalization [34].
Evidence regarding the effects of restricted caloric intake on reproductive hormones is still scant. Previous studies revealed an increase in serum androgen levels [35–38], sometimes associated with E decrease [39] after low-calorie diet (LCD) programs (Table 1). In particular, Kaukua and colleagues [35] found an increase of SHBG, T, HDL cholesterol and a decrease of insulin and leptin serum levels after a 12-week very LCD, maintained also during the follow-up period. Furthermore, the decrease in IR resulted in the parameter being more significantly related to serum T levels in the backward regression analysis, suggesting a role of insulin sensitivity in the retrieval of the eugonadal status in obese men. Analogously, a strong correlation between insulin sensitivity improvement and T levels after LCD has been described both in obese men with and without T2DM [36, 37]. Besides WL and changes in sex hormones profile, they also showed an improvement in sexual function (SF), assessed by the international index of erectile function 5 (IIEF-5) and the sexual desire item (SDI) scores, and LUTSs assessed through the International Prostatic Symptom Score (IPSS) [36, 37]. Since these scores were directly correlated with WC reduction, both sexual dysfunctions and LUTSs in obese men might be considered features of visceral obesity potentially improved by WL. Also, WL and WC reduction may decrease inflammatory markers such as CRP and IL-6 and improve endothelial function [37], contributing to sexual health amelioration. Contrariwise, the study conducted by Klibanski et al. [40] reported a negative effect of diet on sex hormones serum levels (FSH, LH and T) after fasting, suggesting excessive and detrimental effects of fasting and fasting-associated ketosis on metabolism and sex hormones. Therefore, both overfeeding/obesity and prolonged fasting seem to have detrimental effects on the HPT axis. It might be assumed that malnutrition and prolonged fasting hamper the pituitary responsiveness to hypothalamic-releasing hormones. Moreover, fasting might increase cortisol levels with a negative impact on the HPT axis function [41].
Studies analyzing the effects of WL through diet programs on male hormonal profile, sexual function and reproductive health [7]
| Author | n | Diet protocol | Hormonal changes | Sexual/reproductive effects |
|---|---|---|---|---|
| Kaukua et al. [35] | 38 | VLCD (800 kcal/day, n = 19), 10 weeks + behavior modifications + follow up, 22 weeks; TG vs. CG | ↑ TT, ↑ SHBG | = SF |
| Khoo et al. [36] | 70 | LCD (850–900 kcal/day), 8 weeks T2DM group vs. no T2DM group vs. CG | ↑ TT | ↑ IIEF, ↓ IPSS, ↑ erectile function and sexual desire |
| Khoo et al. [37] | 31 | LCD (~1,000 kcal/day) and HP, 8 weeks + 52 weeks LCD group (n = 19), HP group (n = 12) | ↑ TT, ↑SHBG | ↑ IIEF, ↓ IPSS, ↑ FMD |
| Niskanen et al. [38] | 58 | VLCD (800 kcal/day) + maintenance period, 9 weeks + 12 months | ↑ TT, ↑ FT, = E, ↑ SHBG | Not investigated |
| Stanik et al. [42] | 24 | Fasting program (320 kcal/day liquid diet), 8–20 weeks | ↑ TT, ↓ E, ↑ SHBG | Not investigated |
| Strain et al. [43] | 11 | Weight reduction, 5 months and 39 months | ↑ TT, ↑FT, = E, = LH, ↑ SHBG | Not investigated |
| Håkonsen et al. [44] | 43 | Weight reduction, 14 weeks | ↑ TT, ↑ SHBG | ↑ AMH, semen volume, total sperm count, normal sperm morphology |
| Klibanski et al. [40] | 6 | Effect of fasting on sex hormones serum levels | ↓ TT, ↓ LH, ↓ FSH | Not investigated |
| Jaffar and Ashraf [45] | 105 | Weight reduction | NA | ↑ progressive sperm motility, static sperm percentage |
| Caruso et al. [46] | 160 | MD (n = 80) or low-fat diet (n = 80) | NA | ↑ sperm concentration and sperm count, mainly in the MD group |
n: number of subjects; TT: total testosterone; FT: free testosterone; TG: treatment group; CG: control group; VLCD: very low-calorie diet; FMD: brachial artery flow-mediated dilatation; HP: high protein-low fat-reduced carbohydrate diet (reduction by 600 kcal/day); AMH: anti mullerian hormone; MD: Mediterranean diet; NA: not available. ↓: decrease; ↑: increase; =: unchanged
Note. Adapted from “How much does obesity affect the male reproductive function?,” by Bellastella G, Menafra D, Puliani G, Colao A, Savastano S; Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Group. Int J Obes Suppl. 2019;9:50–64 (https://www.nature.com/articles/s41367-019-0008-2). © 2019, The Author(s), under exclusive licence to Springer Nature Limited.
