Affiliation:
1Laboratorio de Neuroinmunología, Fundación Ciencia & Vida, Ñuñoa, Santiago 7780272, Chile
2Facultad de Medicina y Ciencia, Universidad San Sebastián, Providencia, Santiago 7510156, Chile
Email: rpacheco@cienciavida.org; rodrigo.pacheco@uss.cl
ORCID: https://orcid.org/0000-0001-8057-9806
Explor Neuroprot Ther. 2021;1:72–85 DOI: https://doi.org/10.37349/ent.2021.00007
Received: July 21, 2021 Accepted: September 08, 2021 Published: October 29, 2021
Academic Editor: Raymond Chuen-Chung Chang, The University of Hong Kong, China
Current evidence indicates that neurodegeneration of dopaminergic neurons of the substantia nigra associated to Parkinson’s disease is a consequence of a neuroinflammatory process in which microglial cells play a central role. The initial activation of microglial cells is triggered by pathogenic protein inclusions, which are mainly composed by α-synuclein. Importantly, these pathogenic forms of α-synuclein subsequently induce a T-cell-mediated autoimmune response to dopaminergic neurons. Depending on their functional phenotype, these autoreactive T-cells might shape the functional features of activated microglia. T-cells bearing pro-inflammatory phenotypes such as T-helper (Th)1 or Th17 promote a chronic inflammatory behaviour on microglia, whilst anti-inflammatory T-cells, such as regulatory T-cells (Treg) favour the acquisition of neuroprotective features by microglia. Thus, T-cells play a fundamental role in the development of neuroinflammation and neurodegeneration involved in Parkinson’s disease. This review summarizes the evidence indicating that not only CD4+ T-cells, but also CD8+ T-cells play an important role in the physiopathology of Parkinson’s disease. Next, this review analyses the different T-cell epitopes derived from the pathogenic forms of α-synuclein involved in the autoimmune response associated to Parkinson’s disease in animal models and humans. It also summarizes the requirement of specific alleles of major histocompatibility complexes (MHC) class I and class II necessaries for the presentation of CD8+ and CD4+ T-cell epitopes from the pathogenic forms of α-synuclein in both humans and animal models. Finally, this work summarizes and discusses a number of experimental immunotherapies that aim to strengthen the Treg response or to dampen the inflammatory T-cell response as a therapeutic approach in animal models of Parkinson’s disease.
Parkinson’s disease is a neurodegenerative disorder that affect mainly the dopaminergic neurons of the nigrostriatal pathway. Since these neurons are involved in the control of voluntary movement, the most characteristic symptom associated to this pathology is a motor impairment, including bradykinesia, postural instability, tremors, and rigidity. This disorder also involves the generation of pathogenic protein inclusions in the brain, which are composed mainly by a pre-synaptic protein called α-synuclein (αSyn) [1]. It has been described that these pathogenic deposits of αSyn are generated by misfolded and post-translational modifications of αSyn induced by oxidative stress and aberrant phosphorylation [2].
Current evidence indicates that neurodegeneration involved in Parkinson’s disease is a consequence of a chronic inflammatory process of the nigrostriatal pathway [3]. Importantly, microglial cells are the central players in this neuroinflammatory process. In this regard, it has been described that initial microglial activation is triggered by pathogenic forms of αSyn, which stimulate toll-like receptors (TLRs) on these cells [4, 5]. Afterwards, microglia might acquire a pro-inflammatory behaviour, called M1 microglia, which produces inflammatory mediators and neurotoxic factors such as glutamate, reactive oxygen species (ROS), reactive nitrogen species (RNS) and tumour necrosis factor α (TNFα), all of which might induce neuronal death directly [6]. On the other hand, microglia might acquire an anti-inflammatory functional phenotype, called M2 microglia, which release anti-inflammatory mediators and neurotrophic factors, such as glial cell-derived neurotrophic factor, brain-derived neurotrophic factor, insulin-like growth factor 1 and others, which dampen inflammation and promote neuronal survival and tissue repair [6]. Thus, the acquisition of a particular functional phenotype by microglia might define the development of neuroinflammation and the consequent neurodegeneration in Parkinson’s disease.
