Affiliation:
1Instituto de Neurociencias, Universidad Miguel Hernández-CSIC, Avda de Ramón y Cajal s/n, San Juan de Alicante, 03550 Alicante, Spain
2Red Temática de Investigación Cooperativa en Salud (RETICS), Red de Trastornos Adictivos, Instituto de Salud Carlos III, MICINN and FEDER, 28029 Madrid, Spain
ORCID: https://orcid.org/0000-0001-6106-6247
Affiliation:
1Instituto de Neurociencias, Universidad Miguel Hernández-CSIC, Avda de Ramón y Cajal s/n, San Juan de Alicante, 03550 Alicante, Spain
2Red Temática de Investigación Cooperativa en Salud (RETICS), Red de Trastornos Adictivos, Instituto de Salud Carlos III, MICINN and FEDER, 28029 Madrid, Spain
ORCID: https://orcid.org/0000-0003-1251-6357
Affiliation:
1Instituto de Neurociencias, Universidad Miguel Hernández-CSIC, Avda de Ramón y Cajal s/n, San Juan de Alicante, 03550 Alicante, Spain
2Red Temática de Investigación Cooperativa en Salud (RETICS), Red de Trastornos Adictivos, Instituto de Salud Carlos III, MICINN and FEDER, 28029 Madrid, Spain
ORCID: https://orcid.org/0000-0002-9646-9975
Affiliation:
1Instituto de Neurociencias, Universidad Miguel Hernández-CSIC, Avda de Ramón y Cajal s/n, San Juan de Alicante, 03550 Alicante, Spain
2Red Temática de Investigación Cooperativa en Salud (RETICS), Red de Trastornos Adictivos, Instituto de Salud Carlos III, MICINN and FEDER, 28029 Madrid, Spain
Email: jmanzanares@goumh.umh.es
ORCID: https://orcid.org/0000-0002-4681-1533
Explor Neuroprot Ther. 2021;1:55–71 DOI: https://doi.org/10.37349/ent.2021.00006
Received: March 27, 2021 Accepted: May 06, 2021 Published: August 05, 2021
Academic Editor: Peter Illes, University of Leipzig, Germany; Rafael Franco, Universidad de Barcelona, Spain
Since the identification and cloning of the cannabinoid receptor 2 (CB2R), several studies focused on the characterization of its physiological and pathological role. Initially, CB2R was considered as the peripheral cannabinoid receptor due to its detection in the rat spleen and leukocyte subpopulation in humans. Later, CB2R was identified in different brain regions significantly modifying the landscape and pointing out its role in a wide variety of central physiological functions and pathological conditions. Additional research also detected the expression of CB2R in neurons, microglia, and astroglia in different brain regions. Indeed, the findings collected to date support a significant function of CB2R in anxiety, depression, schizophrenia, and additional neuropsychiatric disorders. This review gathers the most relevant literature regarding new advances about the role of CB2R in a variety of neuropsychiatric conditions, with special emphasis on its potential as a new therapeutic target for the treatment of different psychiatric disorders.
The cannabinoid receptor 2 (CB2R) along with the cannabinoid receptor 1 (CB1R) are the receptors of the endogenous cannabinoid system (ECS). Early research focused on elucidating the physiological role and therapeutic utility of CB1R in neuropsychiatry since it was the first cannabinoid receptor identified in the central nervous system (CNS). However, the detection of CB2R in neurons and glia in different brain regions has increased the number of investigations to elucidate its role at the central level, beyond its initial consideration as a “peripheral cannabinoid receptor”. This review summarizes the main findings highlighting the involvement of CB2R in different physiological processes such as stress response, emotional reactivity, and cognitive processing that are affected in different psychiatric disorders (anxiety, depression, schizophrenia, and bipolar disorder), as well as the evidence supporting its therapeutic potential in the pharmacological approach to these pathologies.
ECS components are endogenous ligands, including anandamide (AEA) [1] and 2-arachidonoyl glycerol (2-AG) [2, 3], synthesis enzymes, such as N-acylphosphatidylethanolamine phospholipase D (NAPE-PLD) and diacylglycerol lipase (DAGL), metabolizing enzymes, such as fatty acid amide hydrolase (FAAH) [4, 5] and monoacylglycerol lipase (MAGL) [6–9], and cannabinoid receptors CB1R [10], CB2R [11] and, more recently, G protein-coupled receptor 55 (GPR55) [12, 13], known as non-CB1 non-CB2 cannabinoid-related receptor (Figure 1). Besides, cannabinoids act on transient receptor potential (TRP) channels, particularly on TRP vanilloid 1 (TRPV1), causing these receptors to be called ionotropic cannabinoid receptors [14, 15].
