Affiliation:
State Key Laboratory of Natural Medicines and Jiangsu Key Laboratory of Drug Discovery for Metabolic Diseases, Center of Advanced Pharmaceuticals and Biomaterials, China Pharmaceutical University, Nanjing 211198, Jiangsu, China
†These authors contributed equally to this work.
Affiliation:
State Key Laboratory of Natural Medicines and Jiangsu Key Laboratory of Drug Discovery for Metabolic Diseases, Center of Advanced Pharmaceuticals and Biomaterials, China Pharmaceutical University, Nanjing 211198, Jiangsu, China
†These authors contributed equally to this work.
ORCID: http://orcid.org/0009-0001-2450-6132
Affiliation:
State Key Laboratory of Natural Medicines and Jiangsu Key Laboratory of Drug Discovery for Metabolic Diseases, Center of Advanced Pharmaceuticals and Biomaterials, China Pharmaceutical University, Nanjing 211198, Jiangsu, China
ORCID: http://orcid.org/0000-0002-9093-5947
Affiliation:
State Key Laboratory of Natural Medicines and Jiangsu Key Laboratory of Drug Discovery for Metabolic Diseases, Center of Advanced Pharmaceuticals and Biomaterials, China Pharmaceutical University, Nanjing 211198, Jiangsu, China
Email: xiaoxuanliu@cpu.edu.cn
ORCID: http://orcid.org/0000-0002-7503-8359
Affiliation:
State Key Laboratory of Natural Medicines and Jiangsu Key Laboratory of Drug Discovery for Metabolic Diseases, Center of Advanced Pharmaceuticals and Biomaterials, China Pharmaceutical University, Nanjing 211198, Jiangsu, China
Email: zhudandan@cpu.edu.cn
ORCID: http://orcid.org/0000-0002-1683-3571
Explor Drug Sci. 2024;2:38–49 DOI: https://doi.org/10.37349/eds.2024.00035
Received: August 18, 2023 Accepted: October 12, 2023 Published: February 01, 2024
Academic Editor: Xiangyang Shi, Donghua University, China
Nucleic acid therapeutics are emerging as a promising class of medicines, offering unique therapeutic options for cancer at the gene level. However, the druggability of nucleic acid therapeutics is fundamentally restricted by their low stability, poor membrane permeability, and low bioavailability, necessitating the use of delivery vectors. Various delivery vectors have been developed for nucleic acid therapeutics. The fate of established nucleic acid delivery systems (NADS) in vivo substantially affects the delivery efficiency and therapeutic efficacy. The physicochemical properties of NADS (such as size, charge, shape, etc) are crucial for the interaction of NADS with various biological barriers in the body, thereby determining the fate of NADS in the body. Nanoparticle (NP) size is an important parameter defining the blood circulation, distribution, tumor accumulation, and cellular uptake of NADS. This mini-review briefly introduces the various biological barriers of NADS in cancer treatment and focuses on the influence of the particle size of delivery vectors on the in vivo fate of NADS and their therapeutic efficacy, which provides new insights into the rational design of NADS.
Nucleic acid therapeutics, with the aid of delivery techniques, have emerged as promising and effective approaches to treat human incurable diseases (rare genetic disorders, cancer, and chronic diseases) at the genomic and/or transcriptomic level in the 21st century. Nucleic acid therapeutics exhibit remarkable specificity, high efficacy, intelligent digital design, shortened research and development cycles, and high clinical translatability [1–3]. In particular, a majority of clinical trials (> 3,000) have been conducted based on nucleic acid therapeutics, and many have been successfully approved by the Food and Drug Administration (FDA) from 1998 to 2022, such as nine antisense oligonucleotides (ASOs), two messenger RNA (mRNA) vaccines and five small interfering RNA (siRNA) drugs (Figure 1) [4–6]. A new era of nucleic acid therapeutics has emerged and been witnessed. Novel treatments based on nucleic acid therapeutics (siRNA, mRNA, DNA, etc.) are the most promising cancer strategies [7, 8].