During the last decade, several studies have shown the involvement of a T-cell response specific to pathogenic forms of αSyn in Parkinson’s disease patients and in animal models of this disorder [7]. Importantly, the functional phenotype of microglial cells strongly depends on the particular subsets of T-cells that infiltrate the brain. Thus, whereas the pro-inflammatory T-cells subsets T-helper (Th)1 and Th17 promote the acquisition of the M1 phenotype by the microglia, the regulatory T-cells (Treg) that exert an anti-inflammatory activity promote the M2 phenotype in the microglia [3]. Thereby, the T-cell response plays a key role determining the evolution of the neurodegenerative process involved in Parkinson’s disease.
T-cell activation requires the presentation of antigenic peptides on the major histocompatibility complexes (MHC) by antigen-presenting cells. In this regard, whereas class I MHC present peptides recognized by CD8+ T-cells, class II MHC present antigenic peptides recognized by CD4+ T-cells [8]. Class I and class II MHC are encoded by a number of highly polymorphic genes called histocompatibility leukocyte antigens (HLA). Since MHC encoded by different HLA alleles display different preference of peptide-binding and different affinities, the specific HLA alleles able to present αSyn-derived antigens constitutes a key genetic factor to be considered in the risk of Parkinson’s disease [9].
This review aims to integrate, summarize and analyse the current evidence involving not only CD4+ T-cells, but also CD8+ T-cells that play an important role in the autoimmune response involved in Parkinson’s disease. Moreover, this work also aims to summarize the specific T-cell epitopes derived from the pathogenic forms of αSyn recognized by the autoreactive CD8+ and CD4+ T-cells and their HLA alleles restriction. Finally, this review aims to summarize and discuss a number of experimental immunotherapies involving the potentiation of αSyn-specific Treg response or dampening the αSyn-specific inflammatory T-cell response in preclinical models.
Many studies have provided evidence that T-cells infiltrate the brain in animal models of Parkinson’s disease, including the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model in mouse [10–12], 6-hydroxydopamine (6-OHDA) induced neurodegeneration in mice [13] and rats [14], transgenic mice overexpressing the human αSyn (hαSyn, Thy1-SNCA) [15, 16], the stereotaxic delivery of adeno-associated viral vectors (AAV) encoding for the wild-type hαSyn in mice [17, 18] and rats [19–21], and the stereotaxic delivery of AAV encoding for the A53T mutant form of hαSyn in rats [22]. Moreover, several studies have shown that T-cell deficient mice [10, 12, 23] and rats [21] are significantly protected of neurodegeneration development in animal models of Parkinson’s disease, thereby indicating that T-cell response seems to play a fundamental role in this disorder. Importantly, a recent longitudinal case study in Parkinson’s disease patients revealed an elevated T-cell response specific to αSyn-derived antigens, which may be detected prior motor impairment. This T-cell response was highest early after motor diagnosis and then declined progressively [24]. Thus, these findings suggest an important role of T-cell-driven inflammation in early stages of Parkinson disease development in human individuals.
Some studies carried out in animal models of Parkinson’s disease have suggested that despite both CD4+ and CD8+ T-cells infiltrate the central nervous system (CNS), only CD4+ T-cells are required for neurodegeneration, whilst CD8+ T-cells are of lesser relevance for the development of this disease. In this regard, Brochard and colleagues [23] observed that Cd4–/– mice but not Cd8–/– mice were protected from MPTP-induced degeneration of dopaminergic neurons of the substantia nigra (SN). In addition, Williams and colleagues [17] recently observed that neurodegeneration of dopaminergic neurons of the SN induced in mice by the stereotaxic deliver of AAV-hαSyn is associated with increased expression of class II MHC on myeloid cells in the CNS and a sharp infiltration of interferon-gamma (IFN-γ) producing CD4+ T-cells and CD8+ T-cells into the SN. Nevertheless, only T-cell receptor (TCR)β or CD4 deficiency, but not CD8 deficiency, attenuated neuroinflammation and neurodegeneration in this mouse model [17].
