Affiliation:
1Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton, FL 33431, USA
ORCID: https://orcid.org/0000-0003-1638-2325
Affiliation:
1Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton, FL 33431, USA
ORCID: https://orcid.org/0000-0003-0195-2524
Affiliation:
1Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton, FL 33431, USA
ORCID: https://orcid.org/0000-0002-0977-0365
Affiliation:
1Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton, FL 33431, USA
ORCID: https://orcid.org/0009-0006-9029-2933
Affiliation:
3Department of Neurosurgery, University of Florida, Gainesville, FL 32611, USA
Email: Brandon.Lucke-Wold@neurosurgery.ufl.edu
ORCID: https://orcid.org/0000-0001-6577-4080
Explor Drug Sci. 2023;1:107–125 DOI: https://doi.org/10.37349/eds.2023.00009
Received: January 11, 2023 Accepted: April 13, 2023 Published: April 28, 2023
Academic Editor: Rangasamy Jayakumar, Amrita Vishwa Vidyapeetham University, India
Malignant brain tumors are the leading cause of cancer-related death in children and remain a significant cause of morbidity and mortality throughout all demographics. Central nervous system (CNS) tumors are classically treated with surgical resection and radiotherapy in addition to adjuvant chemotherapy. However, the therapeutic efficacy of chemotherapeutic agents is limited due to the blood-brain barrier (BBB). Magnetic resonance guided focused ultrasound (MRgFUS) is a new and promising intervention for CNS tumors, which has shown success in preclinical trials. High-intensity focused ultrasound (HIFU) has the capacity to serve as a direct therapeutic agent in the form of thermoablation and mechanical destruction of the tumor. Low-intensity focused ultrasound (LIFU) has been shown to disrupt the BBB and enhance the uptake of therapeutic agents in the brain and CNS. The authors present a review of MRgFUS in the treatment of CNS tumors. This treatment method has shown promising results in preclinical trials including minimal adverse effects, increased infiltration of the therapeutic agents into the CNS, decreased tumor progression, and improved survival rates.
Over 260,000 people are diagnosed with primary malignant central nervous system (CNS) tumors annually. Malignant brain tumors are the leading cause of cancer-related death in children and the third largest cause of death in young adults [1, 2]. Currently, brain tumors are typically treated with surgical resection and radiotherapy in addition to chemotherapy or other therapeutic molecules [3–5]. However, the chemotherapeutic molecules must first cross the blood-brain barrier (BBB) to treat CNS tumors [5, 6]. The BBB is a semipermeable system comprised of capillary endothelial cells interlinked by tight junctions [6–8]. The BBB functions as a protective mechanism that defends the brain from exogenous and endogenous substances. Small lipid soluble molecules with a molecular weight of less than 400 Da can cross the BBB via passive diffusion or active transport channels [7, 8]. Large and hydrophilic molecules are blocked by the BBB [7].
Unfortunately, the therapeutic efficacy of chemotherapeutic agents is limited due to the BBB [5, 6, 9, 10]. Treatment with therapeutic molecules is largely limited by the molecular size of the drugs (e.g., doxorubicin ∼540 Da and bevacizumab 149 kDa) [11]. In contrast, temozolomide (TMZ), a lipophilic molecule with a molecular size of 194 Da, is one of few chemotherapeutic agents that can cross the BBB and treat CNS tumors [12]. The concentration of TMZ in the brain and cerebrospinal fluid (CSF) can reach up to 20% of plasma concentrations. TMZ is limited by a half-life of 1.8 h and requires continuous administration to maintain therapeutic levels [13]. Additionally, TMZ has many adverse side effects, and some CNS tumors have been known to develop resistance to TMZ [6, 14]. To overcome these limitations, intra-arterial infusion of mannitol, direct injection, and convection enhanced delivery may be used. However, these approaches are invasive and non-targeted [11]. This poses a therapeutic dilemma as the efficacy of treatment does not outweigh the systemic effects.
Focused ultrasound (FUS) is a promising intervention for CNS tumors, which has shown success in preclinical trials. The therapeutic capacity varies depending on the intensity of the ultrasound waves. High-intensity focused ultrasound (HIFU) has the capacity to serve as a direct therapeutic agent in the form of thermoablation and mechanical destruction of tumor cells. Mechanical destruction requires intravenously administered microbubbles (MBs), which have a gaseous core and are coated by a polymeric or lipid shell [15]. When targeted with ultrasound, MBs oscillate at the endothelium and disrupt the cell membrane thereby decreasing the required energy threshold and reducing the possible adverse effects seen in thermal ablation [16]. In comparison, low-intensity focused ultrasound (LIFU) enhanced with MBs has been shown to disrupt the BBB and enhance the uptake of chemotherapeutic agents in the brain and CNS. Additionally, LIFU has been used in liquid biopsies by disrupting the BBB thereby allowing for the release of tumor biomarkers into the peripheral circulation. We present a review of the mechanism of action of magnetic resonance guided focused ultrasound (MRgFUS), preclinical evidence, and current clinical studies in the treatment of CNS tumors.