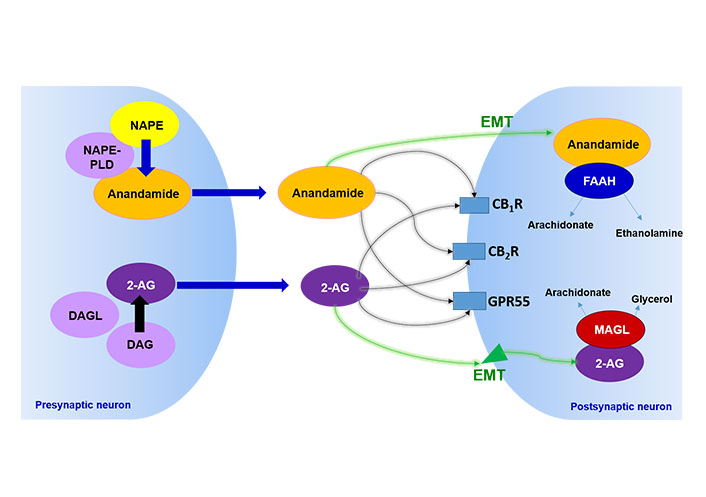
Characterization of the ECS. The image represents schematically the main elements of the ECS described to date. NAPE: N-acylphosphatidylethanolamine; DAG: diacylglycerol; EMT: endocannabinoid membrane transporter
Contrary to most neurotransmitters, endogenous cannabinoids are not stored in synaptic vesicles but are synthesized and released on demand by postsynaptic neurons and accompanying glial cells in response to changes in neuronal activity [16–18]. Once released, they act on specific cannabinoid receptors, predominantly CB1R and CB2R.
CB1R is widely distributed in the brain (cingulate cortex, entorhinal cortex, caudate nucleus and putamen, hippocampus, amygdala, thalamus, substantia nigra, medial hypothalamus, globus pallidus, nucleus tractus solitarius, cerebellum, and substantia gelatinosa of the spinal cord) [10, 19, 20]. This receptor has been proposed as a therapeutic target in the treatment of chemotherapy-associated nausea and vomiting [21, 22], in the treatment of anorexia in patients with acquired immunodeficiency syndrome [23, 24], chronic pain [25], spastic pain in multiple sclerosis [26], psychosis [27] and in anxiety disorders and depression [28–30].
The first attempts to identify the expression of CB2R in the brain under normal conditions resulted unsuccessful [31–35]. In 2005, Van Sickle et al. [36], published the first information identifying the neuronal expression of CB2R in the brainstem of rats, mice, and ferret under physiological conditions. These results served as a precedent for the full characterization of CB2R expression in rat and mouse brains years later [37–39].
The wide distribution of CB2R, both in neurons and glia, in various brain areas (striatum, cerebral cortex, amygdala, hippocampus, substantia nigra, olfactory tubercle, thalamic nuclei, ventromedial nucleus of the hypothalamus, paralemniscal nucleus, pontine nuclei, red nucleus, inferior colliculus, spinal nucleus, paratrochlear nucleus, and cerebellum), ended the idea that CB2R presents an exclusively peripheral role, opening news avenues to research on its potential involvement in different brain functions and neuropsychiatric disorders [37, 39–44].
The presence of CB2R in brain areas closely related to the response to stress and anxiety, such as the hippocampus and the amygdala, has led to the study of its possible involvement in these conditions [37, 39]. One of the main tools to evaluate the involvement of this receptor in anxiety has been the development of animal models that allow us to assess the response of rodents to anxiogenic stimuli. These models have been employed to evaluate whether genetic or pharmacological manipulation of CB2R modifies the response to anxiogenic stimuli in different experimental paradigms.
Interestingly, our group showed that transgenic mice overexpressing CB2R in the CNS (CB2xP mice) present a resistant phenotype against anxiogenic stimuli in the light-dark box and the elevated plus-maze [43]. Complementarily, mice lacking the CB2R gene (CB2–/– mice) develop higher levels of anxiety in both behavioral tests [44].
Another approach to study the role of CB2R in anxiety has been to evaluate the effects of intracerebroventricular (icv) administration of an antisense probe designed against CB2R messenger RNA in rodents [45]. In this study, the authors demonstrated that the blockade of the CB2R gene is associated with a reduction in anxiety-like behaviors, in contrast to the results obtained with genetically modified mice. These discrepancies could be related to the antisense probe administered icv, which may affect different brain regions and in a different manner (more acutely) in comparison with the genetic deletion, in addition to the distinct genetic background of the strains of mice used in these studies [DBA/2, C57BL/6J, BALB/c, and institute of cancer research (ICR)] (Table 1).