Although the studies above indicated suggest that CD8+ T-cells are not fundamental for neurodegeneration in some mouse models of Parkinson’s disease, increasing evidence obtained from animal models and humans points toward an important role of CD8+ T-cell response in the pathogenesis of this disorder. For instance, the treatment of SH-SY5Y neuroblastoma with 1-methyl-4-phenylpyridinium (MPP+), the toxic form of MPTP which inhibits mitochondrial respiration promoting the generation of ROS, induced increased class I MHC expression [25], thus suggesting that oxidative stress favours the expression of class I MHC in neurons. Accordingly, the MPTP treatment of mice induced increased class I MHC expression on dopaminergic neurons of the SN, which correlated with CD8+ T-cell infiltration into the brain. Moreover, the knockdown of class I MHC induced by the stereotaxic delivery of AAV resulted in decreased extent of CD8+ T-cells infiltration and presented a trend in the reduction of neurodegeneration of dopaminergic neurons of the SN [25]. Furthermore, mechanistic analyses indicated that PTEN-induced kinase 1 (PINK1) was repressing the class I MHC expression in neurons [25]. PINK1 is involved in the clearance of damaged mitochondria by mitophagy and PINK1 mutations have been associated with Parkinson’s disease [26].
Interestingly, Matheoud and colleagues [26] provided evidence that PINK1 inhibits the presentation of mitochondria-derived auto-antigens in class I MHC upon stimulation by lipopolysaccharide (LPS) or gram-negative bacteria in vivo. Thus, the absence of PINK1 triggers the presentation of mitochondrial autoantigens in class I MHC and the consequent activation of autoreactive CD8+ T-cells. Accordingly, the infection of Pink1-deficient mice with Citrobacter rodentium, a mouse intestinal gram-negative pathogen that is used as a model of human enteropathogenic Escherichia coli infection, induces a CD8+ T-cell-mediated autoimmune response directed to mitochondrial antigens [26]. This autoimmune response affected specially to dopaminergic neurons of the SN, which display high mitochondrial activity, thereby promoting the loss of dopaminergic terminals in the striatum and motor impairment.
According to the involvement of CD8+ T-cells in the loss of dopaminergic neurons in some animal models of Parkinson’s disease, it has been described that catecholaminergic neurons of the SN and Locus Coeruleus in humans express MHC class I, rendering them susceptible to cytotoxic attack by antigen-specific CD8+ T-cells [27]. Accordingly, a recent study analysed the extent of T-cell infiltration into the SN and the association with neuronal death and synucleinopathy at different disease stages in Parkinson’s disease patients. The results showed a robust CD8+ T-cell infiltration into the SN at early stages of the disease, when the neuronal death was still absent and the αSyn aggregation was confined to the olfactory bulb but absent in the SN [28]. In later stages of the pathology, these authors found a lower CD8+ T-cell infiltration associated to neuronal loss and accumulation of αSyn aggregates in the SN [28]. Interestingly, most CD8+ T-cells infiltrating the SN had a phenotypic profile of tissue resident memory T-cells. Thus, this study suggests that a cytotoxic CD8+ T-cell response may be involved in the initiation and propagation of neuronal death and synucleinopathy in Parkinson’s disease [28]. Altogether, the above evidence suggests that despite some animal models show the CD8+ T-cell response is not necessary for the neurodegenerative process, recent studies in animals and humans indicate that not only CD4+ but also CD8+ T-cells play important roles in the development of neurodegeneration in Parkinson’s disease.
