Affiliation:
1State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Shanghai Engineering Research Center of Nano-Biomaterials and Regenerative Medicine, College of Biological Science and Medical Engineering, Donghua University, Shanghai 201620, China
ORCID: https://orcid.org/0009-0007-8555-318X
Affiliation:
1State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Shanghai Engineering Research Center of Nano-Biomaterials and Regenerative Medicine, College of Biological Science and Medical Engineering, Donghua University, Shanghai 201620, China
Affiliation:
1State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Shanghai Engineering Research Center of Nano-Biomaterials and Regenerative Medicine, College of Biological Science and Medical Engineering, Donghua University, Shanghai 201620, China
ORCID: https://orcid.org/0000-0002-1065-0854
Affiliation:
2Nanyang Ecological Environment Monitoring and Safety Center, Nanyang 473000, Henan, China
Affiliation:
1State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Shanghai Engineering Research Center of Nano-Biomaterials and Regenerative Medicine, College of Biological Science and Medical Engineering, Donghua University, Shanghai 201620, China
3CQM-Centro de Química da Madeira, Universidade da Madeira, 9020-105 Funchal, Portugal
Email: xshi@dhu.edu.cn
ORCID: https://orcid.org/0000-0001-6785-6645
Explor Drug Sci. 2023;1:454–467 DOI: https://doi.org/10.37349/eds.2023.00030
Received: July 30, 2023 Accepted: October 17, 2023 Published: December 27, 2023
Academic Editor: Fernando Albericio, University of KwaZulu-Natal, South Africa; University of Barcelona, Spain
With the continuous development of nanomaterials, nanofibers prepared by electrospinning have gradually occupied people’s vision because of their unique advantages, such as crisscross network and extracellular matrix-mimicking structure, high drug loading efficiency, and sustained release kinetics. Traditionally, electrospun fibers are mainly used as filter materials, wound dressings, and tissue engineering scaffolds, while their wide applications are limited to cancer nanomedicine applications due to their dense network structure. In recent years, two-dimensional fiber membranes have been transformed into short fibers that can be reconstructed to form fibrous rings or microspheres for cancer theranostics. Herein, this paper provides an overview of the recent advances in the design of electrospun short fibers that retain the advantages of nanofibers with good dispersibility for different nanomedicine applications, including cancer cell capture, cancer treatments, and cancer theranostics. The rational preparation of electrospun short fibers that are available to boost the development of nanomedicine is also discussed.
With the development of nanotechnology and the emergence of polymer materials [1–3], synthetic nanofibers are gradually known and widely used in catalysis, energy, biomedicine, and other fields [4, 5]. Among many preparation methods for synthetic nanofibers, electrospinning has been paid much attention because of its simple fast process and low cost [6, 7]. The electrospinning method possesses lots of advantages, such as low equipment cost, simple device, and easy operation. It can also be extensively utilized to obtain fiber scaffolds with nanometer-sized diameters under the set parameters [8, 9]. The orientation, structure, and morphology of fibers can be controlled to prepare composite/multifunctional nanofiber materials [10, 11]. A typical electrospinning device is mainly composed of a high-voltage (HV) power supply, an injection pump, a spinneret, and a receiver [12, 13]. When the polymer liquid (or melt) is pushed from the injection pump and passes through the spinneret, the electrostatic repulsion generated on the liquid surface overcomes the surface tension to make the liquid stretch into a cone shape to form a jet [14]. In the process of jet eruption, with the reduction of jet diameter and the volatilization of solvent, the final substances are solidified into a non-woven fiber mat collected on the receiver [15–17].
Most of the synthetic polymers with good biocompatibility and biodegradability, such as polycaprolactone (PCL), polylactic acid (PLA), and poly(lactic-co-glycolic acid; PLGA), as well as naturally occurring biopolymers including silk fibroin, chitosan, alginate, collagen, and gelatin, are commonly used as substrates to construct electrospun nanofibers for biomedical applications [18–20]. Due to their large specific surface area, high porosity, easy modification, and other excellent characteristics, electrospun nanofibers are used as filter materials to capture sol particles [21–23]. The improved and optimized hybrid nanofibers with good mechanical properties and cell response have been widely used in biomedical fields such as drug-controlled release, tissue engineering, and cancer research in recent years [24–27].
Although electrospun fibers have been extensively studied in the biomedical field, their small pore size limits the delivery of oxygen and nutrients and hinders the penetration of cells, suggesting that traditional two-dimensional (2D) structures are unable to fully simulate the 3D microenvironment that allows cells to survive [28, 29]. Moreover, these 2D structures fail to achieve the effect of minimally invasive treatment in the field of cancer treatment. Therefore, in order to solve the above problems, researchers have attempted to adjust the morphology and structure of the prepared short fibers (SFs) by adjusting the concentration of polymers, the propulsion speed of the injection pump, the receiving distance, and the voltage difference of electrospinning, or adopting various post-processing methods to endow nanofibers with good biocompatibility and unique therapeutic functions. Compared with 2D nanofibrous mats, electrospun SFs can be used as substrates to fabricate nanoskeletons with biomimetic structures and functional surface modifications.
