Affiliation:
Cardiology Division, Fatebenefratelli Hospital, 82100 Benevento, Italy
Email: emma.cerracchio@gmail.com
Affiliation:
Cardiology Division, Fatebenefratelli Hospital, 82100 Benevento, Italy
ORCID: https://orcid.org/0000-0003-4775-8380
Explor Cardiol. 2025;3:101256 DOI: https://doi.org/10.37349/ec.2025.101256
Received: October 16, 2024 Accepted: April 09, 2025 Published: May 07, 2025
Academic Editor: Undurti Das, UND Life Sciences, USA, Sri Ramachandra Medical University, India
The article belongs to the special issue Multimodality Imaging in Ischemic Heart Disease
An electrocardiogram (ECG) is a vital diagnostic tool used during cardiac imaging stress testing to evaluate the heart’s electrical activity under stress conditions. This combination of ECG and stress imaging testing provides comprehensive insights into cardiac function, particularly in detecting coronary artery disease (CAD) and assessing overall heart health. An ECG continuously monitors the heart’s electrical signals, capturing data on heart rate, rhythm, and electrical conduction patterns. The value of the ECG in this context lies in its ability to detect ischemic changes, which occur when there is insufficient blood flow to the heart muscle due to narrowed or blocked coronary arteries, but also for coronary vasospasm or coronary microvascular disease. Specific ECG changes, such as ST-segment depression or elevation, and the appearance of arrhythmias, can indicate myocardial ischemia. These findings, when correlated with symptoms like chest pain or shortness of breath during the test, may provide strong evidence for CAD even in the absence of diagnostic abnormality of cardiac imaging with regional wall motion or perfusion changes. Additionally, the ECG helps identify other conditions that may manifest under stress, such as arrhythmias or conduction abnormalities, which might not be apparent at rest. The ECG’s role extends beyond diagnosis. It helps stratify patients based on their risk of adverse cardiac events. For example, an abnormal ECG during a negative cardiac stress imaging test can suggest an increased likelihood of coronary calcification or abnormal coronary flow reserve and increased risk in the long term for cardiac events. In summary, the ECG is a valuable component of cardiac imaging stress testing. It provides real-time, non-invasive monitoring of the heart’s electrical activity under stress, aiding in the diagnosis and risk assessment of CAD and other cardiac conditions. This enhances patient management by guiding treatment decisions and preventive strategies.
The development of electrocardiogram (ECG) stress testing was a major advance in clinical cardiology [1]. Feil and Siegel recognized that myocardial ischemia and, thus, coronary artery disease (CAD) could be diagnosed by ST depression during exercise. Master and Oppenheimer developed the first standardized exercise test, a 1.5-minute step test to evaluate cardiac capacity and cardiovascular disease. Bruce developed the multi-stage treadmill test, now termed the Bruce protocol. An outcome study of Ellestad demonstrated that 1 mm of ST depression predicted cardiac events and death. Following one hundred years of development, the ECG remains the foundation of exercise stress testing [2, 3]. In the seventies, stress-ECG was also the natural approach of first-generation exercise-independent testing, such as dipyridamole [4, 5] or dobutamine stress [6], later popularized with cardiac imaging. In the cardiac imaging era, ECG stress testing progressively lost ground in favor of other markers of ischemia, more sensitive and earlier in the classical ischemic cascade of events in the presence of epicardial CAD [7–9]. In the last decade, it became progressively more obvious that even in the imaging era, ECG changes can offer surprisingly important diagnostic and prognostic information. Ischemia has multiple phenotypes in the real world [10], including ECG changes without cardiac imaging abnormality. The present narrative review will address the added value of ECG changes during stress in the cardiac imaging era, with a special focus on the different patterns of ECG changes (ST-segment depression, ST-segment elevation) in the presence of cardiac imaging normal or abnormal findings, the prevalence and meaning of arrhythmias during stress, and the pathophysiological and prognostic meaning of chronotropic incompetence during physical stress testing and during pharmacological stress testing where stress ECG is rarely use.