Interestingly, despite most studies indicate that T-cell mediated autoimmune response to αSyn plays a detrimental role in the physiopathology of Parkinson’s disease, there is also evidence indicating that T-cell response might be beneficial under some circumstances. For instance, it has recently been shown that the transfer of T-cells into immunodeficient mice (NOD SCID gamma-chain deficient, NSG) reduces the deposits of phospho-S129-αSyn, one of the pathogenic forms of αSyn, in the striatum and frontal cortex in a model of Parkinson’s disease induced by the injection of hαSyn preformed fibrils (PFF) into the striatum [29].
In addition, it has been shown that lymphocyte deficiency exacerbates the motor impairment and loss of dopaminergic neurons of the nigrostriatal pathway in mice lesioned with unilateral stereotaxic injection of 6-OHDA in the medial forebrain bundle [30]. Moreover, other studies have shown that the immunization of rats or mice with a low dose of αSyn in a mix with complete Freund’s adjuvant (CFA), but not with CFA alone, resulted in the generation of αSyn-specific Treg cells and the consequent attenuation of neurodegeneration in models of Parkinson’s disease induced by the stereotaxic injection of AAV-hαSyn in the SN [19, 31]. Although therapies involving the usage of CFA are not allowed for the treatment of humans due to its high toxicity [32], these studies encourage the design of immunotherapies for Parkinson’s disease based in the generation of Treg responses specific to pathogenic forms of the αSyn. Current evidence indicating how T-cells participate in the development of Parkinson’s disease is integrated in the Figure 1.
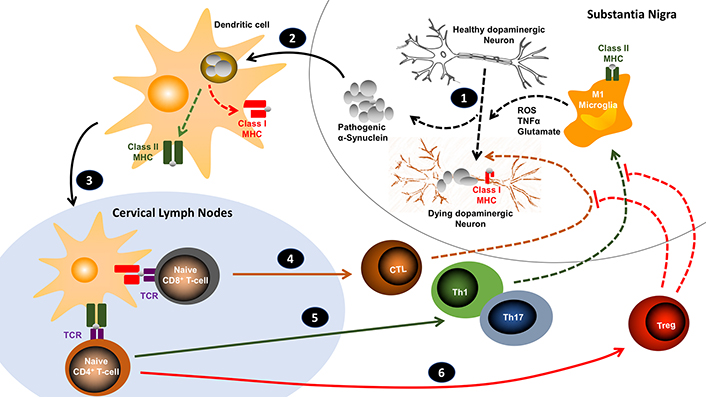
Working model for the role of the T-cell response in the development of Parkinson’s disease. 1. An initial generation of ROS (i.e. by mitochondrial dysfunction) promotes the oxidation and aggregation of αSyn, inducing the generation of pathogenic forms of αSyn (oligomers, fibrils, Lewy bodies). Pathogenic αSyn also stimulate TLRs on microglia, triggering further ROS production and secretion of pro-inflammatory cytokines and glutamate, which favour the expression of class I MHC on neurons and eventually neuronal death. 2. Pathogenic αSyn is captured and processed by dendritic cells and subsequently presented on class I and class II MHC molecules. 3. Once dendritic cells arrive to cervical lymph nodes, they present αSyn-derived antigens to naive T-cells. 4. When naive CD8+ T-cells recognize their specific antigens (through the T-cell receptor; TCR), they become activated and differentiate into cytotoxic T lymphocytes (CTL), which subsequently infiltrate the brain and directly kill neurons presenting their specific antigens on surface class I MHC. 5. Naive CD4+ T-cells specific for the antigens presented by immunogenic dendritic cells become activated and acquire inflammatory phenotypes (i.e. Th1 and Th17), which then infiltrate the brain where are restimulated by microglial cells presenting their specific antigens on class II MHC. Upon this restimulation, cytokines produced by CD4+ T-cells act on microglial cells potentiating their M1 phenotype and favouring further production of ROS, glutamate and TNFα, inducing neuronal death. 6. The manipulation of dendritic cells (with tolerogenic properties) might favour the differentiation of naive CD4+ T-cells into Treg. These lymphocytes subsequently attenuate the effects exerted by CTLs, Th1 and Th17, dampening the neuroinflammation and stopping neurodegeneration. Solid arrows implicate migration; lined arrows involve processes
Similar to other autoimmune diseases, the genetic factors associated with Parkinson’s disease risk include the polymorphism of MHC molecules. In this regard, different MHC alleles involve distinct preferences of peptide-binding and affinities, thereby implicating the activation of different T-cell clones [33]. According to the involvement of both CD4+ and CD8+ T-cell responses, this disorder has been associated with some particular alleles of class II and class I MHC genes respectively, including first the DRB1*15:01 and DRB5*01:01 alleles [34] and later the DQB1*03:04 and A*11:01 alleles [9].