Generally, FUS in the brain can be classified based on intensity. HIFU is a direct treatment approach that directly induces tissue necrosis (Figure 1), while LIFU induces BBB disruption for a more effective administration of pharmaceutical treatments (Figure 2) [17, 18]. The mechanism of HIFU treatment of peripheral brain tumors is through thermoablation and mechanical destruction while the mechanism of LIFU treatment of brain tumors is through the induction of functional and mechanical changes to the BBB and blood tumor barriers [19].
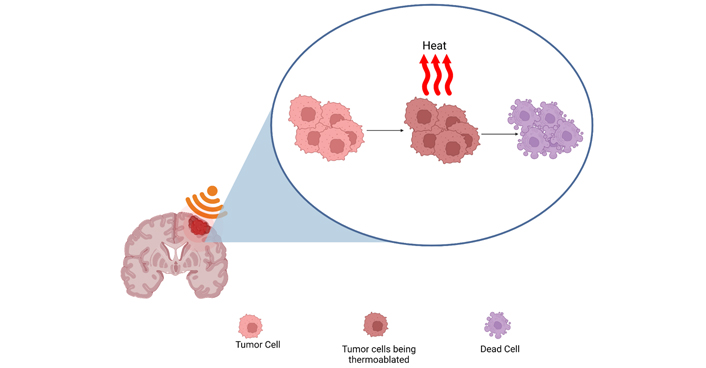
Continuous HIFU waves produce a thermal effect that reaches the threshold for protein denaturation and subsequent cell death. Created with BioRender.com
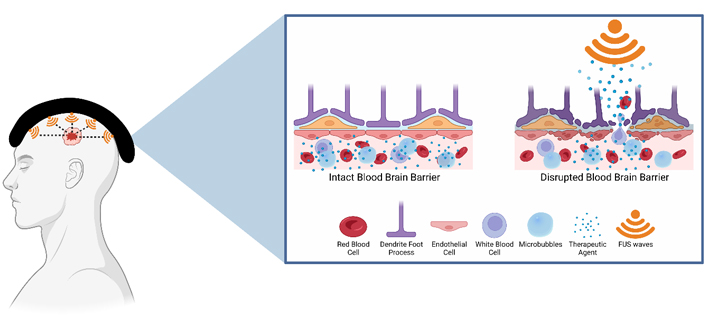
BBB opening for drug delivery: LIFU is used in conjunction with injected intravenous therapeutic agents. LIFU stimulates the MBs causing them to expand and contract. This leads to the opening of tight junctions and increased numbers of transcytotic vesicles in addition to decreased concentration of efflux pumps. Overall, it enhances the ability for therapeutic agents to cross the BBB. Created with BioRender.com
For HIFU, pretreatment magnetic resonance imaging (MRI) volumetric computed tomography (CT) scans are taken for MRgFUS planning. MRI is used to identify the size of the target, and CT scan is used to determine bone density and thickness for phase aberration correction [20, 21]. Exact focusing metrics are determined, and the patient is prepared for the procedure. The head is shaved to prevent hair interference on ultrasound delivery and then immobilized by a stereotactic head frame. A silicon barrier seals cooled gasless water within the transducer cavity to prevent thermal damage and enhance ultrasound delivery [20]. The duration of the sonication, power, phase, and number of the array elements are adjusted throughout the treatment. HIFU is delivered as a single exposure of 2–10 s continuous waveform at 650 kHz with a short 2.3 mm wavelength to establish a tight 6–10 mm tissue ablation focus [22, 23]. Administered ultrasound intensity ranges from 100 W/cm2 to over 10,000 W/cm2 [24]. Passive cavitation detectors and MRI thermometry are used to monitor cavitation and thermal rise, respectively.
Similar to HIFU, pretreatment MRIs are obtained for MRgFUS planning in LIFU, and a stereotactic frame is used to fix the ultrasound transducer to the scalp of the patient [18, 25]. Ultrasound attenuation is minimized by the application of degassed water between the scalp and the transducer [26]. MRI is used to identify the target tissue and magnetic resonance thermometry is used to detect and control tissue temperature at the target region [21, 26]. Contrast enhanced MRI and dye leakage is the most common method used to determine the extent of BBB or blood tumor barrier disruption to assess treatment efficacy [27]. Evans blue is a popular option for assessing barrier disruption. However, other proposed options include FITC-Dextran, Trypan blue, Nile blue, and sodium fluorescein [28–34]. Because of the narrow and precise aperture of the converging LIFU wave, many different targeting methods have been developed to cover the entirety of the target tissue volume and associated areas [19]. This can be achieved using a grid system to target specific points that utilize the additive effect of merging waves to produce the intended therapeutic effect [35–38]. Neuronavigation is another newly explored option for precise targeting that does not require MRI pretreatment planning [39–41]. Ultrasonic pressure waves are applied in phases to induce size changes of exogenous MBs localized at the target zone. To establish the appropriate and safe degree of barrier disruption for the intended therapeutic effects, specific parameters must be established [42]. These include ultrasound frequency, acoustic pressure and duration, burst pulse repetition frequency, duty cycle, exposure duration, and MB type, size, and dosage [6, 18, 42–44]. Unfortunately, there is a lack of consensus regarding the optimal parameters required for LIFU treatment [44, 45]. The discrepancy in the literature is likely attributed to ultrasound wave impendence, attenuation, distortion, scattering, reflection, and absorption when interacting with the varying thickness and density of the skull [18, 44, 46–50] and hair [18]. The suggested delivery of LIFU is as 0.74% to 5% duty cycle for 50–150 s [22]. Frequencies of 220 kHz with a large 6.8 mm wavelength are administered for transient opening of the BBB [24]. Intensities range from 0.125–3.0 W/cm2 and a mechanical index of 0.48, 0.58, and 0.68 demonstrated transient opening of the BBB [24].