In about 50% of patients with anatomically verified epicardial CAD and stress imaging positivity [with perfusion changes or regional wall motion abnormality (RWMA)], a diagnostic ST-segment depression develops during stress. In 178 patients with dipyridamole stress echo positivity, Cortigiani et al. [11] found that 59% of patients showed concomitant ST-segment depression, and these patients were 2 times more likely to have 3-vessel or left main CAD and 3 times more likely to have major adverse events in a 3-year follow-up. In the SE2030 study, Kobal et al. [12] found that stress-induced ischemic ECG changes during physical or pharmacological stress testing are associated with more extensive RWMA and blunted global left ventricular contractile reserve, with reduced coronary flow velocity reserve in the left anterior descending artery. These data were observed with different types of stress (exercise, dobutamine, vasodilators) and with the recruitment of 641 patients from 39 different sites in 10 countries, increasing the generalizability of the findings [12].
ECG changes are relatively frequent during stress, and all physical and pharmacological tests currently employed with imaging techniques started their clinical and scientific life in combination with ECG in the pre-imaging era [1, 4, 6]. However, they were progressively abandoned in the imaging era since their diagnostic sensitivity and specificity are significantly lower if compared to cardiac imaging markers such as RWMA or perfusion changes [8, 9]. In a competitive view of the clinical use of different markers, cardiac imaging based on RWMA and/or perfusion outperforms ECG [13]. However, over the years it was evident that not a single marker was capable of capturing the diversity of phenotypes of inducible ischemia, and that ECG changes during vasodilators are even more suitable than RWMA in capturing a reduction in systemic endothelial dysfunction [14] in symptomatic patients with chronic coronary syndromes, or initial abnormalities of coronary calcification in asymptomatic subjects [15]. More than 20 years ago, a distinguished clinical cardiologist commented on these results, suggesting a revival of the ECG in the imaging era [16]. Subsequent extensive evidence in different patients’ subsets corroborated this visionary editorial comment [17]. ST-segment depression during dobutamine, exercise, or dipyridamole is associated with a worse prognosis even when it occurs in the absence of RWMA or perfusion changes [18].
Nine studies with a total of 23,715 patients were included in a recent meta-analysis. The primary outcome was a composite of all-cause death or myocardial infarction. The primary endpoint was more common with discordant results (ECG positivity with stress imaging negativity) with exercise stress echocardiography [relative risk 1.33, 95% CI (1.08–1.63)] or pharmacological SPECT [relative risk 6.53, 95% CI (2.31–18.48)] [19].
Recently, a paper was published on JACC showing that ECG abnormality with ST-segment depression during stress is frequently associated with a reduction of coronary flow velocity reserve and abnormal coronary vasomotor function in patients with angiographically normal coronary arteries [20]. Interestingly, the concept of ECG lies (false positivity for a coronary anatomic gold standard) becoming physiologic truth when a physiology or prognostic gold standard is adopted is at least 40 years old, and well described in the alternative ischemic cascade already 20 years ago [21–25].
Dipyridamole-induced ST-segment depression is frequently elicited in hypertensive patients when angiographically assessed coronary disease is absent. This ischemic-like electrocardiographic response can be found in left ventricular hypertrophy. However, even when the left ventricular mass is normal, dipyridamole-induced ST-segment depression is associated with an impaired coronary flow response to pacing, which is consistent with microvascular disease.
In special subsets beyond CAD, such as patients with hypertrophic cardiomyopathy without CAD, a solitary (without RWMA) ST-segment depression during stress identifies patients at high risk of subsequent death [26, 27]. In patients with heart transplants, a solitary ST-segment depression identified during pharmacological stress identifies patients with acute rejection, even without inducible RWMA [28].