Importantly, the specific MHC alleles associated to Parkinson’s disease are required to the proper presentation of antigens derived of the αSyn. Evidence obtained from human and animal models has shown the presence of αSyn containing 3-nitrotyrosines (NαSyn) in the SN of individuals with Parkinson’s disease [10, 35, 36], a modified form of αSyn that is found mainly in the αSyn inclusions contained in Lewy bodies. It is noteworthy that the nitration of αSyn is a consequence of the oxidative stress induced by ROS and RNS, and that this kind of posttranslational modification involves the generation of a neo-antigens [37].
In this regard, it has been described a C-terminus region of αSyn including the residues 101–140 (αSyn101–140) involving four 3-nitrotyrosines which represents antigenic T-cell reactivity in the mouse model of Parkinson’s disease induced by MPTP [10]. Interestingly, the epitopes derived from the nitrotyrosine-containing mouse NαSyn101–140 were properly presented on the murine class II MHC molecules encoded by alleles IAk and IAb. Accordingly, CD4+ T-cell responses specific to NαSyn101–140 were evoked in mice bearing the H2K (B10.BR strain) and H2b (C57BL/6) haplotypes when treated with MPTP [10].
With regard to the presence of T-cell epitopes in unmodified αSyn, Davtyan and colleagues [38] evaluated the activation of T-cells in C57BL/6 mice (haplotype H2b) immunized with a battery of overlapping peptides obtained from the aminoacidic sequence of unmodified αSyn, and they found only the αSyn76–95 and the αSyn106–125 peptides were able to induce IFN-γ production by T-cells. Thus, taken together these results indicate that, compared with unmodified αSyn, pathogenic forms of αSyn contains different T-cell epitopes, an important point to be considered at the moment of developing T-cell mediated immunotherapies geared to target selectively only pathogenic forms of αSyn, but not healthy αSyn.
The T-cell epitopes derived from αSyn have also been studied in humans. A systematic study analysed the T-cell epitopes involved in Parkinson’s disease patients using a battery of overlapping peptides derived from the hαSyn. The results revealed two antigenic regions of the human hαSyn involved in the T-cell response in Parkinson’s disease patients, a N-terminus antigenic region involving hαSyn31–46 and a C-terminus antigenic region involving the hαSyn116–140 [9].
The N-terminus antigenic region was presented with high affinity by the class II HLA alleles DRB1*15:01, DRB5*01:01 and DQB1*03:04 as well as by the class I HLA allele A*11:01. Interestingly, despite the N-terminus antigenic region of the hαSyn includes the Y39 which may be phosphorylated in Parkinson’s disease, the phosphorylation of this residue was not required for antigenic T-cell response [9].
In contrast, the C-terminus antigenic region of the hαSyn associated to T-cell response in Parkinson’s disease was not strongly restricted to the HLA alleles described above, and the phosphorylation at the S129 was required for antigenic T-cell response [9]. Of note, the S129 phosphorylation constitutes a pathogenic post-translational modification of αSyn that has been found in Lewy bodies [39]. According to the poor HLA restriction of the C-terminus antigenic region of the hαSyn, a recent study performed with six Parkinson’s disease patients revealed that the TCR repertoire of αSyn-specific T-cells was as diverse as the TCR repertoire of T-cells specific for pertussis, a representative foreign antigen that most individuals have been exposed to [40]. Altogether these results provide evidence that pathogenic forms of αSyn contain epitopes recognized by autoreactive T-cells, some of them strongly restricted by HLA alleles and others just poorly dependent of HLA alleles (Figure 2).