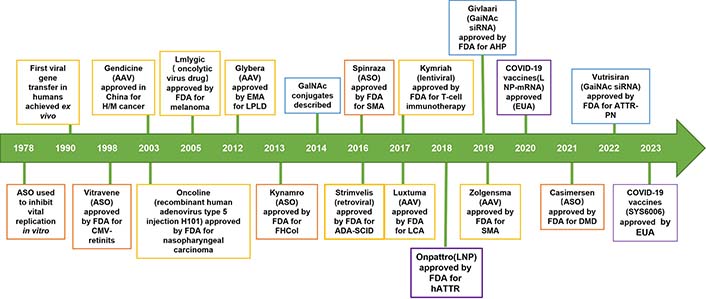
The development history of nucleic acid therapeutics. ASO: orange; siRNA: blue; lipid: purple; virus: yellow; AAV: adeno-associated virus; CMV: cytomegalovirus; EMA: European Medicines Agency; GalNAc: N-acetylgalactosamine; ADA-SCID: adenosine deaminase deficient severe combined immunodeficiency; LCA: life cycle assessment; hATTR: hereditary transthyretin familial amyloidosis; LNP: lipid NP; ATTR-PN: transthyretin familial amyloid polyneuropathy; EUA: emergency use authorization; H/M: hematologic malignancy; LPLD: lipoprotein lipase deficiency; FHCol: familial hypercholesterolemia; SMA: spinal muscular atrophy; AHP: acute hepatic porphyrias; DMD: Duchenne muscular dystrophy; COVID-19: coronavirus disease 2019
However, nucleic acid therapeutics have their unique obstacles to cancer treatment, such as excessive poly-anionic nature, hydrophilicity, and poor stability in vivo. Different nucleic acid therapeutics have different sequences, conformations, sites, and mechanisms of action. Recently, various delivery vectors have been extensively developed to overcome the druggability challenges of nucleic acids [9]. These vectors could effectively package nucleic acids, protect them from enzymatic degradation, enhance the accumulation of nucleic acids at tumor sites through the enhanced permeation and retention (EPR) effect, and significantly improve transfection efficiency [9–11].
As all know, the efficacy of carrier-based nucleic acid delivery systems (NADS) is closely related to their physicochemical properties. Among them, particle size is an essential part of the physicochemical properties of NADS, and it would partially influence protein corona formation, clearance by the reticular endothelial system (RES), tumor distribution, and cellular uptake, thereby significantly affecting their efficiency for cancer treatment [12–15]. Therefore, understanding the particle size of NADS and their influence on biological identity is essential to elucidate the in vivo fate of NADS and to design more effective delivery vectors.
In this short review, the authors first briefly summarize the different biological barriers encountered by NADS in tumor therapy, including complex humoral components, the RES, the tumor microenvironment, and cellular or subcellular membranes, as well as a specific barrier blood-brain barrier (BBB) in brain tumor. Furthermore, the authors focus on the particle size of delivery vehicles and briefly summarize how particle size affects the interaction between NADS and these biological interfaces, which further affects the fate and therapeutic efficacy of nucleic acid drugs in vivo, thereby providing some information and inspiration for the development of more efficient and safer delivery vehicles.
NADS must cross the various biological barriers, penetrate the thickness of the tumor tissue, reach the target tumor cell, facilitate the release of the cargo to the site of action, and ultimately regulate the causative gene (Figure 2). The significant barriers to the NADS include complex body fluid components, RES, tumor microenvironment, and cell or subcellular organelle membrane [16, 17]. Especially, for primary brain tumors, the BBB—the interface between the brain and the vascular system—restricts the influx of biomolecules from the vasculature to the brain parenchyma, allowing almost allows only small lipophilic molecules to enter by passive diffusion. The BBB is a major barrier to NADS to improve outcomes for brain cancer treatment [18].
NADS faces an immensely complex environment when administered in vivo, especially during blood circulation. Nucleic acid/vectors complex with positive charge often adsorbs plasma proteins on their surface to form an interfacial layer, called the protein corona [19, 20]. The composition and physicochemical properties of the vectors affect the interaction and binding position between the plasma proteins and the vectors; the plasma proteins will provide a “biological label” for the nucleic acid/vector complex, thereby affecting the organ targeting performance [21–23].
Also, the nano complexes with protein corona are easily captured by the RES, carried out by macrophages in the liver and spleen, and eliminated from the body by the renal, thus influencing bioavailability and delivery efficacy. Engineered vectors could prolong the blood circulation time of nano complexes and reduce their clearance [11, 20]. Subsequently, nano complexes may selectively extravasate and accumulate at the tumor site through the enhanced EPR effect or active transcytosis, while sparing healthy tissues.