In recent years, many novel nanofibers such as nanofiber hydrogels, nanofiber balls or rings, injectable SF drug carriers, and 3D nanofiber filling materials and other bionic materials have been developed to be used for tissue engineering, cancer therapy, and biosensors. Therefore, based on a comprehensive understanding of the SF systems, this article summarizes the preparation methods of electrospun SFs and their cancer nanomedicine applications in the areas of circulating tumor cell (CTC) capture, cancer therapy, and cancer theranostics in recent years, and discuss some of the key prospects of electrospun SFs in biomedical applications (Figure 1).
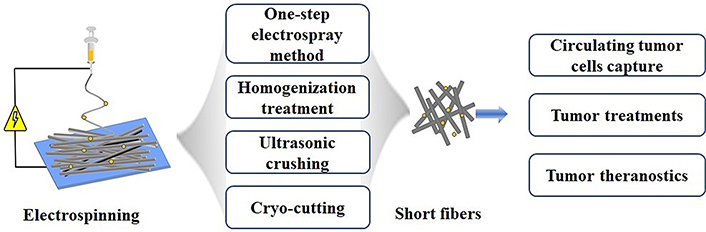
Schematic diagram of preparation of electrospun SFs and their cancer nanomedicine applications
Currently, SFs can be obtained by one-step electrospinning and subsequent processes. The subsequent processes include homogenization, ultrasonic crushing, and frozen sectioning. The most suitable method for the preparation of SFs must be selected according to the requirement of a specific biomedical application. Through different functionalizations, the designed SFs can be used in various biomedical applications, including food packaging, biosensing, cell/tissue engineering, and drug delivery.
The factors that affect the morphology of electrospun fibers include the relative molecular weight, concentration, viscosity, and solvent of polymers, and the viscosity has become the key factor [30, 31]. Generally, in the process of the one-step electrospray method, the polymers are difficult to extend to continuous slender fibers at a low viscosity of polymer solution, while breaking into SFs or spherical particles. The length of the prepared SFs can be changed by adjusting the voltage, flow rate, collection distance, and other parameters. The addition of micro-spherical silica particles to the polymer solution has a certain impact on the length of electrospun polymer fibers. Depending on the concentration of added silica particles, the morphology of nanofibers can be divided into four types: SFs, short bead fibers, short aggregated beaded fibers, and continuous bead fibers [27]. The average length of short nanofibers decreases from 52.4 μm to 15.3 μm with the increase of silica particle concentration from 0–8 wt% [27]. The nanofiber length is almost inversely proportional to the concentration of silica particles, suggesting that the length of the short nanofibers can be simply controlled by the amount of the added microspherical silica particles.
Although the one-step electrospray method possesses many advantages, such as simple operation and no subsequent secondary processing steps, there are still many variables in the electrospinning process, including the shape and size of fibers that are difficult to control, usually accompanied by the production of beaded fibers, liquid droplets, or spherical particles. Therefore, it is still necessary to optimize the processing conditions from other aspects to complete the preparation of SFs with desired properties.
Homogenization treatment aims to break the nanofiber membrane into SFs of a certain length with the help of the mechanical shear force generated by the homogenizing cutter head when running at a high speed. For example, Li et al. [32] prepared electrospun gelatin/PLA fibrous mats. The mats were then cut into small pieces, dispersed in tert-butanol solution, and then homogenized to ensure even dispersion, followed by high-speed centrifugation. In another research, Li et al. [33] prepared graphene/Fe3O4/PLGA short nanofibers with a length of 11.90 μm ± 2.03 μm and a diameter of 256.5 nm ± 13.7 nm by an in-situ deposition method after homogenizer treatment. In general, SFs with good uniformity can be prepared at a high rotation speed and long homogenization time. The type of dispersion and the hydrophilicity of fiber materials are also considered to be important factors affecting the size and morphology of SFs. When hydrophobic fibers are homogenized in water, hydrophobic substances such as hexane with a poor density need to be added to the aqueous solution to ensure the uniform dispersion of fibers.