Major findings supporting the involvement of CB2R in anxiety disorders
| Animal studies | |||||||
|---|---|---|---|---|---|---|---|
| Genetic studies | Genetic manipulation | Animal specie | Experimental test | Behavioral changes | References | ||
| CB2xP | Mouse, ICR | LDB | ↓ vulnerability(↓ time in the lighted box and open arms) | [39] | |||
| EPM | |||||||
| CB2–/– | Mouse, ICR | LDB | ↑ vulnerability(↑ time in the lighted box and open arms) | [44] | |||
| EPM | |||||||
| Cnr2 KO | Mouse, C57BL/6J | SD | ↑ aggressive behavior | [49] | |||
| Pharmacological studies | Acute treatment | Drug | Animal specie | Experimental test | Dosis | Behavioral changes | References |
| CB2R messenger RNA antisense probe | Mouse, DBA/2, C57BL/6J, BALB/c | EPM | 4 µg/µL per 12 h (3 days) | ↓ anxiogenic response | [45] | ||
| JWH015 (CB2R agonist) | Mouse, DBA/2, C57BL/6J, BALB/c | EPM | 1–20 mg/kg | ↑ anxiogenic response | [38] | ||
| JWH133 (CB2R agonist) | Mouse, ICR | LDB, EPM | 0.5, 1, 2 mg/kg | No effect | [47] | ||
| Mouse, C57BL/6J | SD | 1, 2 mg/kg | ↓ aggressive behavior | [49] | |||
| GW405833 (CB2R agonist) | Mouse, C57BL/6J | MB | 10, 30, 100 mg/kg | ↓ anxiogenic response | [46] | ||
| Rat, Sprague-Dawley | Rotarod | 100 mg/kg | |||||
| AM630 (CB2R antagonist) | Mouse, ICR | LDB | 1, 2, 3 mg/kg | ↑ anxiogenic response | [47] | ||
| Chronic treatment | Drug | Animal specie | Experimental test | Dosis | Behavioral changes | References | |
| AM630 (CB2R antagonist) | Mouse, ICR | LDB EPM | 1, 2, 3 mg/kg per 12 h (7 days) | ↓ anxiogenic response | [47] | ||
| JWH133 (CB2R agonist) | Mouse, ICR | LDBEPM | 0.5, 1, 2 mg/kg per 12 h (7 days) | ↑ anxiogenic response | [47] | ||
| Human studies | |||
|---|---|---|---|
| Variable | Population | Results | References |
| rs2070956 polymorphism | Caucasian (infant) | ↓ response to the treatment | [50] |
Cnr2 KO: mouse lacking CB2R gene in the CNS with C57BL/6J background; LDB: light-dark box test; EPM: elevated plus-maze test; SD: experimental paradigm of social defeat; MB: marble-burying behavioral test. ↑: increases; ↓: decreases
The effects of the pharmacological modulation of CB2R by agonists and antagonists were also evaluated. Acute administration of the CB2R agonists GW405833 (100 mg/kg) and JWH015 (1–20 mg/kg) show anxiolytic effects on the rotarod [46] and the elevated plus-maze [38], respectively. However, depending on the agonist, the dose used, and the test employed, some authors find no differences [46, 47] or, on the contrary, anxiogenic effects are observed [45]. These discrepancies show that the effects would be influenced by several factors such as 1) the drug used, 2) the route and pattern of administration (acute vs. chronic) evaluated, 3) the behavioral test, and 4) the species and animal strains studied (rat, mouse; Table 1).
The effects induced by the blockade of CB2R by antagonists, such as that produced by compound AM630, were also evaluated. Its acute administration (1, 2, or 3 mg/kg) in ICR mice induces anxiogenic effects in the LDB. Interestingly, these effects are not found in the previous administration of a CB2R agonist (JWH133), supporting the involvement of CB2R in anxiety [47]. The change of the administration pattern from acute to chronic also modifies the effects mediated by CB2R agonist or antagonist compounds. Indeed, chronic administration of the antagonist AM630 (1, 2, or 3 mg/kg) exhibits anxiolytic effects in the light-dark box and the EPMs, while the agonist JWH133 (0.5, 1, or 2 mg/kg) presents anxiogenic actions. These effects are accompanied by increased CB2R gene expression in the amygdala and cortex, key regions involved in emotional response, in AM630-treated mice. Moreover, chronic activation of CB2R by the agonist JWH133 reduced CB2R gene expression in both regions [47]. These findings agree with the results found in studies performed with genetically modified mice, in which CB2R overexpression is associated with resistance to developing anxiety-like behaviors [43] and, on the contrary, its absence is associated with greater vulnerability [44].