ST-segment depression is by far the most common ECG pattern but 1% to 3 % of ECG positivity is accompanied by ST-segment elevation (in absence of resting Q wave) which indicates coronary vasospasm, most frequently at peak of stress (exercise or dobutamine) [29, 30] or immediately in the early recovery phase at the stop of exercise or early recovery phase, or with dobutamine after beta-blockers [31, 32], or with dipyridamole after aminophylline [33, 34]. This information is clinically important since, if missed, the further administration of an antidote can exacerbate vasospasm, favoring the transition to myocardial infarction. The vasospastic mechanism, if recognized, leads to the administration of intravenous nitrates or sublingual nifedipine, promptly resolving the coronary vasospasm in most cases.
Heart rate reserve is one of the simplest markers of risk during stress. It is easy to acquire since a simple one-lead ECG on the echo monitor is sufficient to obtain all the information needed. It is extraordinarily simple to analyze, since only the rest and peak value of the heart rate are utilized, and the heart rate is displayed automatically online in the echo monitor. It has an impressive amount of evidence behind its clinical use since it was first shown to predict all-cause death in 1975 by the father of stress testing, Ellestad. In a landmark paper published in Circulation, Ellestad and Wan [35] assembled follow-up data in 2,700 patients with exercise stress tests and showed that those with chronotropic incompetence had a high incidence of coronary events even though the ECG response to exercise was normal. This finding was confirmed over the subsequent decades [36, 37], and progressively extended to models beyond CAD such as heart failure [38, 39] or hypertrophic cardiomyopathy [40, 41] or transthyretin amyloidosis [42] and to stresses other than exercise such as dobutamine [43, 44] and vasodilators [45–53]. A blunted heart rate reserve is a strong predictor of all cause-death, especially of sudden death, and its value is additive and incremental over cardiac stress imaging information provided by perfusion defect or wall motion abnormality [54, 55].
Insufficient heart rate increase due to chronotropic incompetence is considered a major contributor to exercise and functional intolerance in heart failure, resulting from an imbalanced autonomic nervous system, which is chronically shifted toward sympathetic overactivation, leading to down-regulation and desensitization of cardiac β-receptors [56, 57].
It is addictive because it focuses on a specific vulnerability, different from critical epicardial coronary artery stenosis or coronary microvascular dysfunction. It is the cardiac sympathetic reserve, elicited by endogenous catecholamines or exogenous catecholamines such as dobutamine or endogenous or exogenous adenosine accumulation stimulating A2a receptors present on the carotid body and increasing efferent sympathetic efflux to the heart, eventually increasing heart rate [58–62]. The greater the increase in heart rate, the higher the cardiac sympathetic reserve, and this problem is a universal feature of vulnerability across all types of disease (Figure 1). This simple parameter has 100% feasibility, 0% variability, and prognostic strength higher than RWMA and coronary flow velocity reserve, and much more user-friendly than all methods used to assess cardiac autonomic function in the clinical arena [63, 64].