Electrical signals are converted to ultrasonic waves and focused using a lens, phased array, or a concave/curved transducer to target a precise volume of tissue [18, 43]. Continuous waves of ultrasound are applied to produce a high-pressure thermal effect with a small target focus, which results in tissue destruction. This thermal effect is achieved when the local temperature reaches the threshold for protein denaturation and tissue damage and subsequent lesion formation [18, 51]. This thermoablative process further increases tumor sensitivity to radiation by damaging deoxyribonucleic acid (DNA) repair enzymes [2].
Treatment of different volumes can be achieved by modulating volume dimension by transducer selection, ultrasound frequency, or repeated ultrasound exposure of multiple overlapping focal volumes [52, 53]. The administration of ultrasound mediated thermoablation is challenging because of skull induced disruption of ultrasound wave propagation [54, 55]. Historically, the administration of FUS treatment for thermoablation required a craniectomy because of the limitations introduced by the cranium [51, 56], which causes phase aberrations and beam distortions that can change the shape and location of focus [57–59]. Current techniques allow for noninvasive transcranial administration of HIFU, reducing the risk of infection while simultaneously addressing factors that influence the efficacy of the treatment [21, 54, 60, 61].
Real time MRI thermometry monitoring, MRgFUS, allows for the delivery of FUS transcranially with stereotactic precision. When administering HIFU with a phased array transducer, phase and amplitude can be adjusted to correct for skull introduced aberrations as well [18, 60, 61]. This is achieved by initiating a distorted wavefront that, upon disruption by the skull, will result in the desired shape and location of the focus. Phased arrays also allow for increased focal placement and accuracy via electronic focus steering [62]. Clinically, the use of both MRgFUS with electronic focus steering capacity of phased array transducers has demonstrated glioblastoma tumor ablation in a patient without adverse effects or neurological deficits [63].
Ultrasound induced mechanical destruction occurs through pressure wave increases created by the charge components of the ultrasound mechanical wave [64]. Positive components induce compression and negative components induce expansion of gas filled crevices or bubbles. These bubbles increase in oscillation between compression and expansion as the ultrasound pressure wave increases. When the desired ultrasound threshold for treatment is reached, the bubbles explode and produce high velocity shock waves that induce cavitation of the focus [65, 66]. This phenomenon is described as inertial or acoustic cavitation (Figure 3). In addition, ultrasound waves produce a direct mechanical stress along the direction of the beam onto the target focus. This secondary mechanism of mechanical destruction can be induced when radiation forces are intense enough to cause tissue displacement and strain [67]. Shear forces induced by liquid movement also play a role in damaging focal tissue based on the presence of liquid [68]. This phenomenon is described as acoustic streaming.