In animal models of social defeat, paradigms of psychosocial anxiety, a reduction of CB2R gene expression was found in different regions of the hippocampus [cornu ammonis-1 (CA1) and dentate gyrus] both in mice that develop submissive behavior towards the aggressor and in those that develop avoidance behavior [48]. Additionally, it has been noted that the absence of CB2R produces increased aggressiveness in the social interaction test and the social defeat model [49]. In this same study, the acute administration of the agonist JWH133 (1, 2, or 4 mg/kg) reduces the aggressive behavior of wild-type mice exposed to this model. Taken together, these results support the involvement of the CB2R in the regulation of adaptive responses to environmental factors.
Few clinical studies have been performed but with promising results. A relationship was found between the rs2070956 polymorphism of CB2R and a poorer response to treatment in children with anxiety disorders [50]. Taking together, more studies in both mice and humans are needed to further investigate the role of CB2R in anxiety disorders, especially from the perspective of its potential therapeutic suitability.
In this section, it is essential to highlight the findings of experimental animal and human studies that revealed the potential therapeutic role of CB2R in depressive disorders. Genetic and pharmacological studies in rodents indicate that overexpression of CB2R is associated with increased resistance to stimuli that promote depressive-like behaviors. CB2xP mice (overexpressing CB2R) showed reduced immobility time in acute models of depression (tail suspension and novelty-suppressed feeding tests) [39]. Similarly, mice lacking this receptor (CB2–/– mice) present a higher degree of vulnerability to develop depressive-like behavior in the tail suspension test [44]. The results found with the conditional knockout mouse lacking CB2R in dopaminergic neurons (DAT-Cnr2–/–) are similar, that is, they present increased immobility time in the tail suspension and forced swimming tests, suggesting increased vulnerability to stimuli promoting depressive-like behaviors in rodents [51] (Table 2).
Major findings supporting the involvement of CB2R in depressive disorders
| Animal studies | ||||||
|---|---|---|---|---|---|---|
| Genetic studies | Genetic manipulation | Animal specie | Experimental test | Behavioral changes | References | |
| CB2xP | Mouse, ICR | TST | ↓ immobility time | [39] | ||
| NSFT | ↓ latency time and ↑ food (g) consumption | [39] | ||||
| CMS | ↑ resistance(↑ sucrose 1% consumption and ↓ immobility time in TST) | [39] | ||||
| CB2–/– | Mouse, ICR | TST | ↑ immobility time | [44] | ||
| DAT-Cnr2–/– | Mouse, C57BL/6J | TST, FST | ↑ immobility time | [69] | ||
| Pharmacological studies | Drug | Animal specie | Experimental test | Dosis | Behavioral changes | References |
| AM630 (CB2R antagonist) | Mouse, ICR | CMS | 1 mg/kg per 12 h (4 weeks) | Antidepressive actions (↑ immobility time in TST and ↑ sucrose 1% consumption) | [39] | |
| Mouse, BALB/c C57BL/6J | CMS | 3 mg/kg per 24 h (4 weeks) | No effect | [45] | ||
| Human studies | |||
|---|---|---|---|
| Variable | Population | Results | References |
| Q63R polymorphism | Japanese | ↑ incidence | [45] |
| CB2R expression | Caucasian | ↓ in DLPFC and amygdala | [56] |
TST: tail suspension test; NSFT: novelty suppressed feeding test; FST: forced swimming test; CMS: chronic mild stress
Another important strength supporting the role of CB2R in the development of depressive behaviors is provided by studies using the CMS model, the most relevant animal model of depression. This model induces behavioral and neurochemical alterations in rodents similar to the different signs and symptoms observed in depressive patients, such as anhedonia, reduced sexual activity, increased aggressiveness, reduced grooming, and detriment of neurogenesis processes, especially in the hippocampus [52, 53]. Exposure to this model does not induce any alteration in CB2xP mice in any of the parameters evaluated, immobility time in the tail suspension test, and consumption of a 1% sucrose solution (anhedonia), both typical traits of depressive-like behaviors in rodents [39].