The principle of the ultrasonic crushing method to prepare SFs is that the ultrasonic probe stimulates the liquid medium to produce dense small bubbles that can burst immediately to release energy and break the electrospun nanofiber membranes into SFs of micrometer length [34, 35]. The parameters of ultrasonic crushing include amplitude, frequency, and time. The increase in amplitude and time is conducive to the formation of SFs. It should be noted that during the ultrasonic process, higher power often leads to faster temperature elevation. Therefore, ultrasound crushing is usually carried out in ice baths to minimize the effect of temperature elevation on the mechanical properties or chemical properties of the created SFs. Furthermore, the toughness of electrospun fibers is an important factor affecting the ultrasonic preparation of SFs. Nanofibers with lower toughness are much easier to form SFs under ultrasound treatment than those with higher toughness. For instance, polystyrene (PS) nanofibrous mat with low toughness can be processed to form SFs with a length of 10.5 μm ± 6.2 μm after 60 s ultrasound treatment [34]. Fibrous mats with strong toughness are quite resistant to the deformation generated in the process of ultrasonic treatment and are difficult to form SFs directly and effectively from simple ultrasonic crushing. Additionally, the tendency of polymer nanofibers to become SFs is largely dependent on the ductility of the polymer during the ultrasound treatment. Brittle electrospun polymer films, such as PS and polymethyl methacrylate, are prone to form SFs with a length of about 10 microns [34]. Lastly, both the initial diameter of the fibers and the degree of nanofiber alignment for the electrospun fibrous mat affect the final length of the resulting SFs. For instance, electrospun nanofibrous mats composed of ductile polymers like poly(l-lactide) and poly(acrylonitrile) require additional treatments such as interaction with surfactants prior to sonication to make them conducive to the sonication-based cleavage [36].
To prepare SFs through cryo-cutting, the fiber films are generally folded to a width of 1~2 cm, frozen with the embedding agent, and fixed in the frozen slicer before the cryo-cutting. The fiber films are cut into slices with a certain thickness in the direction perpendicular to the fiber orientation and finally dispersed in a solution to obtain SFs. Compared with the methods of electrospray and homogenization mentioned above, the most obvious advantage of cryo-cutting is that the length of SFs can be easily set to obtain a uniform morphology. In a recent study, Wei et al. [37] dipped the electrospun PLGA fiber mats into water, let them be frozen, sectioned them at a thickness of 50 μm, and then ultrasonically dispersed them to form SFs. In another study, Omidinia-Anarkoli et al. [38] prepared aligned electrospun PLGA fibers containing magnetic particles with a diameter of 689.7 nm ± 88.5 nm, which were then cut into SFs with four different lengths at 25.5 μm ± 1.8 μm, 51.1 μm ± 2.8 μm, 78.5 μm ± 1.2 μm, and 101.1 μm ± 5.0 μm, respectively.
Generally speaking, cryo-cutting is often adopted to prepare SFs when the glass transition temperature of polymers is lower than room temperature. Ultrasonic crushing method using a simple mechanical shear force hardly overcomes the viscoelasticity of fibers to make them fracture, while the cryo-cutting method can effectively break the fibers. However, the preparation of SFs by cryo-cutting has the defects of time-consuming, high cost, and poor dispersion. This reflects that cryo-cutting has certain requirements on the thickness, mechanical strength, and wettability of the oriented nanofibers.
Electrospun SFs have been designed to be used for various cancer nanomedicine applications, such as rapid capture of CTCs, efficient cancer therapy, and cancer theranostics. Here, we briefly discuss some of the key developments in this area (Table 1).
Summary of platforms for tumor diagnosis and treatment with representative electrospun SFs
| Application | Platforms | Method | Cell models | Mechanism of action | References |
|---|---|---|---|---|---|
| CTC capture | Fe3O4@PEI/PVA-Apt SFs | Homogenization treatment | MCF-7 cells | DNA Apt-based ligand-receptor binding to capture breast cancer cells in vitro | [23] |
| Tumor therapy | PDA-PLA SFs | Cutting and filtering | B16F10/T cells | Immunotherapy of melanoma | [10] |
| Coumarin-PELA containing fiber rods | Ultrasonication treatment | 4T1 and RAW 264.7 | Chemotherapy of breast cancer | [36] | |
| DOX@LDH/α-TOS/PLGA SFs | Homogenization treatment | MCF-7/ADR cells | Chemotherapy of breast cancer cells in vitro | [39] | |
| Tumor theranostics | DOX@PLGA-PEI-Gd/Apt | Homogenization treatment | 4T1 tumor cells | Chemotherapy and MR imaging of breast cancer | [40] |
| DOX-PLGA-PEI-DTPA-Gd/HA fiber microspheres | Homogenization treatment | 4T1 tumor cells | Chemotherapy and MR imaging of breast cancer | [41] |
Apt: aptamer; DOX: doxorubicin; DTPA: diethylenetriaminepentaacetic dianhydride; Gd: gadolinium; HA: hyaluronic acid; LDH: layered double hydroxide; MR: magnetic resonance; PDA: polydopamine; PEI: polyethylenimine; PELA: polyethylene glycol PLA; PVA: polyvinyl alcohol; α-TOS: α-tocopheryl succinate
CTCs refer to cancer cells that detach from tumors, enter the circulatory system and various organs of the body with blood, and become the main cause of cancer metastasis. Therefore, the detection of CTCs in the peripheral blood is vital for early cancer diagnosis and prognosis after treatment. However, the concentration of CTCs in a large number of blood cells is extremely low, hence capturing CTCs rapidly and efficiently in blood samples is still challenging.