Nonetheless, nano complexes then also face additional challenges: They need to be efficiently internalized by the tumor cells and rapidly escape from the endosome into the cytosol to release their loaded nucleic acid therapeutics. However, the endosomal escape efficiency of the clinically advanced lipid vectors is only 1–2%. Therefore, these barriers have seriously restricted the delivery efficiency of NADS in tumor sites and cells [24].
Nanoparticle (NP) size represents a critical parameter defining the blood circulation, biodistribution, tumor accumulation, and cellular uptake of NADS. Notably, NPs size profoundly influences their fate in vivo. For example, small NPs (< 5 nm) are extremely easy to pass through the renal filtration membrane and are eliminated by the kidney, whereas NPs greater than 10 nm can be enriched with tumors through EPR effects. Furthermore, NPs within the size range of 50–200 nm exhibit a propensity for spleen accumulation, likely due to “protein coronas” formed by proteins adsorbed on the surface of the NPs (Figure 3) [25].
Particle size is a key physicochemical property of NADS that directly affects the contact area of NADS interaction with blood components, thereby influencing protein corona formation and blood circulation time of NADS [26]. In general, the protein adsorption of NPs is size-dependent, especially for lipid-based vectors. Larger NPs with a larger volume tend to adsorb more proteins, resulting in greater protein coverage, while smaller NPs exhibit reduced interactions with blood proteins due to their higher surface curvature [27]. For instance, Cui et al. [28] found that the 150 nm automated LNPs had higher mRNA lacking efficacy and stronger serum adsorption ability during blood circulation than the smaller ones (70 nm), highlighting the contribution of particle size to protein corona formation and excellent mRNA delivery. This underscores the role of particle size in protein corona formation and efficient mRNA delivery. These automated LNPs could facilitate cytoplasmic mRNA encoding luciferase (Luc-mRNA) delivery and mediate efficient and prolonged luciferase expression in vivo. However, there are exceptions, as suggested by Partikel’s [29] study, which indicates that polymeric NPs exhibit protein adsorption independent of particle size within the 100–200 nm range. Therefore, the influence of the particle size of NADS on its protein crown adsorption is intricately related to the properties of the carrier itself.
Particle size also has a significant influence on the distribution and accumulation of NADS in tumors. Generally, larger NPs generally exhibit more excellent tumor accumulation and prolonged drug retention, while smaller ones exhibit better tumor penetration [30, 31]. For instance, Hu et al. [32] constructed a multifunctional polyamidoamine (PAMAM)-incorporated pH-sensitive liposome for specific co-delivery programmed cell death ligand 1 (PD-L1) siRNA and doxorubicin (DOX). This co-delivery system with suitable size (160 nm) maintains preferable stability in blood circulation, increases the accumulation of PD-L1 siRNA and DOX in breast cancer michigan cancer foundation-7 (MCF-7) tumor via the EPR effect, and exhibits significant MCF-7 tumor growth inhibition by efficiently preventing tumor immune evasion through the downregulation of PD-L1 expression and synergistic antitumor activity of DOX.
Younis et al. [33] constructed subminiature LNPs (usLNPs) composed of pH-sensitive lipids, phospholipids, and targeted peptides for selective delivery of the cytotoxic drug sorafenib (SOR) and siRNA targeting the Midkine gene (MK-siRNA) to mouse hepatocellular carcinoma (HCC). Through size optimization, achieving NPs of approximately 60 nm, NADS exhibits favorable pharmacokinetics and stability following intravenous administration, which can efficiently and selectively deliver siRNA to liver cancer cells, showing the most excellent selective gene knockout activity.