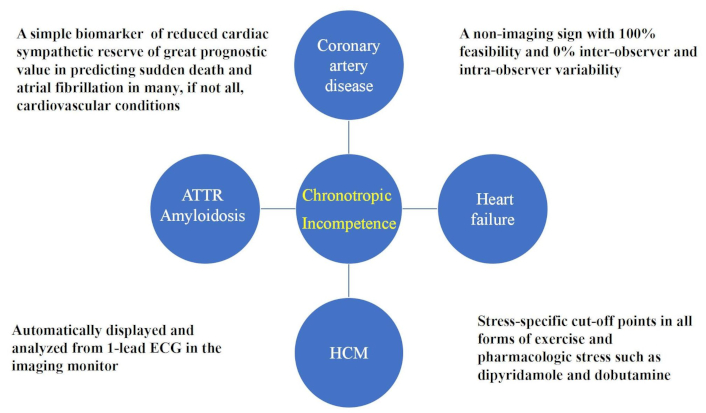
HRR as a marker of sympathetic dysfunction in several cardiovascular conditions. ECG: electrocardiogram; HRR: heart rate reserve
In the 2016 joint recommendation of the European Association of Cardiovascular Imaging and the American Society of Echocardiography of stress echo applications beyond CAD, chronotropic incompetence during exercise is considered an alternative explanation for symptoms of exercise and an exclusion criterion for the correct diagnosis of heart failure with preserved ejection fraction [65]. Before including this diagnosis, one should exclude exercise-induced ischemia with new wall motion abnormalities, dynamic severe mitral regurgitation during stress, development of left ventricular outflow tract obstruction, and insufficient increase in heart rate. In the recently released 2024 recommendations of European Association of Cardiovascular Imaging on the clinical use of stress echocardiography, heart rate reserve is incorporated as a pillar of the ABCDE protocol, with E standing for ECG-based assessment of chronotropic incompetence measured with heart rate reserve with all stresses in all patients, from chronic coronary syndromes to heart failure and hypertrophic cardiomyopathy [9]. Guidelines of the European Society of Cardiology on chronic coronary syndromes, in individuals with an initially low (> 5–15%) likelihood of obstructive CAD, exercise ECG, and detection of atherosclerotic disease in non-coronary arteries may be considered to adjust the pre-test likelihood estimate (IIb C). For the diagnosis of disease progression in patients with established chronic coronary syndrome, with new or worsening symptoms, it is now recommended to use stress imaging (I C). The second, still legitimate, choice of using “alternatively, exercise ECG”—present in the 2019 ESC recommendations, has been deleted. For the initial evaluation of patients with chest pain, exercise-ECG is recommended “in selected patients” [66]. Guidelines state that “Exercise ECG testing may modify the likelihood of obstructive CAD and can be used in patients with low (> 5%–15%) clinical likelihood, in whom a negative test allows reclassification to the very low (≤ 5%) clinical likelihood group with a favorable prognosis. Exercise ECG testing has a lower diagnostic performance of obstructive CAD compared with modern functional imaging and CCTA, which, therefore, should be preferred as a first-line test in subjects with suspected CCS.”
Exercise ECG is recommended in selected patients for the assessment of exercise tolerance, symptoms, arrhythmias, blood pressure response, and event risk. Interestingly, guidelines completely miss the information on heart rate reserve. However, if we consider the value (outcome/cost) [67] and even more, the sustainability (outcome/cost + radiation + carbon footprint) of testing, as we probably should [68], exercise ECG is the first-line obligatory cornerstone of diagnosis and risk stratification of the patient with known or suspected chronic coronary syndromes. Exercise is the only physiologic and by far the safest stress, and is fundamental not only for diagnosis but also for risk stratification. A maximal exercise ECG assesses ischemic vulnerability (with ST-segment and angina), arrhythmic vulnerability, hemodynamic vulnerability (with excessive hypertension or blood pressure drop), and cardiac autonomic system vulnerability (with heart rate reserve for sympathetic reserve and heart rate recovery for parasympathetic reserve) (Figure 2).
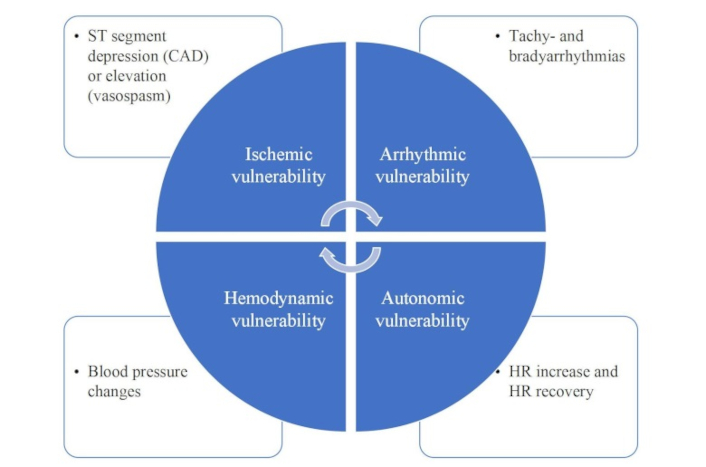
The many functional vulnerabilities identified by a simple exercise-ECG test. HR: heart rate; ECG: electrocardiogram
Even in modern cardiology, it is difficult to obtain so much with so little investment of time and money.