Establishing a platform for efficient screening and collection of CTCs is an important strategy to improve the survival rate of diagnosed patients. Numerous studies have demonstrated that electrospun nanofibers can improve the accuracy and sensitivity of specific CTC detection due to the biomimetic structure of the porous network and surface modification [23, 42]. Recently, SFs have also been developed as a capture platform for CTCs. In our previous work, we prepared Apt-modified magnetic short nanofibers (MSNFs) with magnetic response and targeting recognition functions (Figure 2A) [23]. MSNFs could effectively capture cancer cells with the assistance of an external magnetic field, and the capture efficiency reached about 87%, while the capture efficiency of white blood cells (WBCs) was only 2%, showing the specific capture performance of Apt-modified MSNFs (Figure 2B). The cancer cell capture ability can be visually demonstrated by scanning electron microscopic (SEM) observation of MCF-7 cells (a human breast cancer cell line) that are surrounded by MSNFs (Figure 2C). In addition, the universality of Apt-modified MSNFs was verified by capturing a variety of epithelial cell adhesion molecule-positive MCF-7, A549, and HepG2 cells due to the Apt modification on the surface of the MSNFs. After the decomposition of DNA Apt by Benzonase nuclease, the captured CTCs can be released with an efficiency of up to 90% and still have desired viability, which is convenient for subsequent monitoring and analysis of CTCs. Finally, a comparative study with commercial magnetic beads (MBs) showed that the capture efficiency of Apt-modified MSNFs (87%) is more or less equivalent to that of MBs (91%), while the CTC release efficiency of Apt MSNFs is significantly higher than that of MBs, proving the great potential of short nanofibers in CTC capture and release for cancer diagnosis applications [23].
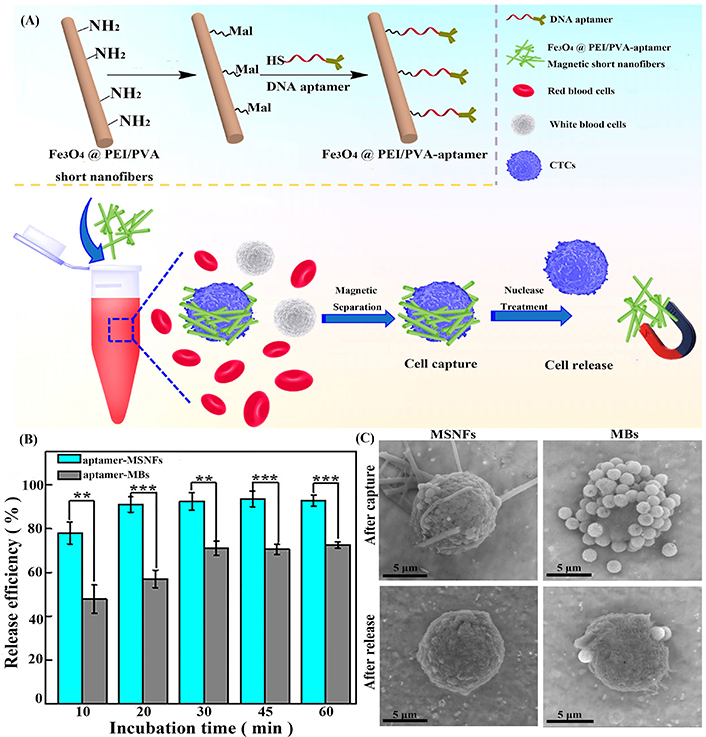
Preparation of composite SF materials and evaluation of their release efficiency on tumor cells. (A) Schematic illustration of the surface modification of MSNFs for cancer cell capture and release applications; (B) capture efficiency of MCF-7 cells and WBCs after incubation with Apt-MSNFs for different time periods; (C) SEM images of MCF-7 cells after capture by (I) Apt-MSNFs and (II) Apt-MBs and after release treatment (III) to remove the MSNFs and (IV) to remove the MBs, respectively. Mal: maleimide; -SH: sulfhydryl
Note. Adapted with permission from “Design of DNA aptamer-functionalized magnetic short nanofibers for efficient capture and release of circulating tumor cells,” by Xiao Y, Lin L, Shen M, Shi X. Bioconjugate Chem. 2020;31:130–8 (https://pubs.acs.org/doi/10.1021/acs.bioconjchem.9b00816). © American Chemical Society 2020.
Chemotherapeutic drugs can be loaded onto nanomaterials through physical encapsulation, non-covalent interaction, and covalent bonding to improve their water solubility and bioavailability and achieve effective chemotherapy due to the adjustability of size, shape, and surface characteristics of nanomaterials. In this regard, electrospun SFs meet the development requirements of most chemotherapeutic drug carriers. For instance, Ma et al. [39] successfully constructed dual drug-loaded short nanofibers as a drug delivery system for efficient chemotherapy of multidrug-resistant (MDR) cancer cells (Figure 3A). The DOX-loaded LDH nanodisks were incorporated into the α-TOS-doped PLGA nanofibers, which were then homogenized to form a SF-based drug delivery system with an average diameter of 830.2 nm and a mean length of 17.4 μm ± 7.3 μm. The short nanofibers display a pH-dependent multistage sustained drug release profile with a fast α-TOS release (Figure 3B) and a slow DOX release (Figure 3C). Due to the unique design, DOX has a more extended diffusion distance than α-TOS. Drug-free LDH/PLGA SFs have good cytocompatibility and can be taken up by MDR cancer cells. The dual drug-loaded LDH/PLGA SFs could effectively inhibit the growth of cancer cells in vitro through different mechanisms.