Furthermore, the optimized smaller NADS possess superior BBB penetration than larger NPs, making them promising therapeutic candidates for incurable and difficult-to-penetrate glioblastoma (GBM) treatment. Drawing on the median size of the brain extracellular space (pore size: 30–60 nm), Guo et al. [34] designed a brain-targeted siRNA delivery system based on 50 nm cationic lipid-polymer hybrid nanovectors with microbubble-enhanced focused ultrasound (MB-FUS) for treating brain tumors. This delivery system with MB-FUS could effectively traverse the brain extracellular space, leading to significantly enhanced siRNA accumulation in pediatric and adult preclinical brain tumor models. This delivery system with optimal particle size successfully delivered smoothened (SMO) protein targeting siRNA into medulloblastoma, inhibited SMO protein expression, and induced apoptosis of brain tumor cells, ultimately suppressing medulloblastoma growth. Similarly, Zou et al. [35] developed a super-small Angiopep-2 (Ang-2) functionalized siRNA nanocapsule [Ang-NCss (siRNA)] to promote siRNA-based GBM therapy. The Ang-NCss (siRNA) with a unique small size (25 nm) and Ang modification could boost efficient BBB permeation and enhance the superior drug accumulation and retention in GBM. Such Ang-NCss (siRNA) significantly inhibited the GBM cell (U87MG) grown with a favorable safety profile. Furthermore, Ang-NCss (siRNA) substantially improved the survival benefit of mice, providing a new strategy for GBM treatment (Figure 4).
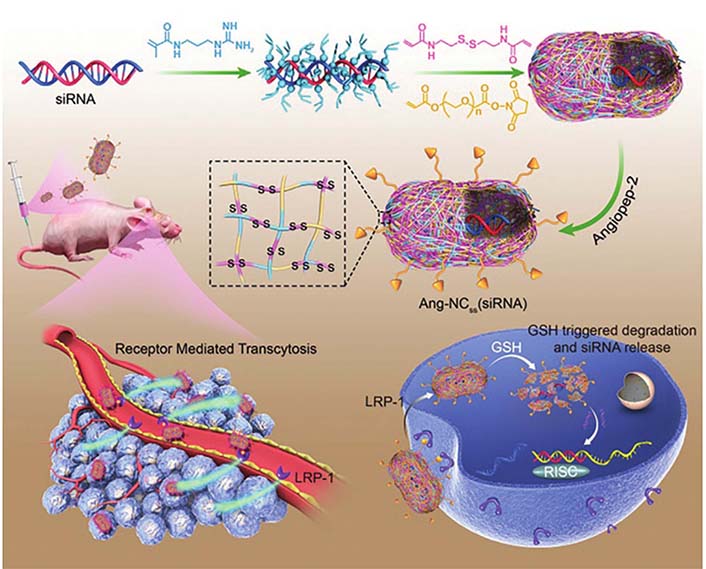
Illustration of Ang-NCss(siRNA) for GBM therapy due to efficient BBB penetration and gene silence. LRP-1: low-density lipoprotein receptor-related protein-1; GSH: glutathione; RISC: RNA-induced silencing complex
Note. Reprinted with permission from “Single siRNA nanocapsules for effective siRNA brain delivery and glioblastoma treatment,” by Zou Y, Sun X, Wang Y, Yan C, Liu Y, Li J, et al. Adv Mater. 2020;32:e2000416 (https://doi.org/10.1002/adma.202000416). © 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Exploiting the particle size dependence of nanocarriers through rational design is crucial for constructing ideal NADS with tumor-specific enrichment and penetration [25]. Based on this, Shi et al. [36] successfully engineered an acidity-triggered size-switchable NADS [mitoxantrone (MIT)/siR-PPA/PLM NP] to deliver indoleamine 2,3-dioxygenase 1 (IDO1) siRNA and MIT for tumor immunotherapy. The larger MIT/siR-PPA/PLM NP (140 nm) prolonged the blood circulation time, thereby enhancing the delivery of IDO siRNA and MIT into triple-negative breast cancer 4T1 tumors through the EPR effect due to the negatively charged PLM shell. Conversely, the positively charged smaller MIT/siR-PPA NPs (60 nm) enhanced intratumoral penetration of IDO siRNA and MIT by facilitating the detachment of PLM from the negatively charged MIT/siR-PPA/PLM NPs in tumor acidic pathological microenvironment (pH 6.8). This size-switchable delivery system, in turn, exerted synergistic anti-tumor effects against aggressive cancers through IDO1 pathway blockage by IDO1 siRNA and inducing immunogenic cell death by MIT (Figure 5A). Building upon similar principles, Dai et al. [37] developed a pH/reactive oxygen species (ROS) cascade-responsive prodrug micelle with size contraction and charge reversal characteristics to deliver tumor growth factor-beta (TGF-β) siRNA for collaborative tumor microenvironment reconstruction (Figure 5B). When exposed to a tumor acidic microenvironment, this nanosystem greatly improved endocytosis efficiency and tumor penetration depth through charge inversion and size reduction.