Only few studies investigated the effects of diet and WL on semen quality (Table 1). Some authors have shown that nutritional factors are critical determinants of male fertility [47, 48]. Macronutrients and group foods including trans-fatty acids, high glycemic index food, high carbohydrate diet and high animal protein intake have been reported to compromise fertility. Contrariwise, omega-3 and omega-6 fatty acids, low glycemic index food and low carbohydrate diet, vegetable proteins, and antioxidants seem to improve fertility [48]. Indeed, it has been shown that a diet rich in fruits and vegetables, legumes and fish (source of antioxidants and polyunsaturated fatty acids) and poor in processed/red meat and full-fat dairy products (sources of saturated fats), may have positive effects on semen parameters [47]. Recently, Caruso and colleagues [46] have evaluated the effects of a MD, as compared with a low-fat diet, on seminal parameters of young healthy adults. After six months of diet, participants in both groups displayed a significant increase in sperm concentration and total sperm count with significant differences in favor of the MD group [46]. Furthermore, WL programs based on a healthy diet and physical activity have been associated with an improvement in semen volume, total sperm count, normal sperm morphology [7, 44], progressive sperm motility and static percentage [45], T, AMH and SHBG serum levels [7, 44].
All the evidence emphasizes the importance of WL in the reduction of IR and chronic inflammation and highlights the central role of lifestyle changes as first-line treatment of MOSH and impaired male fertility [7].
In light of the detrimental effects of fat mass excess, IR and systemic low-grade chronic inflammation on testicular function, KD programs such as a very low-calorie ketogenic diet (VLCKD) may be useful to revert MOSH and improve male fertility.
KD is a high-fat, low-carbohydrate and adequate protein diet and it has been primarily used in the treatment of children’s refractory epilepsy since 1920 [1]. In the last decades, an evolution of the first KD (e.g., VLCKD) is being largely used in the treatment of obesity, with beneficial effects not only on body composition but also on blood pressure, glycemia, lipid profile, IR, non-alcoholic fatty liver disease (NAFLD), gut microbiota, atherosclerosis and polycystic ovary syndrome [1, 5, 6].
VLCKD is characterized by marked carbohydrate restriction (≤ 30 g/day, ≈ 13% of total energy intake), with a relative increase in the proportions of fat (≈ 44%) and protein (≈ 43%, 1.2–1.5 g/kg of high biological value proteins which is not high-protein content) and a total daily energy intake < 800 kcal [1].
The marked carbohydrate restriction induces a condition of physiological ketosis and the production of ketone bodies (KBs; e.g., acetoacetate, β-hydroxybutyric acid and acetone) within 3–4 days. Ketogenesis mainly occurs in the mitochondrial matrix in the liver and KB is used as fuel by many tissues, including the central nervous system, the heart and the skeletal muscle [1]. KBs show anorexigenic effects, reducing hunger and food intake and improving patient’s compliance, tolerability, motivation and adherence to treatment [1].
Although VLCKD has been reported to induce WL and improve MetS parameters, its effects on testicular function, T levels and semen quality have been poorly investigated. While recent Italian studies [1, 5, 6] have described an improvement of T levels, MOSH and LUTSs after WL program with VLCKD, there are currently no significant data on the impact of VLCKD on male fertility (Table 2).