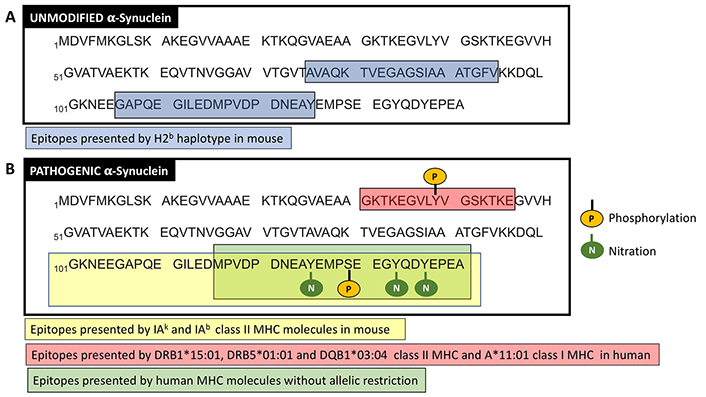
T-cell epitopes from unmodified and pathogenic αSyn. The amino acidic sequence of unmodified (A) and pathogenic (B) forms of αSyn are shown in one-letter code (obtained from UniProt; access number P37840). In (A), blue boxes show the T-cell epitopes presented by MHC molecules of the H2b haplotype in mice. In (B), the yellow box shows a 3-nitrotyrosine rich region with T-cell epitopes presented by class II MHC molecules IAk and IAb in mice. The green box shows a region with T-cell epitopes presented by human MHC molecules without a strong allelic restriction, but dependent on the phosphorylation on S129. The red box represents a region containing T-cell epitopes presented by some particular alleles of class I and class II MHC (the phosphorylation of Y39 is not required for presentation on the MHC alleles indicated). Phosphorylations and nitrations are indicated. For better clarity, only hαSyn sequences are shown
Another important aspect of pathogenic forms of αSyn as a source of T-cell antigens in Parkinson’s disease lies on the physicochemical nature of αSyn oligomers. Interestingly, even in the absence of sequence similarities, oligomers from different proteins often share some structural features, including hydrophobic surfaces [41–43]. These hydrophobic surfaces present in protein aggregates typically represent major stimulator of innate immune cells [44–46]. In this direction, a recent study evaluated the physicochemical features of oligomers relevant to stimulate human T lymphocytes using the N-terminal domain of the HypF protein from E. coli as a model of misfolded protein oligomers [47]. Of note, two different oligomeric structures might be induced with this model due to a differential organization in the core region, type A and type B oligomers. Type A oligomers display higher degree of surface hydrophobicity compared to type B oligomers [48]. Importantly, Leal-Lasarte and colleagues [47] showed that type A oligomers induced greater degree of Treg differentiation in human peripheral blood mononuclear cells (PBMC) and more potent suppressive activity in isolated human Treg compared with the effect of type B oligomers. Nevertheless, type A oligomers also promoted higher degree of Th1 and Th17 differentiation in total PBMC compared to type B oligomers [47]. Thus, it seems the greater the extent of hydrophobic surfaces on the oligomer, the more potent the T-cell response either inflammatory or anti-inflammatory. With this in mind, it would be useful to develop αSyn oligomers with high degree of hydrophobic surfaces in therapies geared to generate Treg responses specific to αSyn-derived antigens as a treatment for Parkinson’s disease.