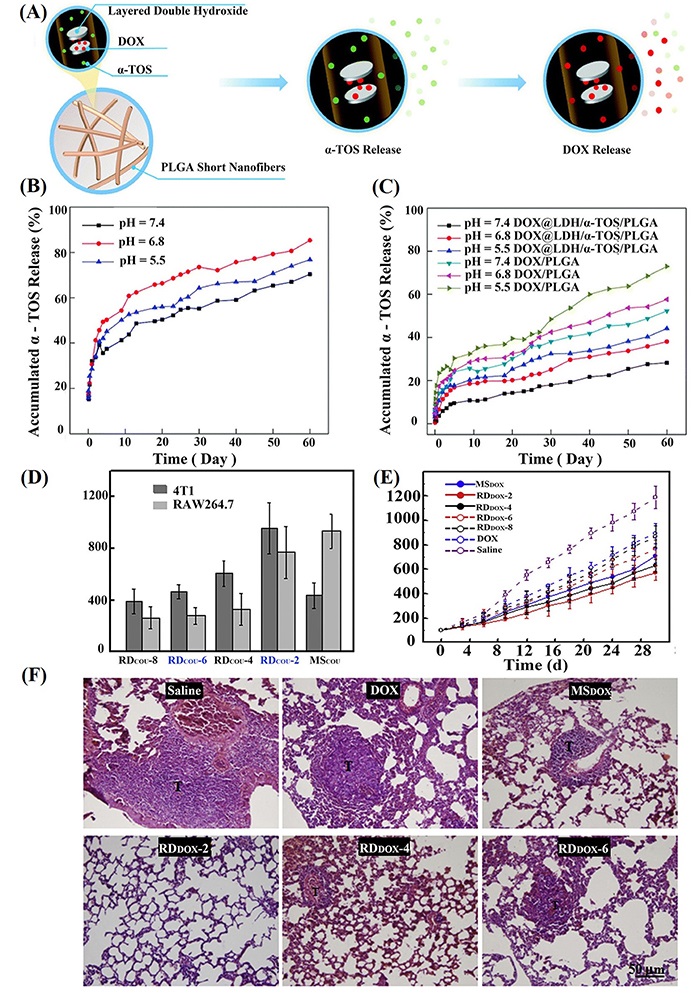
Preparation and drug release of drug-loaded SFs, as well as evaluation of in vitro and in vivo properties. (A) DOX@LDH/α-TOS/PLGA short nanofibers for the treatment of MDR cancer cells; in vitro release of α-TOS (B) and DOX (C) from the DOX/PLGA or DOX@LDH/α-TOS/PLGA short nanofibers with the same α-TOS or DOX amount; (D) the uptake amount of RDCOU with different lengths and MSCOU by 4T1 and RAW 264.7 cells, normalized to the total proteins of the cell lysate (n = 5); (E) tumor growth of 4T1 tumor-bearing mice after intravenous administration of free DOX, RDDOX with different lengths and MSDOX, using saline injection as the control (n = 6); (F) HE staining images of lung sections retrieved on day 21 after intravenous administration of free DOX, RDDOX with different lengths and MSDOX, using saline injection as the control. “T” represents metastatic tumors. HE: hematoxylin-eosin; MSCOU: coumarin-loaded poly (styrene-maleic anhydride; PSMA) microsphere; MSDOX: DOX-loaded PELA microsphere; RDCOU: fiber rod containing coumarin; RDDOX: DOX-loaded fiber rod
Note. Figure 3A, B, and C was adapted with permission from “LDH-doped electrospun short fibers enable dual drug loading and multistage release for chemotherapy of drug-resistant cancer cells,” by Ma Y, Li D, Xiao Y, Ouyang Z, Shen M, Shi X. New J Chem. 2021;45:13421–8 (https://doi.org/10.1039/D1NJ02159A). © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2021. Figure 3D, E, and F was adapted with permission from “Shape effects of electrospun fiber rods on the tissue distribution and antitumor efficacy,” by Zhang H, Liu Y, Chen M, Luo X, Li X. J Control Release. 2016;244:52–62 (https://doi.org/10.1016/j.jconrel.2016.05.011). © 2016 Elsevier B.V.
