Affiliation:
2Department of Endocrinology, Changi General Hospital, Singapore 529889, Singapore
3Medicine Academic Clinical Program, Duke-NUS Graduate Medical School, Singapore 169857, Singapore
ORCID: https://orcid.org/0000-0002-5593-907X
Affiliation:
4Department of Laboratory Medicine, Changi General Hospital, Singapore 529889, Singapore
5Pathology Academic Clinical Program, Duke-NUS Graduate Medical School, Singapore 169857, Singapore
6Yong Loo Lin School of Medicine, National University of Singapore (NUS), Singapore 119228, Singapore
Email: tarchoon@gmail.com
ORCID: https://orcid.org/0000-0002-7814-8836
Explor Endocr Metab Dis. 2025;2:101428 DOI: https://doi.org/10.37349/eemd.2025.101428
Received: January 23, 2025 Accepted: March 31, 2025 Published: April 15, 2025
Academic Editor: Marijn Speeckaert, Universitair Ziekenhuis Ghent, Belgium
Diabetes mellitus is a major risk factor for both cardiovascular and chronic kidney disease (CKD) while CKD is also associated with cardiovascular morbidity. In fact, cardiovascular disease is the leading cause of death in patients with diabetes mainly from heart failure or myocardial infarction. The newer therapeutic agents in diabetes have positive impact on both cardiovascular and renal outcomes. Thus, the American Diabetes Association (ADA)’s annual update on the Standards of Medical Care in Diabetes is an important resource for all caregivers involved in diabetes management as it incorporates the latest scientific research, clinical evidence, and emerging technologies in diabetes management. The 2025 guidelines present significant updates that reflect a deeper understanding of diabetes management, emphasizing expanded usage of technologies such as continuous glucose monitoring, personalized pharmacological approaches, and lifestyle interventions. This commentary provides an analysis of the key updates in the 2025 ADA guidelines exploring implications for clinical practice, laboratory assessments, and public health policy. Where relevant, comparisons to the 2024 version will be made.
Diabetes continues to pose a significant global health burden, affecting millions of individuals worldwide. The International Diabetes Federation estimated that there were 537 million people with diabetes mellitus (DM) globally in 2021 and projects this disease to increase to 643 million by 2030 and 783 million by 2045 [1]. Caring for patients with diabetes requires addressing many issues, particularly cardiovascular and renal, besides glycemic control. Recent guidelines for the management of cardiovascular disease (CVD) in diabetes and diabetes management in chronic kidney disease (CKD) are available from specialty experts [2, 3]. The American Association of Clinical Endocrinology has also published a recent consensus statement on the comprehensive management of type 2 DM (T2DM) [4]. The prevalence of diabetes is rising and so, effective, evidence-based strategies for diagnosis, monitoring, and management are needed. The 2025 American Diabetes Association (ADA)’s Standards of Medical Care in Diabetes, a hefty 343-page document, serve as a critical resource for clinicians, laboratorians, and policymakers, offering recommendations that are updated annually to reflect the most current scientific advancements.
The 2025 ADA guidelines introduce transformative updates that address gaps in the 2024 standards. This commentary highlights these key changes, focusing on continuous glucose monitoring (CGM) for broader populations, advancements in pharmacological therapies, resistance training, and expanded recommendations for lifestyle and dietary interventions. Additionally, the guidelines also address emerging topics such as the implications of recreational cannabis use in diabetes management and the enhanced screening for presymptomatic T1DM.
Though the 2024 ADA Standards of Care recommended autoantibody screening for T1DM and emphasized its role in identifying high-risk individuals, it has not been widely adopted in clinical practice [5]. The 2025 update expands on this and underscores the value of antibody-based screening for presymptomatic T1DM in individuals with a family history of the disease. This addition reflects advances in understanding T1DM as a progressive autoimmune condition that can be detected early through specific markers. Screening for autoantibodies to insulin, glutamic acid decarboxylase (GAD), islet antigen 2 (IA-2), or zinc transporter 8 (ZnT8) allows for the identification of high-risk individuals at high risk prior to the onset of hyperglycemia [6]. Early detection can facilitate timely intervention, participation in prevention trials, and preparedness for disease onset, ultimately improving long-term outcomes and management strategies. Besides, teplizumab, a humanized CD3-directed monoclonal antibody, has been approved as a treatment to delay the progression of T1DM [7].