Studies analyzing the effects of VLCKD-induced WL on metabolic and hormonal profile, SF and reproductive health in males. Both mean weight (kg) and BMI (kg/m2) refer to baseline
| Author | n | Age (years) | Weight (kg) | BMI (kg/m2) | Duration of WL (weeks) | IR | D | TT | FT | E | FSH | LH | SHBG | PSA | Effects on SF and RF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| La Vignera et al. [5] | 20 | 49.3 ± 5.2 | 93.0 ± 6.5 | 32.0 ± 3.1 | 12 | ↓ | NA | ↑ | NA | NA | NA | NA | NA | NA | Not investigated |
| Mongioì et al. [6] | 40 | 45.8 ± 2.4 | 112 ± 3.7 | 37.5 ± 1.1 | 8 | ↓ | ↑ | ↑ | NA | NA | NA | ↑ | NA | ↓ | Not investigated |
| Renck et al. [49] | 10 | 39 | - | 40.2 | 8 | ↓ | NA | ↑ | NA | NA | NA | NA | NA | NA | ↑IIEF |
| Renck et al. [50] | 2 | - | 113.65 | 37.05 | - | ↓ | NA | ↑ | NA | NA | NA | NA | NA | NA | ↑ sperm motility and morphology |
| Cignarelli et al. [51] | 22 | 39.3 ± 11.7 | 117.5 | 38.2 ± 6.4 | 4 | ↓ | NA | ↑ | = | NA | = | = | ↓ | NA | ↑ somatic domain of AMS |
n: number of subjects; age: mean age (years); weight: mean weight (kg); BMI: mean body mass index (kg/m2); D: vitamin D; PSA: prostate-specific antigen; RF: reproductive function; NA: not available; AMS: Aging Males’ Symptoms Scale. ↓: decrease; ↑: increase; -: none; =: unchanged
Despite the evidence is still scant, in 2019 the consensus statement from the Italian Society of Endocrinology (SIE) suggested a WL program through VLCKD for male patients with MOSH to increase plasma androgen levels and to improve their SF [1].
Afterward, La Vignera and colleagues [5] showed an improvement in insulin sensitivity and MOSH in a cohort of twenty overweight/obese nondiabetic male patients with hypogonadism treated with VLCKD for 12 weeks. At enrollment, patients presented increased insulin levels, HOMA index and C-peptide levels (markers of IR) as well as elevated proinsulin levels (suggestive of pancreatic β-cell dysfunction), while the proinsulin/insulin ratio was lower than the values suggested to predict β-cell exhaustion. VLCKD treatment induced a significant WL; moreover, serum glycemia, insulin, C-peptide, and proinsulin levels returned within the normal range in all patients, suggesting a recovery of β-cell dysfunction and a decreased risk to develop proinsulin-induced macrovascular complications. No changes were observed in the proinsulin/insulin ratio at the end of the diet since its value resulted within the normal range according to the current literature [52, 53]. Furthermore, diet induced a ~200% increase of serum total T and a complete remission from MOSH since no patient showed total T levels below the normal range after VLCKD. In as much as hypogonadism is a risk factor for metabolic and cardiovascular complications, VLCKD may be useful to restore T levels in overweight/obese men thus decreasing the chance of developing long-term sequels.
Likewise, Mongioì et al. [6] have proved the effectiveness of an 8-week-VLCKD protocol on T levels and LUTSs in 40 overweight/obese male patients. Before VLCKD, the most of patients (n = 32, 80%) showed IR while three (7.5%) and eleven subjects (27.5%) had T2DM and raised FPG, respectively. At baseline, five of forty patients (12.5%) showed overt hypogonadism (TT < 230 ng/dL) and four of them fully recovered the HPT function after the VLCKD treatment. Besides WL and glucose/lipid profile improvement, VLCKD treatment induced a significant increase in serum vitamin D, LH, and total T levels as well as a significant reduction of the prostatic-specific antigen levels when compared with pretreatment values.
Since T deficiency is related to obesity, MetS and IR, which in turn are associated with increased prostate volume, VLCKD could be useful not only to recover the HPT function and MOSH but also to counteract BPH, LUTSs and ED. Indeed, obesity has detrimental effects on prostate function by inducing hormonal imbalance (relative hyperestrogenism) as well as by increasing the lymphocyte infiltration of the prostate tissue, and the adrenergic tone. Condorelli and colleagues [54] have evaluated the effects of body WL in men with male accessory gland infections/inflammations (MAGI) treated with VLCKD or the MD. The authors provided an amelioration in LUTSs and quality of life in subjects treated with MD and a significant improvement not only in the same parameters but also in ejaculatory pain/discomfort and sexual dysfunction in males treated with VLCKD. Therefore, VLCKD might have positive effects on the prostate gland due to the retrieval of the HPT axis function, its anti-inflammatory effects and improvement in IR. However, evidence regarding the effects of VLCKD on obesity and hypogonadism-related prostatic disease as well as on MAGI symptoms is still scant. Future studies are needed to clarify these effects and to hypothesize a potential VCKD administration in patients with prostatic diseases.
