Affiliation:
NGO Praeventio, 50407 Tartu, Estonia
Email: katrin.sak.001@mail.ee
ORCID: https://orcid.org/0000-0003-0736-2525
Explor Drug Sci. 2023;1:287–291 DOI: https://doi.org/10.37349/eds.2023.00019
Received: April 05, 2023 Accepted: June 07, 2023 Published: August 30, 2023
Academic Editor: Marcello Iriti, Milan State University, Italy
Flavonoids present a large group of natural polyphenols with numerous important health benefits for preventing and treating a diverse variety of pathological conditions. However, the actual therapeutic use of these phytochemicals is impeded by their low oral bioavailability. In this commentary article, an interesting paradox is presented: while the ingested flavonoid glycosides can be absorbed by means of sodium-dependent glucose transporters (SGLTs; SGLT1) located in the brush border membrane facing the lumen of the small intestine, certain flavonoid aglycones are able to inhibit these shuttle proteins. It is expected that avoiding the co-intake of such SGLT1 inhibitors concomitantly with flavonoid-rich foods might provide a new option for enhancing the oral bioavailability of flavonoids, thereby preventing the transport of unabsorbed compounds to the large intestine and conversion into catabolites by the colonic microbiota. Altogether, the administration of flavonoids in appropriate combinations is highlighted for getting the maximal health benefits from consuming these bioactive compounds.
Over the recent decades, increasing evidence has suggested multifaceted health benefits of plant-derived polyphenolic compounds, especially from the class of flavonoids. These phytochemicals, including more than 8,000 structurally different substances, can be found in numerous fruits and vegetables, medicinal herbs, nuts, and grains [1]. Their antioxidant, antiinflammatory, antimicrobial, anticancer, cardioprotective, and neuroprotective activities have been well described in a wide array of experimental model systems, but also in several epidemiological studies [2, 3].
In plants, flavonoids typically exist in the form of glycosides, meaning that most of the ingested flavonoids present as different types of sugar conjugates [4]. Within the gastrointestinal tract, the absorption of flavonoids is associated with the cleavage of sugar moiety under the action of a cascade of hydrolyzing enzymes. In this way, lactase phloridzin hydrolase (LPH) catalyzes the cleavage of sugar units in the small intestine, whereupon the released aglycones enter the epithelial cells by means of passive diffusion [5, 6]. An alternative hydrolytic step is mediated by a cytosolic β-glucosidase, necessitating the prior transport of flavonoid glycosides from the intestinal lumen into epithelial cells. This process is carried out by sodium-dependent glucose transporters (SGLTs), SGLT1, expressed in the brush border membrane facing the lumen [5, 6] (Figure 1). Once absorbed, flavonoids undergo an extensive bioconversion (phase II metabolism) before entering the systemic circulation and the target tissues to exert their various advantageous bioactivities [5]. However, unabsorbed flavonoids travel down the gastrointestinal tract reaching the large intestine, where they are exposed to the resident microbiota. Under the action of colonic bacteria, flavonoid glycosides and other conjugates are further hydrolyzed, ultimately leading to a wide variety of low molecular weight catabolites (mostly phenolic acids) which can be effectively absorbed and reach the bloodstream after undergoing phase II metabolism [5]. Therefore, the respective deglycosylation pattern of certain flavonoids usually depends on the nature of aglycone but also the linked sugar groups. For example, rhamnose-linked flavonoids can be hydrolyzed only by α-rhamnosidases in the large intestine, secreted by colonic microbiota [6]. Altogether, the oral bioavailability of flavonoids is very low, being importantly affected by membrane transporters, metabolic enzymes, and intestinal microbiota, representing a great obstacle to getting the maximal health benefits from the intake of food products rich in these natural agents [6]. In recent years, several novel strategies have been proposed to improve the oral bioavailability of flavonoids, including the use of absorption enhancers and inclusion complexes, such as mucoadhesive and mucoadhesive guided systems [7, 8]. In addition, changing the absorption site from the large intestine to the small intestine has been suggested to be another possibility for promoting the bioavailability of some flavonoids [6]. Actually, substituting the rutinose moiety in poorly absorbed hesperidin molecule (hesperetin-7-O-rutinoside) with a glucose group resulting in hesperetin-7-O-glucoside was demonstrated to be accompanied by a significant increase in the oral bioavailability of hesperetin in humans [6].