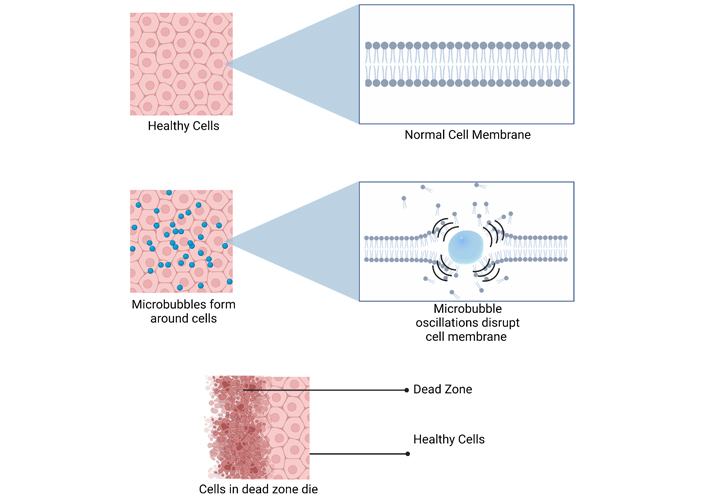
HIFU induces mechanical destruction of cells by creating pressure waves that oscillate the MBs. As the MBs expand and contract, they eventually reach a threshold where they explode, producing shock waves that disrupt cell membranes leading to cell death. Created with BioRender.com
Exogenous MBs are administered and allowed to circulate the blood vessels and localize to the treatment target zone [42]. Ultrasonic pressure waves are applied in phases to induce MB size changes. MBs expand during the compression phase and contract during the rarefaction phase. This process of stable cavitation induces linear and symmetric oscillations that, when combined with the morphological ultrasound induced changes in MB size, result in disruption of BBB and blood tumor barriers [17, 42]. The FUS induced alteration in size and shape of MBs apply physical stress on blood vessel walls. The changes in size and shape also create circumferential fluid streaming that places further stress on endothelial cell walls [6, 42, 69]. In addition, the ultrasound waves can directly increase the permeability of the BBB and blood tumor barriers by facilitating trans- and paracellular transport [45]. The stretching of cerebral endothelial cells along with sonopermeation temporarily alters the integrity of tight junctions by affecting adhesion molecules [17, 70]. The integrity of the barriers may also be disrupted through the release of substances by the neurovascular unit (cerebral endothelial unit, astrocytes, pericytes, nerve endings, and microglia) in response to treatment (Figure 2) [71–73].
LIFU can increase permeability of the BBB and blood tumor barrier by inducing functional changes. The use of LIFU may constrain or otherwise modulate protein expression by elevating local endothelial cell temperatures [74]. Transcellular permeability is amplified through the increased expression of calcium activated potassium channels while pharmacological efflux is reduced through the decreased expression of P-glycoprotein [18, 75–78].
While most pre-clinical research involving MRgFUS has focused on LIFU, recent studies have shown that HIFU can serve as a direct therapeutic agent through thermoablation [11]. One study analyzed RNA and protein expression changes in MRgFUS-induced BBB hyperpermeability using pulsed FUS with MB parameters. They used rat models to evaluate the thermoablation effects on tumor prognosis and found that proinflammatory cytokines and heat shock protein 70 (HSP70) concentrations significantly increased following MRgFUS. Furthermore, they found increased levels of ionized calcium-binding adapter molecule 1 (Iba1), which indicates microglial and macrophage activation [79]. A second study utilized a spherical transducer at a frequency of 551.5 kHz and delivered ultrasound waves in 10 ms bursts, beginning with a starting acoustic pressure of 0.128 MPa, and found that MRgFUS was associated with an upregulation in pro-inflammatory cytokine and chemokine genes in neuronal endothelial cells. Additionally, there was a significant downregulation of BBB transporter genes in the 24 h following MRgFUS exposure [80]. Both studies found increased concentrations of glial fibrillary acidic protein (GFAP), which is associated with astrocyte activation, indicating that HIFU activates the innate immune system to further degrade tumor cells [79, 80]. As a caveat to both preclinical HIFU and LIFU studies, preclinical animal models differ from their human counterparts. For example, the equipment used is often minimized to better facilitate the study resources and parameters which may drastically alter the clinical impact. Such key differences are often referenced as fundamental limitations in bridging preclinical studies to human trials.
Multiple studies have evaluated the efficacy of LIFU to enhance drug delivery into the brain. Drugs that have been studied include bevacizumab [81], TMZ [82, 83], trastuzumab [84, 85], doxorubicin [86–88], methotrexate [89], and carboplatin [90]. Additionally, this technique has been used to promote the migration of immunoglobulins [91–95], viruses [96], and cells across the BBB (Table 1). In animal models, BBB disruption occurs immediately and resolves within 6–8 h. Additionally, multiple studies found that MRgFUS did not cause neuronal injury [6]. A summary of all preclinical trials reviewed is listed in Table 1.