Similarly, Renck et al. [49] have reported a significant increase in T levels and improvement of metabolic parameters in ten obese men after 2 months of VLCKD intervention following the Pronokal® method. Furthermore, the administration of the IIEF-5 questionnaire in a subgroup of six subjects at baseline and after six months revealed a diet-induced ED improvement. In particular, four subjects showed ED improvement from mild normal (n = 3) and from severe to mild/moderate (n = 1).
A recent study [51] has evaluated the effects of VLCKD on serum total T in non-diabetic obese patients treated for 28 days. After 7 days and 28 days of diet, participants showed a significant WL and IR improvement as well as a significant increase in total T and SHBG levels. However, calculated free T and LH did not change after 7 days and 28 days of ketogenic treatment [51]. This evidence supports the hypothesis that VCLKD may restore the testicular and HPT axis function within the first week of treatment.
Globally, the few data currently available prove that VLCKD is a valid and safe tool to boost serum T levels in obese/overweight men through cardio-metabolic profile ameliorations and decreased chronic inflammation. Therefore, the rise in serum T levels may concur to improve general health and sexual/RF.
The effectiveness of VLCKD on testicular function may be due both to the WL related to calorie restriction and KBs production. WL could recover the HPT function and decrease relative hyperestrogenism regardless of the method applied (e.g., diet and lifestyle interventions, weight-lowering drugs, bariatric surgery) [51]. KD regimens may induce a rapid WL with increased patients’ compliance mainly due to the KBs’ anorexigenic effects.
KD protocols should be applied both to reduce body weight and to improve physical performance [55]. Indeed, KBs produce more adenosine triphosphate which could be used as fuel by several tissues such as heart, muscle, brain, and kidneys. KD is particularly useful to sustain metabolic processes by KB production and to remove fat from adipose [55]. KD might improve androgen production through several mechanisms. For instance, fat mass loss may reduce the T storage in the adipocytes. The high-fat content typical of KD might also increase the blood substrate androgen production; moreover, the KD’s low fiber content could increase bioavailable cholesterol which is a substrate for steroidogenesis [55]. The KD-related carbohydrate restriction also leads to amelioration in glucose homeostasis and IR thus resulting in reduced stress oxidative and inflammation and better testicular function. Finally, the liver function amelioration with increased SHBG levels could impact T levels [51].
Notwithstanding some study limitations (e.g., the small sample size, the short-term follow-up period, the absence of a CG, the lack of albumin, E and T/E ratio measurement), these emerging evidence open a new scenario in the prevention and treatment of MOSH.
Despite the documented association between obesity and male infertility, so far no studies have investigated the effects of VLCKD on sperm parameters.
Only a recent report [50] has described the effects of significant WL on sperm parameters in two obese men with MetS. Patients followed a VLCKD according to a commercial WL program (Pronokal® method), which also included lifestyle support and behavior modification. At baseline, both patients showed total T levels within the hypogonadal range which increased after VLCKD (256 ng/dL and 351 ng/dL vs. 623 ng/dL and 388 ng/dL, respectively). Besides body WL, increased insulin sensitivity and T levels, all semen parameters (concentration, motility, and morphology) resulted improved in both patients. The total motile sperm count increased by 20% and 40% in patients 1 and 2, respectively. Additionally, the total and progressive sperm motility increased respectively by 46.4% and 62% in patient 1 and by 2.63% and 24.5% in patient 2. Finally, normal sperm morphology increased from 5% to 8% in patient 1 and from 1% to 5% in patient 2 after diet treatment.
Therefore, the significant improvement in metabolic and hormonal profiles after WL by VLCKD might positively impact semen parameters in overweight/obese patients.
A study conducted on a diet-induced obesity mice model has stated that KD may improve spermatogenesis and enhance sperm parameters (motility, percentage of sperm normal morphology and spermatogenic cell maturation) by reducing lipid peroxidation and oxidative stress [56]. Moreover, KBs may be fuel for sperm motility.