Our review provides an overview of the diagnostic and prognostic role of ECG during stress in the new cardiac imaging era. With the advances in cardiac imaging in both functional and anatomic fronts and the existing limitations of exercise stress testing, this modality appears less favoured worldwide, as reflected in recent guidelines. Instead, the combination of stress-ECG and imaging modalities provides the clinician a wealth of information to identify different phenotypes of inducible ischemia and to better risk-stratify patients (Figure 3). Chronotropic incompetence is another important marker of vulnerability that can emerge during stress. Heart rate reserve is a strong predictor of outcomes, especially sudden death, not only in CAD but in many other cardiovascular conditions, especially heart failure. In summary, the ECG represents a ubiquitous, noninvasive, and low-cost functional test for a comprehensive risk assessment in patients with CAD and other cardiac conditions. This improves patient management by guiding treatment decisions and preventive strategies.
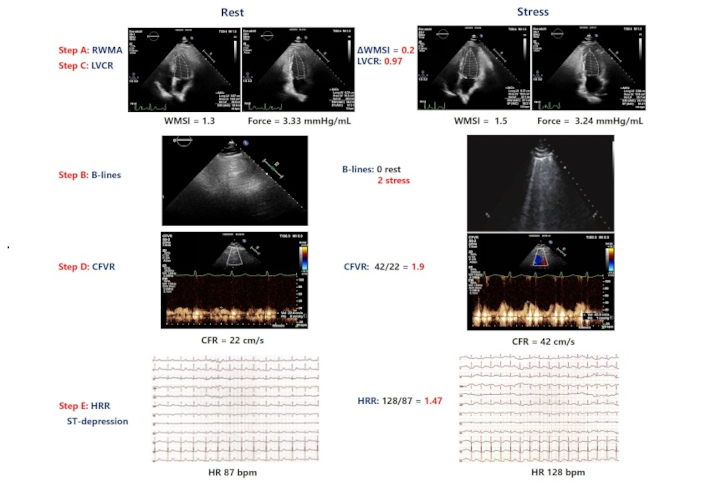
Central illustration. An example of a patient with positive ABCDE dipyridamole stress: induced ST-segment depression, new wall motion abnormalities (WMSI, step A positive), B lines at peak stress (step B positive), abnormal left ventricular contractile reserve (LVCR, step C positive) reduced coronary flow velocity reserve (CFVR, step D positive). RWMA: regional wall motion abnormality; LVCR: left ventricular contractile reserve; CFVR: coronary flow velocity reserve; CFR: coronary flow reserve; HRR: heart rate reserve; AWMSI: average wall motion score index; WMSI: wall motion score index; HR: heart rate
CAD: coronary artery disease
ECG: electrocardiogram
RWMA: regional wall motion abnormality
E Cerracchio: Writing—review & editing. E Campagnano: Data curation. BV: Supervision. QC: Conceptualization, Supervision, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study in the central illustration was approved by the Ethics Committee Lazio 1 [prot n. 295, 8/3/2021] and complies with the Declaration of Helsinki.
Informed consent to participate for the study in the central illustration was obtained from all participants.
Not applicable.
The data for Central Illustration could be available from the corresponding authors upon reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Marco Fabio Costantino ... Luisiana Stolfi
Cristhian Espinoza Romero ... Tatiana Torres Leal
Marco Antonio Rodrigues Torres, Natália Moraes de Quevedo
Praveen Kumar Chandra Sekar, Ramakrishnan Veerabathiran