Summary of existing preclinical trials
| Model | Frequency (mHz) | Burst length (ms) | Repetition frequency (Hz) | Exposure length (s) | Acoustic pressure (MPa) | Estimated acoustic power (W) | MB type | Therapeutic agent | Observation |
|---|---|---|---|---|---|---|---|---|---|
| Mouse (Swiss Webster) [91] | 0.69 | 10 | 1 | 40 | 0.6–1.1 | - | Optison™ | D4 receptor antibody | Minimal damage seen < 0.8 MPa. Damage observed > 0.8 MPa |
| Mouse (Swiss Webster) [84] | 0.69 | 10 | 1 | 40 | 0.6 and 0.8 | - | Optison™ | Herceptin | Greater delivery at 0.8 MPa compared to 0.6 MPa |
| Transgenic mice (B6C3-Tg and PDAPP) [92] | 0.69 | 10 | 1 | 40–45 | 0.67–0.8 | 0.28–0.4 | Optison™ or Definity® | Anti-amyloid β antibodies | 3-fold increase in antibodies localized to plaques |
| TgCRND8 mice [93] | 0.558 | 10 | 1 | 120 | 0.3 | - | Definity® | Amyloid-β antibodies | Mice treated with MRgFUS had a 12% reduction in plaque sizes |
| non-Tg and TgCRND8 mice [94] | 0.5 | 10 | 1 | 120 | 0.3 | - | Definity® | Endogenous antibodies | Reduced plaque sizes and activation of microglia |
| pR5 mice [95] | 1 | 10 | 10 | 6 | 0.7 | - | In-house lipid-shelled | RN2N antibodies | Mice treated with MRgFUS had an 11-fold increase in RN2N delivery to CNS. Reduced anxiety and tau phosphorylation |
| nu/nu mice (intracranial U87mg cells) [81] | 0.4 | 10 | 1 | 60 | 0.4–0.8 | 4–18 | SonoVue® | Bevacizumab | Animals treated with MRgFUS had decreased tumor growth and vessel area as well as increased survival rates |
| nu/nu mice (intracranial U87mg cells) [83] | 0.5 | 10 | 1 | 60 | 0.3–0.7 | 2–5 | SonoVue® | TMZ | MRgFUS caused TMZ accumulation in the brain to increase and have a slower degradation rate compared to controls. MRgFUS also slowed tumor progression |
| Male NOD-scid mice [88] | 1 | - | 1 | 60 | - | 2.86 | SonoVue® | Doxorubicin | MRgFUS increased oxorubicin concentrations in the brain by 2.35-fold compared with the control tumors |
| C57BL/6J mice [96] | 0.558 | 10 | 1 | 120 | 0.53–0.6 | - | Definity® | Virus serotype 9 | A dose of 2.5 × 109 VG/g allowed expression of the transgene in neurons, astrocytes, and oligodendrocytes in brain regions targeted with ultrasound. Nontargeted regions were minimally infected |
| Athymic nude-Foxn1nu mice [90] | 1.05 | 23.8 | 1 | 120 | 0.3 | - | SonoVue® | Carboplatin | Animals who received MRgFUS had a 4.2-fold increase in carboplatin concentration in the brain. MRgFUS also enhanced survival and delayed tumor growth |
| Nude rats (intracranial M.D. Anderson-metastatic breast (MDA-MB)-361 cells) [85] | 0.69 | 10 | 1 | 60 | 0.46–0.62 | 0.4–0.7 | Optison™ | Trastuzumab and pertuzumab | Animals treated with FUS had slower tumor growth rates and higher survival rates |
| Sprague-Dawley rats (intracranial C6 glioma) [5] | 0.5 | 100 | 1 | 90 | 0.36–0.7 | 5 or 20 | SonoVue® | IL-12 | Animals treated with MRgFUS had a 3-fold increase in IL-12 in the CNS. They also had higher CD8+/T-reg ratio and slower tumor growth |
| Sprague-Dawley rats [97] | 0.55 | 1 | 1 | 120 | 0.24 | - | Definity® | GFP-tagged neural stem cells | MRgFUS allowed higher concentrations of neuronal stem cells in specifically targeted locations in the brain compared to controls |
| Male Sprague-Dawley rats [98] | 1.7 | 10 | 1.7 | 60–120 | 1.2 | - | Optison™ | Doxorubicin | MRgFUS increased the antineoplastic efficacy of liposomal doxorubicin in the brain. Animals treated with FUS had better survival rates compared to controls |
| Male Sprague-Dawley rats [80] | 0.55 | 10 | 1 | 120 | 0.128 and increased by a 0.008/s increment | - | Definity® | Gadovist | MRgFUS induced a transient inflammatory response in microvessels |
| Male Sprague-Dawley rats [87] | 1.5 or 1.7 | 10 | 1 | 30 | 0.36–2.5 | 0.06–3.0 | Optison™ | Doxorubicin | Doxorubicin concentrations were significantly higher in areas targeted by MRgFUS than nontargeted areas of the brain |
| Athymic nude rat (intracranial MDA-MB-231 cells) [99] | 0.55 | 10 | 1 | 120 | 0.32–0.35 | - | Definity® | In HER2-specific NK-92 | 10-fold increase in HER2-specific NK-92 cells regions targeted by MRgFUS |
| Athymic nude rat (intracranial MDA-MB-231 cells) [100] | 0.55 | 10 | 2 | 120 | - | - | Definity® | In HER2-specific NK-92 | Rats with MRgFUS had significantly reduced tumor growth and higher survival rates compared to controls |
| Male Fischer rats [82] | 0.5 | 10 | 1 | 60 | 0.6 | 3 | SonoVue® | TMZ | MRgFUS increased the TMZ CSF/plasma ratio from 22.7% to 38.6%, reduced tumor progression, and increased survival rates |
| Male Sprague-Dawley rats [62] | 0.69 | 10 | 1 | 60 | 0.55 | - | Definity® | Doxorubicin | The concentration of doxorubicin decreased by 32% when injected 10 min after MRgFUS compared to when doxorubicin is injected before sonification |
| Female Sprague-Dawley rats [79] | 0.589 | 10 | 1 | 120 | 0.3 | - | Optison™ | - | MRgFUS increased levels of proinflammatory, anti-inflammatory, trophic, neurotrophic and neurogenesis factors |