In the 2024 ADA Standards of Care in Diabetes, significant emphasis was placed on using HbA1c levels as a key diagnostic tool for diabetes, supported by its established correlation with average blood glucose levels and ease of testing [5]. However, the 2025 guidelines expand on this by providing a detailed comparison that addresses critical considerations in the utilization of HbA1c versus plasma glucose for diabetes diagnosis [6]. The comparison between glucose testing and HbA1c highlights notable differences in within-patient variability and standardization. Glucose levels show high variability due to acute influences such as food intake, stress, and diurnal changes, making it less reliable for consistent monitoring [8, 9]. In contrast, HbA1c demonstrates low within-person variability, providing a stable measure of average glucose over time. Additionally, while glucose assays lack universal standardization and can vary by sample type and processing, HbA1c testing is well-standardized, ensuring greater reliability across different laboratories [10]. This shift in focus demonstrates the ADA’s efforts to enhance the accuracy and equity of diabetes diagnostics, integrating these nuances to better tailor diagnostic practices to individual patient needs.
The 2025 guidelines emphasize greater usage of combination therapies, particularly in patients with early-stage T2DM, as opposed to the stepwise treatment strategy advocated in 2024. While the 2024 guidelines already recommended glucagon-like peptide-1 receptor agonists (GLP-1 RAs) for weight loss due to their proven efficacy in reducing body weight and improving glycemic control, the 2025 update broadens their scope of use for their multifaceted benefits in diabetes management, including weight loss, kidney disease, and metabolic dysfunction-associated steatotic liver disease (MASLD)/metabolic-associated steatohepatitis (MASH) [11, 12]. The 2025 guidelines mention the dual receptor agonist of glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 that has been approved for T2DM and obesity management [13, 14]. Additionally, the guidelines underscore the renal-protective effects of GLP-1 RAs, particularly in slowing the progression of diabetic kidney disease [15].
New evidence highlights the potential of GLP-1 RAs in managing MASLD/MASH, conditions that are increasingly prevalent among individuals with T2DM, by reducing hepatic inflammation and improving metabolic parameters [11]. This section of the guidelines has been considerably expanded by the ADA panel of experts. Notably, the 2025 guidelines also introduce the combination of GLP-1 RAs with pioglitazone, emphasizing their synergistic effects in enhancing insulin sensitivity, reducing liver fat, and improving overall metabolic outcomes [16]. Also highlighted is the use of resmetirom, a recently FDA-approved thyroid hormone receptor-β agonist, for moderate and advanced MASLD [16].
Given the increased incidence of heart failure (HF) in patients with diabetes, the 2025 ADA Standards of Care recommend using GLP-1 RAs in individuals with T2DM, obesity, and symptomatic HF with preserved ejection fraction (HFpEF). While sodium-glucose cotransporter 2 (SGLT2) inhibitors remain the first-line therapy for HF in diabetes, GLP-1 RAs are now recognized as complementary agents that can be prescribed concomitantly [17]. Supported by cardiovascular outcome studies, GLP-1-RAs and SGLT2 inhibitors are preferred for patients with DM and CVD [13, 17].
Many patients undergoing surgery have diabetes, prediabetes, or unrecognized diabetes. These patients may also develop adverse events from the surgical stress and hospitalization. Given the known gastrointestinal side-effects of GLP-1 RAs (e.g., nausea, vomiting, and delayed gastric emptying) pulmonary aspiration from general anesthesia is an important concern that the ADA 2025 guide highlights [18]. The 2023 American Society of Anesthesiologists guide is cited wherein GLP-1 RAs are withheld on the day of surgery (for daily dose agents) or 7 days prior to the procedure (for once-weekly drugs) [18].
While the US Endocrine Society advocates vitamin D for the prevention of diabetes in high-risk adults with pre-diabetes, the ADA expert panel is disinclined to do so and awaits further research [7]. They cite 3 studies (Norway, US, and Japan) where there was only modest risk reduction with vitamin D treatment and the results were not statistically significant [7].
The 2025 ADA Standards of Care emphasize the integral role of diabetes self-management education and support (DSMES) in improving diabetes outcomes across the lifespan. A significant update is the increased focus on personalized, culturally tailored DSMES programs that address social determinants of health and ensure equitable access. The guidelines underscore the need for ongoing education at critical life stages or care transitions, such as diagnosis, changes in treatment regimens, and development of complications. Digital platforms and virtual DSMES programs are now highlighted as essential tools to expand accessibility and engagement, particularly for underserved populations [19].