Additionally, several studies were conducted to evaluate whether pharmacological manipulation of CB2R by the administration of a selective antagonist would reproduce a similar phenotype in wild-type mice to that observed in CB2xP mice. This assumption is based on the evidence supporting that administration of receptor antagonists produces long-term up-regulation of the blocked receptor [54, 55]. To this end, the effects of chronic administration of the CB2R antagonist AM630 [1 mg/(kg·12 h)] were evaluated in the CMS model. This study demonstrated how the administration of AM630 reversed depressive-like behavior [evaluated in the tail suspension test and the ingestion of a 1% sucrose solution (anhedonia)] after 4 weeks of treatment, an effect accompanied by an increase of CB2R gene expression in the hippocampus [39]. In contrast to these data, it was previously shown that administration of AM630 [1 mg/(kg·24 h)] failed to exert any effect on sucrose consumption in CMS-exposed mice [41]. Again, these discrepancies could be due to notable differences between the two studies such as 1) the rodent strains employed (BALB/c, ICR), and 2) the dose and administration patterns evaluated [3 mg/(kg·24 h)] [41] in contrast to 1 mg/(kg·12 h) [39].
In humans, the research team led by Onaivi [41] showed that there is a high incidence of the Q63R polymorphism of CB2R in Japanese depressive patients. Furthermore, a postmortem study performed in suicides revealed that there is a reduction of CB2R gene expression in the dorsolateral prefrontal cortex and amygdala, key regions involved in decision making, impulsivity, and emotional reactivity, supporting the role that this receptor could play in several psychiatric diseases with an increased tendency to suicide, such as depression, schizophrenia, and bipolar disorder [56].
Together, these results highlight the involvement of CB2R in the emotional response and its potential utility as a new target for the treatment of depressive disorders.
Currently, a large number of results support the involvement of CB2R in psychotic disorders. Clinically, significant reductions in CB2R gene expression were detected in peripheral mononuclear blood cells of schizophrenic patients in remission [57]. Also, an increased incidence of CB2R polymorphisms rs12744386 and rs2501432 was found in Japanese schizophrenic patients [42], and of rs2501432 and rs22229579 CB2R polymorphisms in the Han ethnic population in China [58]. The results of this latest study suggest that the T allele of the rs2501432 polymorphism would have a protective role, especially in men. Conversely, the T allele of the rs2229549 polymorphism would constitute a risk factor for the development of schizophrenia. However, further studies failed to establish a relationship between additional polymorphisms in CB2R (rs6689530, rs34570472, Cnr2_ht1, Cnr2_ht2, and Cnr2_ht3) and schizophrenia in the Korean population, probably due to the small sample sizes included in them [59] (Table 3).
Major findings supporting the involvement of CB2R in psychotic disorders
| Animal studies | ||||||
|---|---|---|---|---|---|---|
| Genetic studies | Genetic manipulation | Animal specie | Experimental test | Behavioral changes | References | |
| CB2–/– | Mouse, ICR | OF | ↑ sensibility to cocaine-induced hyperlocomotion | [44] | ||
| SDIA | short and long-term memory impairment | [44, 71] | ||||
| PPI | ↑ PPI | |||||
| Pharmacological studies | Drug | Animal specie | Experimental test | Dosis | Behavioral changes | References |
| AM630 (CB2R antagonist) | Mouse, C57BL/6J | PPI | 3 and 30 mg/kg | ↑ alteration induced by MK-801 | [42] | |
| Mouse, ICR | OF | 2 mg/kg | ↑ MK-801-induced hyperlocomotion | [70] | ||
| JWH015 (agonista CB2R) | Mouse, BALB/c | PPI | 1, 3 and 10 mg/kg | ↓ alteration induced by MK-801 | [67] | |
| JWH133 (agonista CB2R) | Mouse, ICR | OF | 20 mg/kg | ↓ cocaine-induced hyperlocomotion | [68] | |
| Mouse, C57BL/6J | OF | 10, 20 and 30 mg/kg | ↓ cocaine-induced hyperlocomotion | [69] | ||
| Human studies | |||
|---|---|---|---|
| Parameter | Population | Results | References |
| CB2R gene expression | Caucasian | ↓ reduction in PBMCs | [57] |
| Polymorphism | Population | Results | References |
| rs12744386 rs2501432 | Japanese | ↑ incidence | [42] |
| rs2501432 | Chinese (Han ethnic group) | Allele T ↑ risk | [58] |
| rs22229579 | Allele T ↓ risk | ||
| rs6689530 rs34570472 Cnr2_ht1 Cnr2_ht2 Cnr2_ht3 | Korean | No effect | [59] |
OF: open field test; SDIA: step-down inhibitory avoidance; PBMCs: peripheral blood mononuclear cells
The use of different animal models of schizophrenia supported the involvement of CB2R in this psychiatric disease. Early maternal deprivation constitutes an animal model for the study of schizophrenia by reproducing several symptoms (cognitive and attention alterations) observed in schizophrenic patients that translate into alterations in prepulse inhibition (PPI), startle reflex, and latent inhibition [60, 61]. These behavioral disturbances are accompanied by important neurobiological alterations in the hippocampus, increased number of astrocytes, neuronal degeneration, elevated corticosterone and 2-AG levels [62, 63], reduced CB1R gene expression, and increased CB2R gene expression [64].