| Rabbit (New Zealand white) [55] | 1.63 and 1.5 | 100 | 1 | 20 | - | 0.55 or 3 | Optison™ | - | Sonication as 0.55 W increased the number of endothelial vesicles and fenestrations on the luminal surface of endothelial cells. Damage occurred at 3 W |
| Rabbit (New Zealand white) [89] | 1.1 | - | - | 10 | - | 6 | SonoVue® | Methotrexate | MRgFUS significantly increased methotrexate concentrations in the brain compared to controls |
GFP: green fluorescent protein; HER2: human epithelial growth factor receptor 2; NK: natural killer; NOD: non-obese diabetic; -: none
Studies in mouse and rat models found that TMZ administered with MRgFUS had higher concentrations of the drug in the brain. Additionally, MRgFUS leads to slower tumor growth rates and prolonged survival [6]. Liu et al. [81] found that nude mice implanted with U87 human glioma cells had more TMZ accumulation in neuronal tissue and slower tumor progression. Additionally, they found that there were higher rates of TMZ degradation in the core of the tumor [83]. Furthermore, Wei et al. [82] used Fisher rat models with implanted 9-L glioma cells and found that MRgFUS facilitated (parameters included acoustic power = 3 W; peak negative pressure = 0.6 MPa; burst length = 10 ms; pulse repetition frequency = 1 Hz; exposure time = 60 s) BBB opening and permitted higher concentrations of TMZ to enter the brain tissue. Overall, the rats with MRgFUS and TMZ had improved survival rates and elevated TMZ CNS/plasma ratios compared to the rats given only TMZ [82].
Despite these findings, the therapeutic efficacy of TMZ is limited by its high rates of tumor resistance. TMZ has minimal effects on tumors with an unmethylated promotor region in the O6-methylguanine-DNA methyltransferase (MGMT) gene [101]. Therefore, MGMT gene modulation has become a growing interest [6, 102]. A study by Papachristodoulou et al. [103] used MB enhanced MRgFUS to deliver MGMT inactivator liposomal O6-(4-bromothenyl) guanine (O6BTG) in mice with TMZ-resistant tumors. They delivered ultrasonic waves in bursts of 10 ms at a repetition frequency of 1 Hz for a total duration of 180 s and found MRgFUS to be correlated with higher rates of MGMT resistance reversal and prolonged survival [103].
Bevacizumab is a monoclonal antibody that inhibits vascular endothelial growth factor (VEGF) [104]. While commonly used to improve peritumoral edema, it has not been known to improve prognosis [6]. However, a study using U87 glioma mouse models found that delivering MRgFUS with MB parameters increased bevacizumab trafficking into the brain. They found that the concentration of bevacizumab in the CNS increased by 57-fold [6, 81]. It was also noted that animals that received MRgFUS had a decrease in tumor vascular distribution and a slower tumor growth rate compared to controls leading to significantly improved survival rates [81].
1,3-bis (2-chloroethyl)-1-nitrosourea (BCNU) is a common second-line agent for recurrent gliomas. However, it lacks efficacy as a monotherapy [6]. Systemic administration of BCNU is highly toxic [6, 105]. However, the addition of MRgFUS enhanced BCNU concentrations in the CNS by 202% in rat C6 glioma models [6, 81]. Furthermore, MRgFUS slowed tumor growth and improved survival [81].
Multiple studies have shown success in delivering antibodies into the CNS via MRgFUS. Chen et al. [5] found that Sprague-Dawley rats with implanted intracranial C6 gliomas had a three-fold increase in IL-12 concentrations in the CNS when exposed to MRgFUS [5]. Additionally, Nisbet et al. [95] found similar results with RN2N antibodies. They noted an 11-fold increase in RN2N antibodies with MRgFUS [95]. With the ability to introduce antibodies to the brain, treatment for malignant CNS tumors can become more targeted and less invasive. Furthermore, this treatment method can potentially be utilized in other diseases such as Alzheimer’s disease [92, 93]. Overall, this treatment approach offers a promising future that warrants further investigation.
Although viruses fail to surpass the BBB, viral therapy remains a promising therapeutic technique for various brain tumors. Current strategies to overcome this include viral vector implantation through stereotactic or open surgery procedures [6]. Despite these approaches, viral therapy is less effective because the current delivery techniques result in uneven coverage throughout the CNS. However, recent studies have shown that MRgFUS combined with intravenous MBs facilitated the transmission of recombinant viral vectors into the brain parenchyma [106–108].
Chimeric antigen receptor T-cell therapy has been shown to decrease tumor progression. However, its efficacy is limited by T-cell migration into the CNS through the BBB. This can be overcome through direct intraventricular administration of the T-cell therapy or, as recent studies have shown, by utilizing MRgFUS to facilitate the trafficking of neuronal stem cells and immune cells past the BBB [97, 99, 100, 109].