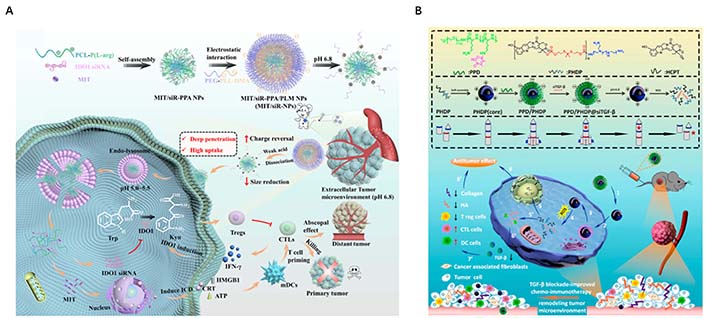
Rational design the size of NP to enhance tumor-specific enrichment and penetration. (A) Schematic illustration of size reduction leads to highly penetrable NPs (MIT/siR-PPA/PLM NPs, MIT/siR-NPs) based on immunogenic cell death and blockage of the IDO1 pathway for enhanced cancer immunotherapy; (B) synthesis routes and disassembly mechanism of PPD/PHDP@siTGF-β micelleplexes, and illustration of PPD/PHDP@siTGF-β drug delivery system for tumor therapy in vivo. ATP: adenosine triphosphate; HMGB1: high mobility group box-1 protein; IFN-γ: interferon-γ; CTLs: cytotoxic T lymphocytes; mDCs: myeloid dendritic cells; PCL-P(L-arg): poly( ε-caprolactone)-poly(L-arginine) micelles; PEG-PLL-DMA (PPD): 2,3-dimethylmaleic anhydride-grafted poly(ethylene glycol)-poly(L-lysine) copolymer; Trp: tryptophan; ICD: immunogenic cell death; CRT: calreticulin; HCPT: chemotherapeutics 10-hydroxycamptothecin; HA: hyaluronic acid; DC: dendritic cell
Note. Figure 5A was adapted with permission from “Blockage of the IDO1 pathway by charge-switchable nanoparticles amplifies immunogenic cell death for enhanced cancer immunotherapy,” by Shi M, Zhang J, Wang Y, Han Y, Zhao X, Hu H, et al. Acta Biomater. 2022;150:353–66 (https://doi.org/10.1016/j.actbio.2022.07.022). © 2022 Acta Materialia Inc. Elsevier Ltd.; Figure 5B was adapted with permission from “TGF-β blockade-improved chemo-immunotherapy with pH/ROS cascade-responsive micelle via tumor microenvironment remodeling,” by Dai L, Li X, Zheng X, Fu Z, Yao M, Meng S, et al. Biomaterials. 2021;276:121010 (https://doi.org/10.1016/j.biomaterials.2021.121010). © 2021 Elsevier Ltd.
Regulating the particle size of NADS can also facilitate organ targeting, such as kidney targeting. Exploiting the physiological characteristics of glomerular structures, including the effective filtration area of glomerular endothelial cells (10–70 nm), the mesangial supporting area (70–130 nm, further expanded to more than 200 nm under pathological conditions), and variations in pore size and permeability, NPs in blood circulation can infiltrate into the glomerular mesangial area through the intercellular space of glomerular endothelial cells. These NPs are passively deposited and retained in the mesangial area without further penetrating the glomerular basement membrane [38]. Based on this, Fang et al. [39] designed a novel kidney-targeted composite nanocarrier for TGF-β1 siRNA delivery. TGF-β1 siRNA was efficiently loaded into gold NPs by Au-S bond, and dexamethasone (DXMS) was dispersed in lipid bilayers to obtain double drug-loaded NPs [DXMS/siRNA@Au-immunoliposomes (ILs)] with the size of 123 nm. These DXMS/siRNA@Au-ILs can penetrate the glomerular endothelial intercellular space (70–130 nm) and enter the glomerular mesangial cell region, but cannot penetrate the glomerular basement membrane (10–70 nm), thereby achieving passive targeting of the glomerular mesangial cell region. This study verified that delivery systems with a particle size in the range of approximately 120 nm can selectively accumulate in the kidney. Thai et al. [40] prepared small-size DNA tetrahedron (STD) by self-assembling oligonucleotides modified with different sugar skeletons, which can be used for renal-targeted siRNA delivery (Figure 6A). The hydrodynamic size of STD is approximately 6 nm, allowing it to pass through the glomerular backbones (Figure 6B). The exudated NPs are filtered through the glomerular basement membrane, and subsequently flow through the renal tubules for excretion in the urine (Figure 6C). When the NPs have the absorption characteristics of renal tubular cells, they can be reabsorbed by renal tubular cells, resulting in their accumulation in the renal parenchyma. Additionally, Hu et al. [41] designed a new liposome formulation consisting of DNA and octa arginine peptide within the core of calcium phosphate, polyethylene glycol on the surface to ensure long circulation, galactose-targeted ligands were also incorporated to facilitate hepatocyte uptake. These 50 nm NPs exhibited rapid accumulation in hepatocytes, greatly enhancing gene expression.