In another work, Zhang et al. [36] loaded coumarin 6 into PSMA and obtained RDCOU by electrostatic spinning and ultrasonic crushing. At the same time, PELA was used to construct RDDOX with different lengths. Then, MSDOX and MSCOU with a diameter close to the fiber diameter were prepared by the lotion method. The authors investigated the effect of the shape of drug-loaded electrospun SFs on the efficiency of anticancer therapy [36]. As shown in Figure 3D, compared with RDCOU-2 and RDCOU-4, the uptake of MSCOU by 4T1 cells (a murine breast cancer cell line) is significantly lower. The uptake of MSCOU by RAW 264.7 cells (a murine macrophage cell line) is 2.5 times higher than those of RDCOU-4, RDCOU-6, and RDCOU-8, and slightly higher than that of RDCOU-2. In addition, in 4T1 and RAW 264.7 cells, RDCOU-6 and RDCOU-8 with a longer length have a lower cellular uptake, likely due to the large amount of energy required for the endocytosis of particles with a high aspect ratio. In vivo treatment of a 4T1 tumor-bearing mouse model reveals that the mice experience rapid tumor growth after 30 days in the saline group, showing a significant difference compared with the free DOX group. In comparison with free DOX, MSDOX and RDDOX significantly inhibit tumor growth due to the slow release of DOX from the microspheres and SFs to tumors, thus having increased DOX bioavailability (Figure 3E). In addition, compared with the saline group, the number of lung metastatic nodules in the MSDOX and RDDOX groups decreased significantly. Especially, there is no obvious tumor metastasis in RDDOX-2 group, indicating that drug-loaded fiber segments with a shorter length possess a higher tumor cytotoxicity than the counterpart materials with a longer length (RDDOX-4, RDDOX-6, and RDDOX-8 groups, see Figure 3F). That is to say, the preparation of effective drug carriers with improved tumor treatment efficacy can be achieved by varying the shape of SFs.
In addition to delivering chemotherapy drugs, short nanofibers have also been designed to efficiently deliver cytokines to inhibit the occurrence and development of tumors. For instance, a sustainable and recyclable delivery system has been designed using short injectable polymer fibers to encapsulate interleukin-2 (IL-2) and IL-15, which can be retained at the tumor site after injection [10]. The electrospun PLA fibrous mats were dispersed in an ethanol/water mixture solution to increase the space between fibers. Then, the dispersed PLA fibers were immobilized with tyrosinase through covalent linking for subsequent enzymatic coating of PDA, onto which streptavidin (SA) and biotinylated protein G (Bio-PtG) were serially bound. Then, the protein G-bound PLA fibers were cut into injectable short PLA (sPLA) with a length of 15–100 μm obtained by filtration. The constructed sPLA fibers were loaded with cytokines through the interaction between protein G and fragment crystallizable, and intratumorally injected. The cytokine-loaded sPLA could effectively inhibit tumor growth by up to 70% through reactivation of nonfunctional T cells to a functional state that can kill tumors in a sustainable and recyclable manner. The injectable sPLA fiber platform can be considered as a carrier to efficiently deliver a variety of immune drugs for cancer treatment.
Besides the therapeutic functions achieved, electrospun SFs can also be designed as a theranostic nanoplatform for simultaneous tumor imaging and therapy. In our recent work, we have shown that DOX-loaded electrospun PLGA microfibrous rings can be formed under a homogenization process in aqueous solution with a high ionic strength ([NaCl] = 2 mol L–1), while under the same condition but without salt, only PLGA SFs can be formed [40]. The generated microfiber rings can be further decorated with PEI partially modified with Gd chelates and targeting ligand DNA Apt through the linkage of 3-(maleimido) propionic acid N-hydroxysuccinimide ester (NHS-Mal) between thiol end group of DNA Apt and the PEI amines (Figure 4A). The prepared microfiber rings have an average diameter of 11.1 μm and fiber diameter of 1.69 μm, possess good biocompatibility, pH-dependent long-term DOX release characteristics, and high r1 relaxivity (4.46 mmol L−1s−1). The experimental results in vivo confirmed that the microfibrous rings can effectively inhibit the growth of tumors, and the therapeutic effect of the targeted group is better than that of the non-targeted group. As shown in Figure 4B, compared with the non-targeted group, the DNA Apt-functionalized fibrous ring (DOX@PLGA-PEI-Gd/Apt) can be retained in the tumor region for a long time so as to achieve MR imaging-guided tumor therapy (Figure 4C). Due to the anchoring effect of the microfibrous rings, simultaneous reduction of the shedding of tumor cells to form CTCs in the blood can also be achieved to simultaneously inhibit tumor metastasis.