The membrane transporter protein SGLT1 is primarily expressed in the small intestine [9, 10]. Under physiological conditions, SGLT1 is essentially involved in the active transport of glucose, derived from a carbohydrate-rich diet, across the intestinal brush border membrane into the enterocytes, thereby contributing to the regulation of postprandial blood glucose levels [9–12]. Somewhat surprisingly, in recent years, the ability of certain flavonoid aglycones to inhibit SGLT1 has been demonstrated in several preclinical in vitro and in vivo models, leading to a decrease in glucose absorption and lowering postprandial blood glucose levels [11–15]. For example, green tea flavanols such as catechin, epigallocatechin (EGC), epicatechin 3-O-gallate (ECG), and epigallocatechin 3-O-gallate (EGCG) reduced glucose absorption by competitive inhibition of the intestinal SGLT1 in vitro, revealing also some ability to retard the intestinal absorption of glucose in healthy subjects [13, 16, 17]. Likewise, the citrus flavanone naringenin was found to suppress the uptake of glucose in everted rat intestinal sleeves through the inhibition of SGLT1 [13, 18, 19], while the flavonoid-rich extract from grapefruit repressed SGLT1-mediated glucose uptake in human intestinal Caco-2 cells and revealed a decrease in postprandial blood glucose level in maltose-fed rats [16]. Moreover, based on in vitro studies, soybean extract containing the isoflavones daidzein and genistein suppressed glucose absorption into the intestinal brush border membrane vesicles of rabbits [15]. Although these findings may provide a promising novel strategy for controlling diabetic hyperglycemia, the ability of flavonoids to inhibit SGLT1 poses another critical question, i.e., could specific flavonoids impede also the absorption of flavonoid glycosides in the small intestine, thereby suppressing the oral bioavailability of these bioactive phytochemicals (Figure 2)? If it is true, it would be prudent to avoid the consumption of these SGLT1 inhibitors together with flavonoid-rich food products, for example, to prevent the co-intake of vegetables and green tea or drinking citrus juice concomitantly with flavonoid supplements.
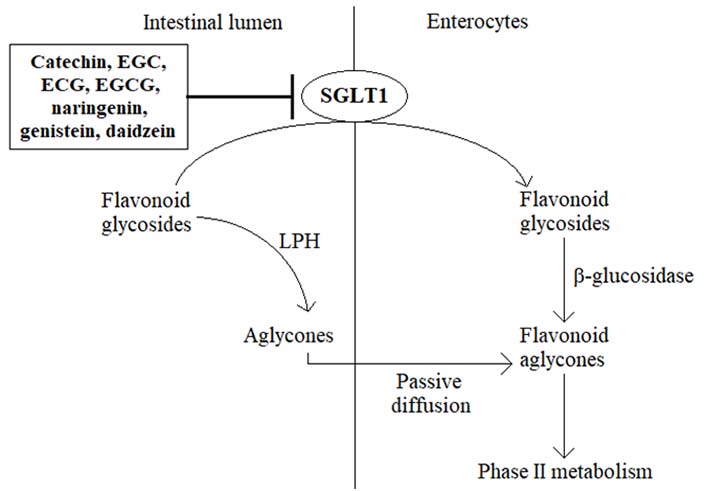
Hypothetical mechanism of the role of SGLT1 inhibition on the low bioavailability of flavonoids
In summary, flavonoids are abundant natural compounds with a huge range of different beneficial activities, relevant to both health as well as disease. However, the low oral bioavailability of these natural polyphenols has still remained a great challenge impeding their potential therapeutic use. In addition to the current strategies developed for enhancing the bioavailability of flavonoids, another important approach might involve the intake of these phytochemicals in appropriate combinations to avoid their loss within the intestinal absorption process.
LPH: lactase phloridzin hydrolase
SGLT1: sodium-dependent glucose transporter-1
KS: Conceptualization, Methodology, Writing—original draft, Writing—review & editing.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