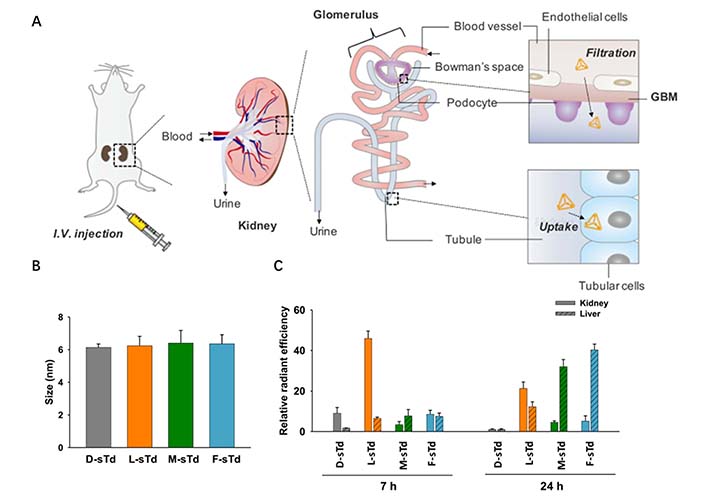
Regulating the particle size of NADS to facilitate organ targeting. (A) Intravenously injected small tetrahedrons (sTds) are filtered through the glomerular basement membrane and Bowman’s space to enter tubules where the nanoconstructs are taken up into tubular cells; (B) hydrodynamic sizes of sTds (1 μmol/L) in TM buffer measured by dynamic light scattering; (C) relative distribution levels of sTds in kidney or liver estimated at 7 and 24 h by fluorescence ex vivo imaging
Note. Adapted from “Kidney-targeted cytosolic delivery of siRNA using a small-sized mirror DNA tetrahedron for enhanced potency,” by Thai HBD, Kim KR, Hong KT, Voitsitskyi T, Lee JS, Mao C, et al. ACS Cent Sci. 2020;6:2250–8 (https://doi.org/10.1021/acscentsci.0c00763). © 2020 American Chemical Society.
The particle size of NADS determines the surface area available for nanoreactors to interact with the tumor cell membrane, which plays a partial but critical role in facilitating cellular uptake by tumor cells. These particles must be able to bind to the cell surface receptors. Smaller particles lack the ability to bind to a sufficient number of membrane surface receptors, hindering cell internalization. Conversely, larger particles pose challenges for cellular internalization. Therefore, the optimal size for NADS falls in the range of approximately 40–50 nm to facilitate cell internalization. For instance, Wei et al. [42] developed a transferrin-functionalized chimeric polymer [transferrin (Tf) @transferrin-binding peptide (TBP)-chimaeric polymersomes (CPs)-polo-like kinase 1 siRNA (siPLK1)] carrying polo-like kinase1-specific siRNA for triple-negative breast cancer (TNBC). The average size of Tf@TBP-CPs-siPLK1 is approximately 50 nm. Thanks to the existence of transferrin and the appropriate size of NPs, Tf@TBP-CPs-siPLK1 can be effectively internalized by human breast cancer cells (MDA-MB-231 cells) and has excellent intercellular effects in the endothelial monolayer model (Figure 7). Thus, the progression of the tumor was effectively inhibited, and the life span of mice was significantly prolonged.