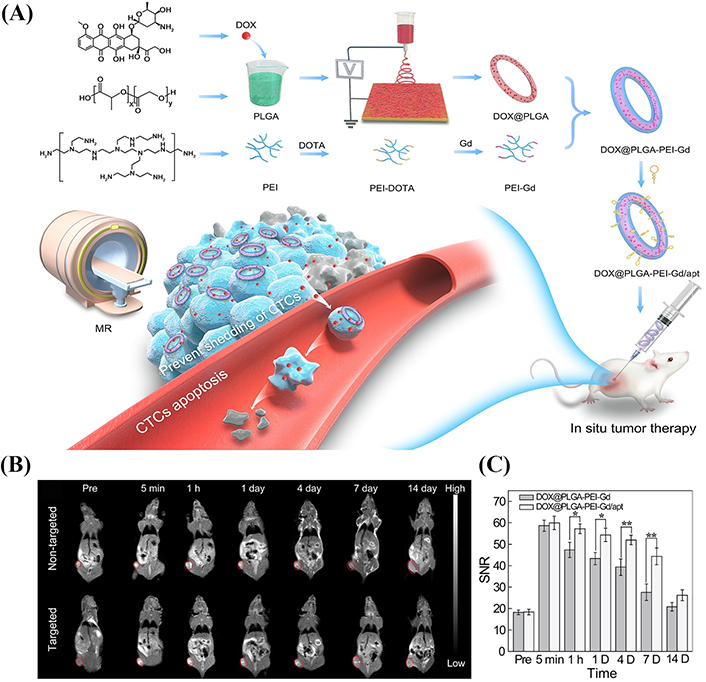
Preparation of drug-loaded PLGA fiber rings for MR imaging and tumor treatment. (A) Schematic illustration of the preparation and surface modification of DOX@PLGA fibrous rings for simultaneous tumor therapy and metastasis inhibition; (B) T1-weighted MR images; (C) the corresponding MR SNR of the 4T1 tumor before injection and at different time points post-injection of the DOX@PLGA-PEI-Gd and DOX@PLGA-PEI-Gd/Apt ([Gd] = 5 mmol L–1, in 100 μL NS for each mouse), respectively (mean ± SD, n = 3). The tumor region was tagged with red dotted circles. The MR signal intensity of blank (air) was used as the background (noise). DOTA: 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid; NS: normal saline; SD: standard deviation; SNR: signal-to-noise ratio
Note. Adapted with permission from “Multifunctional PLGA microfibrous rings enable MR imaging-guided tumor chemotherapy and metastasis inhibition through prevention of circulating tumor cell shedding,” by Xiao Y, Fan Y, Tu W, Ning Y, Shi X. Nano Today. 2021;38:101123 (https://doi.org/10.1016/j.nantod.2021.101123). © 2021 Elsevier Ltd.
The 2D microfibrous rings have been demonstrated as a powerful platform for tumor theranostics. In order to further improve the tumor theranostic efficacy, 3D microspheres composed of short electrospun PLGA nanofibers have been prepared for local tumor drug delivery and MR imaging in our recent work [41]. First, electrospun PLGA fibrous mats prepared were homogenized to obtain the PLGA SFs, which were then covalently linked with DTPA-PEI conjugate. Followed by Gd3+ chelation and HA surface modification, multifunctional PLGA-PEI-DTPA-Gd/HA SFs were obtained. Then, an aqueous dispersion containing 0.1 wt% gelatin, PLGA-PEI-DTPA-Gd/HA SFs, and DOX were electrosprayed and cross-linked to create the multifunctional drug-loaded microspheres [DOX-PLGA-PEI-HA (PGH), Figure 5A]. The prepared DOX-PGH microspheres were comprehensively characterized to have a fiber diameter of 1.7 μm and a whole size of 118.8 μm, respectively, and r1 relaxivity of 17.7 mmol L−1s−1, nearly four times higher than that of Magnevist (4.56 mmol L−1s−1). Meanwhile, the DOX-PGH microspheres show a sustained DOX release profile to achieve accumulated DOX release of 52.2% at 120 h, thus facilitating long-term tumor local drug delivery applications. With the HA-mediated targeting and anchoring to tumor cells overexpressing CD44 receptors, the developed DOX-PGH microspheres enabled targeted tumor MR imaging with imaging contrast enhancement lasting for 5 days (Figure 5B). These results strongly proved that the prepared HA-functionalized fiber microspheres could stay at the tumor site for a long time and be more firmly anchored to the tumor cells through HA binding to the CD44 receptor on the tumor cell surface, which is conducive to DOX accumulation at the tumor site and tumor metastasis inhibition. After local treatment, the tumor volume of mice in the DOX@PGH group is smaller than other groups, indicating the best anti-tumor effect of DOX@PGH (Figure 5C), along with the best inhibition of lung metastasis as reveals HE staining. Overall, the developed DOX@PGH can be used as a promising local drug delivery system with long-term local chemotherapy, tumor metastasis inhibition, and MR imaging functions.