Interestingly, some studies suggest the possibility that autoreactive T-cell responses might be triggered by the herpes simplex virus 1 (HSV1) infection due to molecular mimicry of viral components with αSyn. In this regard, Caggiu and colleagues [49, 50] found a positive correlation for the presence of T-cell responses specific for hαSyn and HSV1 in Parkinson’s disease patients. This correlation seems to be due to molecular similarities in the T-cell epitopes found in hαSyn (hαSyn100–114) and the HSV1 protein UL42 (UL4222–36) [50].
Since the T-cell response to αSyn-derived antigens has been extensively involved in the development of neuroinflammation and consequent neurodegeneration in Parkinson’s disease, many researchers have attempted to attenuate this T-cell response as a therapeutic approach to stop the disease progression. Some studies have sought to decrease the inflammatory response induced by Th1 and Th17 cells, others have attempted to potentiate the immunosuppressive response of Treg, and other researchers have tried to do both.
To inhibit the inflammatory T-cell response involved in Parkinson’s disease, a group of studies has tested the therapeutic potential of the well-known immunosuppressive drug FK506 (also called tacrolimus), which has been broadly used to inhibit the graft rejection. Its immunosuppressive effect lies in the binging to the immunophilin FK506-binding protein (FKBP) to form the FK506-FKBP complex, which inhibits calcineurin activity, thus attenuating T-cell activation [51, 52]. This immunosuppressive drug has shown therapeutic effects reducing the number of T-cells infiltrated into the SN, and attenuating the motor impairment and the neurodegeneration in a rat model of Parkinson’s disease induced by the unilateral stereotaxic delivery of AAV encoding for the A53T hαSyn [22]. FK506 has also shown to protect from the reduction of dopamine contained in the striatum in the mouse model of neurodegeneration induced by MPTP [53].
Another drug used to attenuate the inflammatory T-cell response into the CNS in fingolimod (also called FTY720). This drug is a functional antagonist of the sphingosine-1-phosphate receptor, whose stimulation is required for the egress of T-cells from the secondary lymphoid organs. Consequently, this drug reduces the entrance of T-cells into the CNS [54]. Importantly, fingolimod has shown significant therapeutic effects in multiple sclerosis, an autoimmune disorder that involves a T-cell driven neuroinflammation [54]. Due to the parallelism between multiple sclerosis and Parkinson’s disease, several groups have tested the therapeutic potential of fingolimod in animal models of Parkinson’s disease. These studies have found significant therapeutic effects of fingolimod attenuating the disease development in animal models of neurodegeneration induced MPTP, 6-OHDA, rotenone or by the stereotaxic delivery of AAV-hαSyn in the SN [17, 55–57].
A group of studies have tested the therapeutic potential of drugs improving the Treg response as a treatment for Parkinson’s disease. For instance, a recent study sought to induce the generation of αSyn-specific Treg cells as a treatment of Parkinson’s disease using microparticles loaded with hαSyn and rapamycin in a transgenic mouse model that involves the overexpression of hαSyn. The results of this study show that this therapeutic approach indeed increased the extent of Treg infiltrating the CNS and reduced the deposits of pathogenic forms of αSyn and limited the development of neuroinflammation and neurodegeneration [58].
Another Treg-based experimental therapy for Parkinson’s disease has used a nicotinic agonist of the cholinergic receptor α7 (α7nAChR), PNU-282987, which has been shown to increase Treg response [59]. This study provided data indicating that the intraperitoneal administration of PNU-282987 increased the extent of Treg infiltration into the SN of rats treated with the stereotaxic delivery of 6-OHDA into the medial forebrain bundle. This increased Treg infiltration was associated with a significant reduction in the motor impairment, attenuated loss of dopaminergic neurons in the SN, and decreased astrogliosis [59].