One of the main tests used both in humans and rodents for the evaluation of attention-deficit associated with schizophrenic pathology is the PPI test in which reduced values are representative of the inability to filter the information presented by these patients. In this regard, the administration in rodents of methamphetamine or MK-801, a non-competitive N-methyl-D-aspartate (NMDA) glutamatergic receptor antagonist, are two highly accepted models of schizophrenia producing a reduction in PPI values similar to those observed in schizophrenic patients [65, 66]. While CB2R blockade using the CB2R antagonist AM630 (3 mg/kg and 30 mg/kg) does not trigger alterations per se in PPI, it exacerbates MK-801- or methamphetamine-induced alterations in C57BL/6J mice [42]. Another complementary study shows that the agonist JWH015 (1, 3, and 10 mg/kg) improves MK-801-induced impairment in PPI, without presenting any effects on its own [67]. The fact that this effect is blocked by AM630, but not by the CB1R antagonist AM251, confirms the involvement of CB2R.
Hyperlocomotion induced by psychostimulants or by MK-801 is another animal model of schizophrenia. The results obtained using this model have been promising. On the one hand, the administration of the agonist JWH133 dose-dependently reduces cocaine-induced hyperlocomotion in wild-type mice [68, 69]. On the other hand, AM630 at doses that do not affect motor activity (2 mg/kg) worsens MK-801-induced hyperlocomotion [70]. These results strongly suggest that the inhibition of CB2R function potentially precipitates schizophrenia-like symptoms in rodents when combined with risk factors such as inhibition of glutamatergic transmission induced by NMDA receptor antagonists.
Studies carried out in genetically modified mice have confirmed the involvement of CB2R in schizopsychotic disorders. CB2–/– mice present increased vulnerability to hyperlocomotion induced by acute cocaine administration, in addition to PPI and cognitive alterations [44, 71]. Moreover, treatment with the antipsychotic risperidone normalizes PPI values in CB2–/– mice. All these facts suggest that genetic deletion of CB2R could be a useful tool as an animal model of schizophrenia [44].
Recently, it was shown that CB2R is critical for acetylcholine muscarinic 4 (M4) receptor agonists, such as compound UV0467154, to exhibit their antipsychotic actions [72]. This study indicates that agonist-regulated stimulation of M4 receptors would increase the release of endogenous cannabinoids, in turn activating CB2R, which would eventually inhibit dopamine release, a fact that would be involved in the antipsychotic efficacy of M4 agonists. Consequently, blocking CB2R by the antagonist AM630 would prevent M4 agonists to present antipsychotic effects. This finding highlights that CB2R is indispensable in the regulation of dopamine concentrations altered in this disease, pointing out its potential therapeutic utility in the treatment of psychotic disorders.
Taken together, these findings suggest that CB2R is crucial in the regulation of symptoms related to psychotic disorders. Further pharmacological studies, such as those evaluating the additive effects of CB2R agonist/antagonist combinations with antipsychotics (e.g., risperidone), are needed to clarify the role of pharmacological modulation of CB2R in the treatment of psychotic disorders.
To date, few studies focused on investigating the possible link between CB2R and bipolar disorder. There is only one study in which the incidence of three polymorphisms of the gene coding for CB2R, rs2501432 (315>G; Arg63Gln), rs41311993 (524C>A; Leu133Ile) and rs2229579 (1073C>T; Tyr316His), was evaluated in 80 patients with bipolar disorder [73]. The results allowed to establish a direct relationship between the rs41311993 (524C>A) polymorphism of CB2R and bipolar disorder. This polymorphism consists of the substitution of the amino acid leucine by isoleucine at position 133, involved in the structural stability of the receptor since it allows the formation of hydrophobic interactions between helix III with the rest of the 7 transmembrane domains (except helix I). Helix III, therefore, plays a primordial role in the regulation of CB2R activity, participating in its interaction with the G protein-coupled to it. Consequently, the presence of this polymorphism could affect the structural stability of the receptor, as well as its interaction with the G protein. This finding is the first evidence to indicate that CB2R may be involved in the etiology of bipolar disorder. Further studies, both clinical and preclinical, are needed to investigate the involvement of CB2R in this psychiatric disorder.