The 2025 ADA guidelines recommend targeted screening for HF in individuals with T2DM who present with symptoms such as fatigue, dyspnea, or reduced exercise tolerance, emphasizing the importance of early detection and intervention using natriuretic peptides. However, routine screening for atherosclerotic CVD (ASCVD) in asymptomatic individuals is not recommended, as there is insufficient evidence to support its benefit in this population. Instead, the focus is placed on clinical assessments and managing established cardiovascular risk factors to prevent complications [17].
For the management of CKD in diabetes, the guidelines recommend annual assessments of spot urine albumin-to-creatinine ratio (UACR) and estimated glomerular filtration rate (eGFR), alongside lifestyle interventions like sodium restriction. Key updates include a stronger emphasis on SGLT2 inhibitors for renal and cardiovascular protection, regardless of glycemic control, and the integration of nonsteroidal mineralocorticoid receptor antagonists (e.g., finerenone) for patients with persistent albuminuria despite optimized therapy. The guidelines also highlight the importance of renin-angiotensin system (RAS) blockers (such as angiotensin-converting enzyme inhibitors and angiotensin receptor blockers) in patients with albuminuria and advocate for early nephrology referral for advanced CKD or rapidly declining renal function [20]. The recommendations can be summarized in Figure 1.
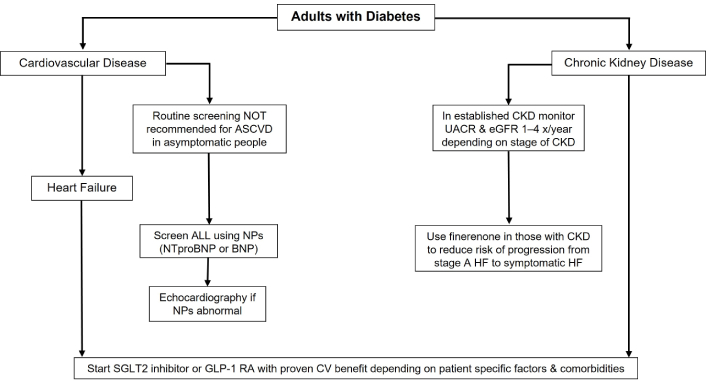
Key recommendations from the 2025 ADA Standards of Care in Diabetes guidelines for the management of major comorbidities in diabetes. ADA: American Diabetes Association; ASCVD: atherosclerotic cardiovascular disease; CKD: chronic kidney disease; UACR: urine albumin-to-creatinine ratio; eGFR: estimated glomerular filtration rate; NPs: natriuretic peptides; HF: heart failure; SGLT2: sodium-glucose cotransporter 2; GLP-1 RA: glucagon-like peptide-1 receptor agonist
While the 2024 guidelines emphasized calorie restrictions for weight management, the 2025 version shifts towards promoting high-quality, sustainable eating patterns, such as plant-based and Mediterranean-style diets, alongside reducing ultra-processed foods. While calorie management remains crucial, the guidelines highlight the importance of adequate water consumption to support hydration and metabolic health [19, 21]. Non-nutritive sweeteners in moderation and for the short term can be used in place of sugar to aid caloric restriction. In fact, habitual intake of sugar-sweetened beverages has been associated with weight gain and a higher risk of T2DM and CVD [22]. This guidance complements the focus on balanced nutrition and individualized approaches tailored to cultural preferences and patient needs, ensuring long-term compliance.
New recommendations advocate for integrating resistance training with aerobic exercises for comprehensive metabolic improvements, moving beyond the general activity recommendations in 2024 [19]. Resistance training, particularly for individuals on weight-loss pharmacotherapies or post-metabolic surgery is emphasized to prevent muscle loss and improve metabolic health [23].
Sleep health in relation to the risk of T2DM is now emphasized in the 2025 recommendations; 6–9 h of sleep per night is encouraged [7]. The ADA experts consider the importance of sleep to be on par with other lifestyle factors like exercise and diet.
The 2025 ADA Standards of Care strongly advise against the use of cannabis in any form for individuals with diabetes due to its potential risks. A specific concern is cannabis hyperemesis syndrome, characterized by persistent nausea, vomiting, and abdominal pain, which can complicate diabetes management and lead to dehydration or electrolyte imbalances [24]. This clinical picture may be confused with other diabetic urgencies like diabetic ketoacidosis and hyperosmolar nonketotic states.
The 2025 ADA Standards of Care introduce several updates to the 2024 guidelines on technological integration in diabetes management [25]. CGM is now strongly recommended for a broader population, including individuals with T2DM not on insulin, thus expanding its use beyond intensive insulin regimens [25, 26]. CGMs are now integrated with automated insulin delivery systems. Based on feedback from the CGM insulin delivery is adjusted. Fully automated systems (end to end), designated as closed-loop systems, are receiving increased focus for their ability to personalize insulin delivery and reflect new evidence of improved glycemic outcomes [25, 27]. The guidelines also place greater emphasis on digital tools, such as mobile apps and virtual platforms, to enhance patient engagement and care accessibility [25].