While the use of carrier vehicles including MBs, liposomes, and nanoparticles to deliver chemotherapeutics offers protection from systemic side effects, it limits release of the agent at the target site (particularly in CNS tumors) and may result in subtherapeutic local drug levels [110]. Preclinical studies have demonstrated the ability of MRgFUS to increase the targeted release of therapeutic agents from drug delivery vehicles through hyperthermia, stable cavitation, and radiation forces [111–113]. In addition to improving delivery of carrier vehicles through the BBB in rodents, MRgFUS has been successfully applied in a trans-skull model to generate controlled hyperthermia and effectively release thermosensitive drugs in glioma [110, 114]. Other studies have examined the frequency and duration of FUS for this purpose, with some results suggesting high intensity bursts may be optimal [115–117].
Blood-based biopsies are used to diagnose and monitor various other cancers. However, this approach is hindered in CNS malignancies due to the BBB blocking biomarker release into the peripheral circulation [6]. Recent studies have investigated the use of MRgFUS in tumor diagnostics. Zhu et al. [118] explored the effect of MRgFUS in tumor biomarkers in mice with orthotopic implanted murine glioma cells that were enhanced with green fluorescent protein (eGFP). They found that MRgFUS at 0.59 MPa resulted in significantly increased plasma eGFP mRNA levels. Furthermore, the levels of biomarker release were directly correlated to the extent of tumor expansion [118]. These findings offer a promising future for cancer diagnostics.
Intracranial MRgFUS has historically been used to treat neurologic conditions such as essential tremor, epilepsy, and Parkinson’s disease. However, its indications are expanding and may now include intracranial neoplasms. LIFU and HIFU are valid options, and both may be used depending on the exact tumor present. LIFU has traditionally been used to open the BBB and allow for delivery of chemotherapeutic agents [6]. In contrast, HIFU uses FUS beams to thermally ablate or mechanically destroy target tissues [119]. Advancing technologies and new modalities of administering ultrasonic energy, such as needle-based therapeutic ultrasound, have improved the ability of HIFU to work through the calvarium [120]. Notwithstanding, much of the existing clinical literature surrounding HIFU for brain tumors surrounds centrally located brain tumors.
A majority of current clinical trials regarding MRgFUS are investigating its use in treating glioblastoma multiforme (GBM). However, other types of tumors currently being investigated include astrocytomas, ependymomas, cranial nerve tumors, meningiomas, metastases, neuronal tumors, pineal tumors, and tumors in the sellar region [121]. Hersh et al. [2] found the reason for optimism in using both modalities to treat GBM [2]. However, limited sample sizes and outdated technologies have obscured the data. The same researchers found that LIFU, when used in conjunction with either chemotherapeutics (e.g., TMZ or carboplatin), nanoparticles (e.g., gold, iron, or platinum), or MBs, was able to locally disrupt the BBB and impair multi-drug efflux pumps, resulting in decreased GBM growth and improved overall survival, although further studies are needed to obtain more conclusive results [2, 22]. In contrast, they found that HIFU still faces technological obstacles to surpass calvarial attenuation and minimize collateral damage. Notwithstanding, current literature includes reports of HIFU and subsequent thermocoagulation resulting in partial high-grade glioma resection and improved treatment margins [63].
Because most gliomas are infiltrative and difficult to distinguish from healthy brain tissue, gross total resection can be difficult, whether through MRgFUS or open surgery. In contrast, well-defined brain lesions, such as metastases or meningiomas, may be easier to ablate using HIFU while simultaneously monitoring temperature to ensure a safe and controllable procedure that limits damage to surrounding structures [121]. This may be most feasible when such benign tumors are in inaccessible or eloquent locations (e.g., adjacent to the brainstem or cranial nerves). In contrast, LIFU can be used to increase the permeability of the BBB for virtually any subtype of brain tumor for which chemotherapy is used, whether that be neoadjuvant or adjuvant [22]. These findings suggest that FUS may play a role in treating any CNS neoplasm, as some tumors may be ablated while others are treated with chemotherapy in conjunction with LIFU.
While LIFU disruption of the BBB for the delivery of therapeutic agents has been demonstrated to be safe and effective for the treatment of brain tumors [122, 123], there has been considerably less investigation into the use of HIFU to treat brain tumors via direct tissue destruction. Early attempts at HIFU for the treatment of brain tumors proved challenging, as ablative temperatures could not be safely reached without performing a craniectomy to provide an acoustic window [56, 124, 125]. However, with the advancement of phased array transducers, interest in transcranial MRgFUS thermoablation and histotripsy has been rapidly increasing [63, 125, 126]. In preclinical studies, both histotripsy and thermoablation have been shown effective through excised human skulls in vitro [127]. More recently, Lu et al. [128] demonstrated successful treatment of swine cerebral tissue through a human skull [128], and later demonstrated successful histotripsy ablation through a human skull in an in vivo swine model [129]. To date, clinical data are limited to case reports of 1–3 patients [54, 63]. Two phase I clinical trials evaluating the safety and efficacy of transcranial MRgFUS thermoablation for the treatment of either brain metastasis or recurrent glioma are ongoing [130, 131]. Though recruitment challenges delayed their timelines, selected early results have been promising and both trials concluded in December 2022 [20, 63, 130, 131]. A further exciting possibility is transcranial MRgFUS without the need for complex phased array devices. Sukovich et al. [132] in 2016 demonstrated targeted lesion generation in red blood cell agarose tissue through a human skullcap without aberration correction, which could simplify the procedure and open a new line of investigation [132].