Indeed, it has been shown that KD may modulate mitochondrial function by reducing oxidative stress and increasing the production of antioxidants and detoxification enzymes [57]. Mitochondria play a crucial role in spermatozoa’s metabolism, energy production, redox equilibrium, calcium regulation and apoptotic pathways which are indispensable for flagellar motility, capacitation, acrosome reaction and gametic fusion [58]. Although there is no evidence yet, modulation of oxidative stress and mitochondrial function induced by KD/VLCKD might have positive effects on spermatozoa and, consequently, on male fertility. Further studies are needed to clarify these effects.
Finally, Condorelli et al. [54] have proved the effectiveness of VLCKD on prostatitis and MAGI which are frequently observed among males with infertility and sexual dysfunctions.
VLCKD has been shown to exert a positive effect on testicular function mainly by restoring the HPT axis function in overweight/obese men. However, evidence regarding the effectiveness of VLCKD in testicular dysfunctions is still poor.
The few studies available show some limitations related to the paucity and heterogeneity of the samples, the short period of follow-up, the lack of a CG and the study design.
The most of studies have focused on changes in serum T levels before and after the VLCKD treatment while LH, FSH, free T and E levels have not always been evaluated. Similarly, only one study has investigated the serum SHBG variations after treatment.
Therefore, the T levels variations seem to be the first endpoint of most studies investigating the effectiveness of KD on testicular function while other hormone measurements represent a secondary endpoint and, consequently, results partial.
Another limitation is related to the lack of trials investigating the potential effectiveness of VLCKD on human spermatogenesis. Future researches are needed to clarify its impact on semen parameters.
It would be interesting to assess the effectiveness of VLCKD on testicular function by studying the whole HPT function, the free T, SHBG and albumin variations, as well as thyroid hormones, prolactin, and cortisol levels before and after treatment. These measurements should be associated with the evaluation of all sperm parameters (number, motility, morphology) to find potential effects on spermatogenesis. Furthermore, the study design should provide an evaluation of the different effectiveness on testicular function of KD/VLCKD and other diet programs such as MD, VLCD, and intermittent fasting.
Finally, it would be interesting to perform scrotal and prostate gland ultrasound examination in patients before and after VLCKD in order to ass any diet-induced volume and echostructure modifications.
Obesity and MetS may impair testicular function by reducing serum T levels and/or spermatogenesis. However, MOSH is a functional and potentially reversible condition that may be recovered by treatments aimed at lower body weight, IR, system low-grade chronic inflammation and hormonal imbalance.
VLCKD is an effective tool against obesity and MetS shows positive effects on body weight, insulin sensitivity, pancreatic β-cell function, cardio-metabolic and hormonal profile. According to emerging evidence, VLCKD may restore HPT function and T levels and improve SF, LUTSs and male fertility in overweight/obese male patients. Therefore, VLCKD could be safely administered to prevent/revert MOSH and, probably, male infertility. Future studies with a larger cohort and a longer follow-up period are warranted to clarify the effects of KD on T production and semen parameters. Furthermore, future studies should also focus on the potential effectiveness of KD in BPH and LUTSs prevention/treatment.
BPH: benign prostatic hyperplasia
CG: control group
E: estradiol
ED: erectile dysfunction
FSH: follicle-stimulating hormone
GnRH: gonadotropin-releasing hormone
HH: hypogonadotropic hypogonadism
HPT: hypothalamic-pituitary-testicular
IIEF-5: international index of erectile function 5
IL: interleukin
IPSS: International Prostatic Symptom Score
IR: insulin resistance
KBs: ketone bodies
KD: ketogenic diet
LCD: low-calorie diet
LH: luteinizing hormone
LR: leptin resistance
LUTSs: lower urinary tract symptoms
MAGI: male accessory gland infections/inflammations
MetS: metabolic syndrome
MD: Mediterranean diet
MOSH: male obesity secondary hypogonadism
OSA: obstructive sleep apnea
SF: sexual function
SHBG: sex-hormone binding globulin
T: testosterone
T2DM: type-2 diabetes mellitus
TT: total testosterone
VLCD: very low-calorie diet
VLCKD: very low-calorie ketogenic diet
WC: waist circumference
WL: weight loss
GI: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. CI: Investigation, Writing—original draft. PM and MV: Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Leo Karl Hanke ... Paola Molettieri
Paola Pellegrini ... Maria D’Elia
Xin Qi, Richard Tester
Büşra Atabilen, Yasemin Akdevelioğlu
Alejandro Borrego-Ruiz, Juan J. Borrego