CB2R was identified in different cell types (neurons, glia, and astroglia) of the as well as its interaction with targets classically related to the response to stress, anxiety, depression, and schizophrenia, such as the hypothalamic-pituitary-adrenal (HPA) axis, processes of neurogenesis, and neuronal plasticity, and the GABAergic, dopaminergic and serotonergic neurotransmitter systems.
The identification of the pharmacological properties of CB2R stimulated great interest in determining the cell type in which this receptor is expressed, not without some controversy. The main limitation in interpreting the results of these studies is antibody selectivity [74], a common problem for many G protein-coupled receptors [75].
Despite these limitations, several studies revealed that CB2R localizes to hippocampal glutamatergic [76, 77], cortical pyramidal [37, 56], cholinergic [78], and dopaminergic [79–82] as well as in other neuronal types in the brainstem [36] and cerebellum [37]. Furthermore, human postmortem studies identified CB2R in microglia in the cerebellum [83] and astrocytes in the dorsolateral prefrontal cortex [56].
In mice, CB2R was detected in the astroglia of the nucleus accumbens and ventral tegmental area [79]. Complementary studies show that this expression is significantly upregulated in the presence of certain types of lesions in both reactive microglia and activated astrocytes [84–86], which has served to refute its role in diseases in which the neuroinflammatory component is important, such as schizophrenia (for review [87, 88]).
The HPA axis is one of the main elements of the response to stressful stimuli, modulating endocrine, autonomic, and behavioral responses to stress. Alterations in the HPA axis were associated with increased levels of anxiety and stress response in rodents and patients with anxiety disorders [89, 90].
Recent studies showed that CB2R is involved in the regulation of the HPA axis. In an animal model of stress induced by movement restriction (in a cylinder for 30 min), it was found that the CB2xP mouse (overexpressing CB2R) shows no changes in corticotropin-releasing factor (CRF) gene expression in the paraventricular nucleus (PVN), and only a slight increase in pro-opiomelanocortin (POMC) gene expression in the arcuate nucleus (ARC) (22%). However, in control mice, there is an 82% increase of CRF in the PVN and a 42% increase of POMC in the ARC [43]. On the other hand, it was found that exposure to certain stressful situations increases CB2R gene expression in the hippocampus, an area closely related to the HPA axis activity [48, 64].
In humans, only one study evaluated CB2R gene expression in human adrenal tissue as well as in cultures of the NCI-H295R cell lines, a widely accepted model for human adrenocortical studies. The presence of CB2R could not be detected in both types of samples [91].
These results highlight the need to perform further experimental and clinical studies that explore the role of CB2R in the regulation of the HPA axis. Evaluation of how the HPA axis response to stressful stimuli is modified by administration of CB2R agonists/antagonists would be a promising starting point.
Neuroplasticity is one of the processes compromised in a wide variety of neuropsychiatric disorders [92–95]. The hippocampus represents a region of interest because of its high plasticity [96]. A reduction of CB2R gene expression was found in the hippocampus of rodents exposed to the animal model of CMS along with a reduction of brain-derived neurotrophic factor (BDNF). Both reductions are blocked by the administration of the CB2R antagonist AM630 (1 mg/kg) [39]. In this study, CB2xP mice presented increased levels of BDNF in the hippocampus. Surprisingly, these levels are not modified after exposure to CMS, as is the case in control mice. These data indicate that the increased resistance of CB2xP mice to develop depressive behaviors could be related, at least in part, to its actions on neuronal plasticity. Indeed, recent studies show that CB2R regulates hyperpolarization phenomena in hippocampal neurons [cornu ammonis 3 (CA3) and cornu ammonis 2 (CA2) regions]. This phenomenon is independent of excitatory glutamatergic and inhibitory GABAergic synaptic transmission and therefore has been proposed as an intrinsic plasticity mechanism that would negatively modify the excitability of neurons in CA3. The reduction of the excitability of these neurons would possibly enhance plasticity [76].
Furthermore, CB2R appears to regulate different key signaling pathways in plasticity processes, such as the phospholipase A and C pathway and phosphoinositol-3 kinase and mitogen-activated protein kinases (MAPKs) [97], supporting their role in the intracellular regulation of calcium homeostasis and mitochondrial functions, as well as the control of trophic processes, excitability and neuronal signaling [98]. Moreover, additional results revealed that activation of CB2R in the brain stimulates glucose reuptake [99] and reduces oxidative stress [100].
Taken together, all these findings suggest that CB2R may result in a new therapeutic target in the treatment of several diseases with impaired neuroplasticity processes.
In parallel, the interaction between CB2R and GABA, the main CNS inhibitory neurotransmitter involved in a wide variety of psychiatric diseases, were studied [101–106].