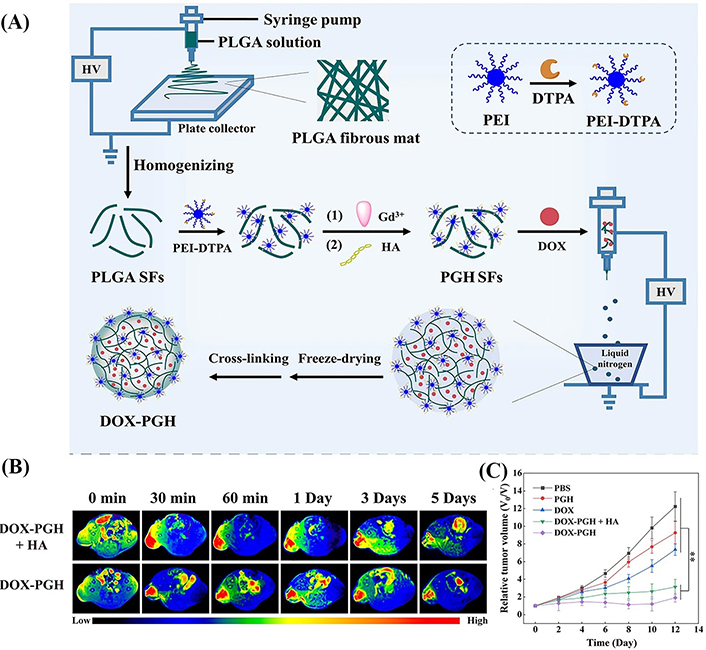
The synthesis route and in vivo treatment efficiency of drug-loaded fiber microspheres. (A) Schematic diagram of the preparation of DOX-PGH microspheres; (B) T1-weighted MR images of 4T1 tumors at different time points postinjection of DOX-PGH + HA or DOX-PGH; (C) relative tumor volume of 4T1 tumor-bearing mice as a function of time post-treatment in different groups (n = 6). PBS: phosphate buffered saline
Note. Adapted with permission from “Tumor-anchoring drug-loaded fibrous microspheres for MR imaging-guided local chemotherapy and metastasis inhibition,” by Zhang B, Gao Y, Yang R, Ouyang Z, Yu H, Wang H, et al. Adv Fiber Mater. 2022;4:807–19 (https://doi.org/10.1007/s42765-022-00137-8). © 2022, Donghua University, Shanghai, China.
In summary, we reviewed the recent progress in the design of electrospun SFs for cancer nanomedicine applications. Electrospun SFs can be generally created through methods such as one-step electrospray of polymer solution, homogenization treatment, ultrasonic crushing, or cryo-cutting of fibrous mats to form aqueous colloidal suspension with desired stability and injectability. Due to the fact that electrospun SFs can be encapsulated with anticancer drugs or imaging agents such as iron oxide nanoparticles, can be further surface-functionalized or immobilized with various biological molecules, or can be further processed to create superstructured fibers such as 2D fibrous rings or 3D fibrous microspheres, a range of cancer nanomedicine applications can be rendered using them as a universal platform including capture of CTCs, tumor chemotherapy, tumor immunotherapy, and tumor theranostics (Table 1).
Although some great progresses have been achieved in the design of electrospun short nanofibers for cancer nanomedicine applications, there are still many challenges and rooms that deserve further development. For instance, the current approaches to load drugs are just based on prior encapsulation of the drugs within the fibrous mats for subsequent SF processing or mixing drugs with SFs for further electrospray. In order to further improve the drug loading and release, coaxial or emulsion electrospun nanofibrous mats may also be used to load drugs for the subsequent formation of SFs. Drugs may also be covalently grafted on the surface of SFs through tumor microenvironment (TME)-sensitive bonds to realize tumor-specific drug release for improved drug bioavailability. Further, different therapeutic components such as chemotherapeutic drugs, photothermal agents, photodynamic drugs [42], immune activators, or genetic materials may be simultaneously incorporated within electrospun SFs, thus providing a range of opportunities for combination tumor therapy applications. What’s more, electrospinning may also be combined with microfluidic technology to provide more opportunities in terms of materials processing for tumor interventional therapy. Overall, much effort is still needed to electrospun SFs as a universal platform for cancer nanomedicine applications.
2D: two-dimensional
Apt: aptamer
CTC: circulating tumor cell
DOX: doxorubicin
DTPA: diethylenetriaminepentaacetic dianhydride
Gd: gadolinium
HA: hyaluronic acid
LDH: layered double hydroxide
MBs: magnetic beads
MDR: multidrug-resistant
MR: magnetic resonance
MSCOU: coumarin-loaded poly (styrene-maleic anhydride) microsphere
MSDOX: doxorubicin-loaded polyethylene glycol polylactic acid microsphere
MSNFs: magnetic short nanofibers
PEI: polyethylenimine
PELA: polyethylene glycol polylactic acid
PGH: poly(lactic-co-glycolic acid)-polyethylenimine-hyaluronic acid
PLA: polylactic acid
PLGA: poly(lactic-co-glycolic acid)
RDCOU: fiber rod containing coumarin
RDDOX: doxorubicin-loaded fiber rod
SFs: short fibers
sPLA: short polylactic acid
α-TOS: α-tocopheryl succinate
YH: Software, Writing—original draft. MZ: Writing—original draft. MS: Supervision, Resources, Project administration, Writing—review & editing. LZ: Writing—review & editing. XS: Supervision, Resources, Funding acquisition, Project administration, Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This study is financially supported by the National Natural Science Foundation of China [52350710203]; the Science and Technology Commission of Shanghai Municipality [23520712500, 21490711500, 20DZ2254900]; the Shanghai Education Commission through the Shanghai Leading Talents Program; and the 111 Project [BP0719035]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