In nearly every surgical subspecialty, the newest trends focus evermore on minimizing the invasiveness of the procedure. Regarding neurosurgery, endovascular and endoscopic procedures are vastly more common than they were in years past. MRgFUS presents the opportunity to eliminate the invasiveness of surgery altogether. MRgFUS provides a glimpse at what the future of tumor therapy might be, which utilizes MRI to locate the lesion and MRgFUS to ablate the lesion without requiring any truly intracranial operation. Such procedures may even be done in the outpatient setting with mild sedation rather than general anesthesia.
FUS has the potential to drastically change how brain tumors are managed. The non-invasive approach would improve access to the deep brain structures while simultaneously decreasing the damage to the healthy surrounding brain tissue, reduce the likelihood of operative complications, and lessen the length of hospital stays and recovery time (and thus the overall cost of care) without compromising therapeutic efficacy or adding increased exposure to ionizing radiation. Additionally, and perhaps more importantly, LIFU has demonstrated clinical efficacy and is currently the modality with the most intrigue. LIFU can temporarily disrupt the BBB to enhance the effects of adjuvant therapies and improve the overall clinical impacts on chemotherapeutics. Such targeted increased permeability to the BBB can allow for maximal chemotherapeutic delivery to the target tissue in the brain with minimal adverse effects throughout the rest of the body.
This treatment modality is faced with the challenge of parameter optimization. MRgFUS has demonstrated treatment efficacy in both LIFU and HIFU. Factors such as ultrasound wave impendence, attenuation, distortion, scattering, reflection, and absorption when interacting with the varying thickness and density of the skull and hair are key contributors to the discrepancies observed in the literature regarding the optimal parameters for treatment. Further clinical studies will be needed to consider these factors and determine the appropriate ultrasound frequency, acoustic pressure and duration, burst pulse repetition frequency, duty cycle, exposure duration, and MB type, size, and dosage. These are important considerations because of the function-mechanical effects of LIFU and thermo-mechanical effects of HIFU. In addition, a standardized monitoring approach of treatment efficacy will likely need to be established considering the variance in determining BBB disruption in LIFU and monitoring mechanical effects. Thus, future directions for this new treatment modality would be focused on controlling external factors, optimizing treatment parameters, and standardizing monitoring modalities.
FUS is an emerging treatment modality for CNS tumors that may be used in isolation or in conjunction with surgical resection and radiotherapy to improve overall outcomes. HIFU serves as a direct therapeutic agent in the form of thermoablation and mechanical destruction of tumor cells and their components while LIFU functionally and mechanically disrupts the BBB and enhances the uptake of chemotherapeutic agents in the CNS. Preclinical studies surrounding LIFU comprise a majority of the literature and have shown that MRgFUS significantly improved the transmission of multiple therapeutic agents such as chemotherapeutics, small molecules, and antibodies to the brain, leading to reduced tumor burden and improved outcomes. Additionally, LIFU was shown to play a role in monitoring neoplastic disease through liquid biopsy. Early preclinical studies with HIFU also showed promise, with evidence of tumor destruction and an improved host immune response. Both therapies have the potential to treat a broad array of CNS tumors, however, further clinical studies will likely need to be pursued before FUS becomes a routine treatment option for CNS tumors. Overall, MRgFUS has shown promising results throughout the existing literature, including minimal adverse effects, increased infiltration of therapeutic agents into the CNS, decreased tumor progression, and improved survival rates. As such, further investigation regarding MRgFUS is warranted.
BBB: blood-brain barrier
BCNU: 1,3-bis (2-chloroethyl)-1-nitrosourea
CNS: central nervous system
FUS: focused ultrasound
GBM: glioblastoma multiforme
HIFU: high-intensity focused ultrasound
LIFU: low-intensity focused ultrasound
MBs: microbubbles
MGMT: O6-methylguanine-deoxyribonucleic acid methyltransferase
MRgFUS: magnetic resonance guided focused ultrasound
MRI: magnetic resonance imaging
TMZ: temozolomide
PMJ: Project administration, Investigation, Writing—original draft, Writing—review & editing. PYH, AAM, and SJG: Investigation, Writing—original draft, Writing—review & editing. MF: Writing—review & editing. BLW: Conceptualization, Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