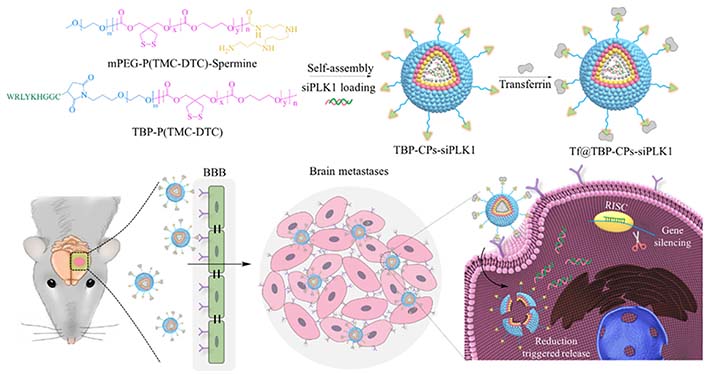
Illustration of the fabrication of Tf functionalized polymersomal siPLK1 (Tf@TBP-CPs-siPLK1), and the targeted delivery of siPLK1 to brain metastases in vivo. mPEG-P(TMC-DTC): poly(ethylene glycol)-b-poly(trimethylene carbonate-co-dithiolane trimethylene carbonate)
Note. Reprinted with permission from “Selective transferrin coating as a facile strategy to fabricate BBB-permeable and targeted vesicles for potent RNAi therapy of brain metastatic breast cancer in vivo,” by Wei Y, Sun Y, Wei J, Qiu X, Meng F, Storm G, et al. J Control Release. 2021;337:521–9 (https://doi.org/10.1016/j.jconrel.2021.07.048). © 2021 Elsevier B.V.
With the rapid advancement of gene therapy, NADS with high specificity and good safety have been increasingly exploited to overcome the unfavorable characteristics of nucleic acid therapeutics for precision cancer therapy. The size of NPs plays a pivotal role in shaping the interactions between NADS and the physiological environment. Current progress suggests that the optimal size design of NADS significantly affects protein corona formation, tumor distribution, cellular uptake, and efficiency in cancer treatment. Firstly, lipid-based NADS with a particle size of 100–150 nm is preferable to efficiently adsorb plasma proteins on their surface, which affects their organ-selective distribution. Secondly, the nanosize (50–100 nm) could enhance the tumor distribution of NADS due to the EPR effect. Furthermore, nanoscale particles falling within the 20–50 nm range are favored for their ability to readily traverse through the BBB and improve the delivery efficiency for brain cancer treatment. Finally, NPs ranging from 10–50 nm efficiently promote the internalization of NADS by cancer cells, which elicits potent antitumor activity. The authors could develop a size-switchable nucleic acid therapeutic delivery system to meet the different demands in diverse biological processes, according to specific molecular characteristics of tumors and their microenvironment. Thus, the rational design vectors and regulation of their NP sizes are critical to meeting the delivery requirement of nucleic acid therapeutics and achieving efficient cancer treatment.
Ang-NCss (siRNA): super-small Angiopep-2 functionalized siRNA nanocapsule
BBB: blood-brain barrier
CPs: chimaeric polymersomes
DOX: doxorubicin
DXMS: dexamethasone
EPR: enhanced permeation and retention
GBM: glioblastoma
IDO1: indoleamine 2,3-dioxygenase 1
LNP: lipid NP
MIT: mitoxantrone
mRNA: messenger RNA
NADS: nucleic acid delivery systems
NP: nanoparticle
PD-L1: programmed cell death ligand 1
PLK1: polo-like kinase 1
RES: reticular endothelial system
siRNA: small interfering RNA
TBP: transferrin-binding peptide
Tf: transferrin
TGF-β: tumor growth factor-beta
MY and JG equally contributed to: Investigation, Writing—original draft. WC: Writing—review & editing. XL: Conceptualization, Writing—review & editing, Supervision, Funding acquisition. DZ: Conceptualization, Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work was financially supported by the Key Program for International S&T Cooperation Projects of China [2018YFE0117800]; the State Key Laboratory of Natural Medicines at China Pharmaceutical University [SKLNMZZ202007]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