The 2025 ADA Standards of Care integrate the latest findings from groundbreaking clinical trials, reflecting the evolving landscape of diabetes management. Research supporting early intervention with SGLT2 inhibitors highlights their effectiveness in slowing the progression of CKD, offering dual cardiovascular and renal protection [19, 28]. Additionally, evidence from trials like SELECT emphasizes the dual benefits of GLP-1 RAs for glycemic control and significant weight loss, addressing the dual burden of obesity and diabetes [17, 29]. These findings underscore the growing importance of tailoring therapies to prevent complications and improve outcomes.
Emerging areas of research also include the role of natriuretic peptides as biomarkers for early diagnosis and risk stratification, expanding diagnostic precision in high-risk populations [30]. Advances in diabetes technology, such as hybrid closed-loop systems and CGM, are further supported by studies demonstrating improvements in time-in-range and patient-reported outcomes [25, 27, 31]. The guidelines also highlight disparities in access to care, urging research into equitable distribution of novel therapies and technologies to maximize their global impact. This evidence-driven approach ensures that diabetes care remains patient-centered and aligned with the latest scientific advancements.
In response to global concerns, the 2025 ADA Standards of Care emphasize reducing global health disparities in diabetes care, particularly in low-resource settings [32]. The guidelines call for improving access to essential treatments, including affordable medications, and expanding the availability of diabetes technologies such as CGM. They also highlight strategies like telemedicine and community-based diabetes education to reach underserved populations. Additionally, the guidelines advocate for collaborative efforts to address cost barriers and ensure equitable healthcare delivery, aiming to bridge gaps and enhance outcomes globally [33].
The 2025 guidelines reflect a comprehensive update informed by recent clinical trials and emerging trends in diabetes management. The shift towards individualized care, integration of technology, and holistic lifestyle recommendations aligns with the evolving needs of patients and healthcare providers. It is not our intent to be comprehensive in our coverage of the 2025 ADA guidelines on standards of medical care in diabetes. We highly commend the reading of the guidelines in its entirety to all involved in diabetes care.
Table 1 summarizes the key revisions and recommendations that we have gleaned from the 2025 ADA guidelines.
Key recommendations from the 2025 ADA Standards of Care in Diabetes guidelines
| Section | Key recommendations (2025) |
|---|---|
| Diagnosis | Consider antibody-based screening for presymptomatic type 1 diabetes in individuals with a family history of the disease |
| Pharmacological advances | GLP-1 RA or dual GIP-GLP-1 RA are recommended for their multifaceted benefits in diabetes management |
| Dietary guidance | Importance of adequate water consumption highlightedRecommend high-quality, sustainable eating patterns such as the plant-based dietReplace sugar with non-nutritive sweeteners in moderation and short term to aid caloric restriction |
| Digital technology | Strongly recommend CGM for individuals with T2DM on non-insulin regimens as well as those on insulin |
ADA: American Diabetes Association; GLP-1 RA: glucagon-like peptide-1 receptor agonist; GIP: glucose-dependent insulinotropic polypeptide; CGM: continuous glucose monitoring; T2DM: type 2 diabetes mellitus
These 2025 ADA updates reinforce the commitment to evidence-based practices and patient-centered care in DM. The broader adoption of innovative therapies and technologies, alongside a deeper focus on sustainability and equity, marks a significant step forward in improving diabetes outcomes globally.
ADA: American Diabetes Association
CGM: continuous glucose monitoring
CKD: chronic kidney disease
CVD: cardiovascular disease
DM: diabetes mellitus
DSMES: diabetes self-management education and support
GLP-1 RAs: glucagon-like peptide-1 receptor agonists
HF: heart failure
MASLD: metabolic dysfunction-associated steatotic liver disease
SGLT2: sodium-glucose cotransporter 2
T2DM: type 2 diabetes mellitus
DT: Conceptualization, Formal analysis, Investigation, Resources, Data curation, Writing—original draft, Visualization. WJL: Writing—review & editing. TCA: Conceptualization, Resources, Writing—review & editing, Visualization. All authors have read and agreed to the final version of the manuscript.
Tar Choon Aw who is the Editorial Board Member of Exploration of Endocrine and Metabolic Diseases had no involvement in the decision-making or the review process of this manuscript. The other authors declare no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