Pharmacological or genetic manipulation of CB2R modifies GABAergic transmission. Chronic administration of the antagonist AM630 increased CB2R levels in the amygdala and enhanced the expression of GABA A receptor subunits alpha 2 (GABAAα2), and gamma 2 (GABAAγ2), related to the anxiolytic effects of GABA. In contrast, the agonist JWH133 produces changes in the opposite direction [47]. Additionally, CB2xP mice show higher gene expression of both GABAergic subunits, both in the hippocampus and amygdala, which explains, at least in part, the absence of anxiolytic effects after alprazolam administration in these rodents [43].
Furthermore, some studies using electrophysiological techniques indicate that CB2R regulates GABAergic inhibition in the medial entorhinal cortex of rats [101]. The administration of the agonist JWH133 abolishes GABAergic inhibition which can be reversed if the antagonist AM630 is administered. Complementarily, the administration of the inverse agonist JTE-907 increases GABAergic transmission in this region. These effects are not observed with the administration of CB1R agonists, confirming the role of CB2R in the regulation of GABAergic transmission in this brain region.
The identification of gene and protein expression of CB2R in certain neuronal types, together with the effects observed after its pharmacological and genetic manipulation, has stimulated studies to elucidate the role of CB2R in dopaminergic and serotonergic neurotransmission.
The absence of CB2R generates a series of alterations in the gene expression of different elements of dopaminergic transmission. The CB2–/– mouse shows increased gene expression of the dopaminergic D2 receptor in the prefrontal cortex, which could contribute, at least in part, to the behavioral alterations observed in this rodent [44]. Also, the use of the conditioned KO mouse demonstrated the inhibitory role of CB2R in dopaminergic neurons [51].
Alterations in gene expression of monoamine oxidase A (MAO-A) enzyme, serotonin transporter, and serotonergic 5-hydroxytryptamine 2C (5-HT2C) receptor in the dorsal raphe of CB2–/– mice have been observed. Also, alterations of the serotonergic 5-hydroxytryptamine 2A (5-HT2A) receptor in the prefrontal cortex and the enzymes MAO-A and catechol-O-methyltransferase and the serotonergic 5-hydroxytryptamine 1B (5-HT1B) receptor in the amygdala of CB2–/– mice are present [44, 49].
Electrophysiology studies suggest that the CB2R agonist GP 1a increases gene and protein expression of the serotonergic 5-HT2A receptor in the rat dorsolateral prefrontal cortex, which is blocked by the administration of the CB2R antagonist PD 198306. This increase appears to be related to activation of the extracellular regulated kinase 1/2 (ERK1/2) signaling pathway induced by CB2R activation [107, 108]. Cortical 5-HT2A receptor activity was linked to stress response, anxiety, depression, and schizopsychotic symptoms present in various disorders.
Taken together, these results provide relevant information on the molecular mechanisms by which CB2R activation or blockade may be relevant in the pathophysiology of different psychiatric disorders.
In summary, the findings included in this review highlight the potential therapeutic suitability of CB2R in a wide variety of psychiatric disorders, including anxiety disorders, depressive disorders, schizophrenia, and disorders involving cognitive impairment. An additional value of CB2R pharmacological manipulation is the fact that, contrary to CB1R activation, does not induce psychotropic effects. The availability of agonist/ antagonist compounds of this receptor, along with recent allosteric modulators, represents a valuable tool for further experimental and preclinical studies to finally elucidate the role of the pharmacological regulation of CB2R in psychiatry. Besides, the few clinical trials conducted to date showed that these compounds are well tolerated, at least at the doses and in the pathologies tested [109, 110]. Nonetheless, future studies are needed to evaluate safety in neuropsychiatric disorders.
2-AG: 2-arachidonoyl glycerol
5-HT2A: 5-hydroxytryptamine 2A
CB1R: cannabinoid receptor 1
CB2R: cannabinoid receptor 2
CMS: chronic mild stress
CNS: central nervous system
ECS: endogenous cannabinoid system
EPM: elevated plus-maze test
HPA: hypothalamic-pituitary-adrenal
ICR: institute of cancer research
LDB: light-dark box test
M4: acetylcholine muscarinic 4
OF: open field test
PPI: prepulse inhibition
TST: tail suspension test
We thank all participants in this study. We greatly appreciated Biorender for helping to create figure 1.
MSGG and JM conceived the presented idea. MSGG took the lead in writing the manuscript. FN and AG wrote the manuscript in consultation with MSGG. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
The authors declare no conflict of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2021.
Copyright: © The Author(s) 2021. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