An interesting example of immunotherapy based in the potentiation of Treg response has used the vasoactive intestinal peptide (VIP), which has been described to increase the Treg number and their activity [60, 61]. According to its immunosuppressive potential, the adoptive transfer of VIP-generated Treg in mice treated with MPTP exerted a strong therapeutic effect attenuating microglial activation and neurodegeneration of the nigrostriatal pathway [11]. In addition to its effect on Treg cells, VIP might also induce the acquisition of tolerogenic features in dendritic cells (DCs) [62]. In this regard, VIP-generated tolerogenic DCs not only favour the expansion of Treg, but also induce the differentiation of naive CD4+ T-cells into Tr1 cells, another subset of T-cells with immunosuppressive activity [63]. Due to the multi-target nature of VIP inducing immunosuppression, Gendelman’s team generated a synthetic version of VIP (LBT-3627) with enhanced resistance to proteases and thereby increased half-life. Accordingly, the treatment of MPTP-mice or rats overexpressing hαSyn with LBT-3627 has shown promising results, attenuating microglial activation and reducing the extent of neurodegeneration [64, 65].
Another immunotherapy proposed for the treatment of Parkinson’s disease is based in the anti-inflammatory effects of granulocyte-monocyte colony-stimulating factor (GM-CSF) on DCs, which induces the acquisition of tolerogenic properties in these cells, thereby favouring the generation of Treg cells [66–68]. Accordingly, Lipid nanoparticle containing mRNA encoding GM-CSF were administered intramuscular in MPTP-mice or rats overexpressing αSyn [69]. The treatment induced a rise in plasma GM-CSF and increased frequency of peripheral Treg cells. Concomitantly, the treatment reduced neuroinflammation and neurodegeneration of dopaminergic neurons in mouse and rat models of Parkinson’s disease [69]. Due to the successful results in preclinical models, the therapeutic potential of recombinant human GM-CSF (sargramostim) for the treatment of Parkinson’s disease is currently under study in a clinical trial (NCT03790670).
Finally, a group of studies have sought both increasing Treg responses and attenuating inflammatory T-cell responses at the same time to promote a therapeutic effect in animal models of Parkinson’s disease. In this regard, an interesting molecular target to dampen T-cell mediated inflammation in Parkinson’s disease is the dopaminereceptor D3 (DRD3) [70]. DRD3-signallingin T-cells has been shown toinduce Th1 differentiation and to promote Th17 expansion [71], thereby potentiating the function of two subsets of inflammatory T-cells involved in Parkinson’s disease [37]. In addition, DRD3-signalling limits the suppressive activity of Treg cells [72], thereby contributing to promote T-cell mediated inflammation. Moreover, DRD3 expression is altered in CD4+ T-cells obtained from Parkinson’s disease patients [73], suggesting the involvement of this receptor in T-cell mediated neuroinflammation in human individuals. DRD3 is selectively stimulated by low dopamine levels found in the nigrostriatal pathway in Parkinson’s disease [23]. Accordingly, the genetic deficiency of Drd3 in CD4+ T-cells results in a strong attenuation of neuroinflammation and neurodegeneration in a mouse model of Parkinson’s disease induced by MPTP [12]. Interestingly, DRD3-signalling in astrocytes also contribute to promote neuroinflammation [74]. According to the pro-inflammatory role of DRD3 in T-cells and astrocytes, the systemic administration of a selective DRD3-antagonist, PG01037, has shown to inhibit the motor impairment and to reduce the extent of neurodegeneration in mouse models of Parkinson’s disease induced by MPTP and 6-OHDA [75].
Another example of immunotherapeutic approach geared to affect both Treg and inflammatory T-cells to attenuate neuroinflammation in Parkinson’s disease involved the prophylactic vaccination with αSyn in combination with the chaperone Grp94 and CFA in the MPTP mouse model [76]. Using direct immunization of MPTP-treated mice or the adoptive transfer of splenocytes isolated from vaccinated animals into MPTP-treated mice, the authors observed a significant reduction of microglial activation in the SN and the striatum. Mechanistical analyses indicated that this formulation induced an αSyn-specific adaptive immune response involving Th1 and Treg cells and a humoral response predominantly mediated by IgG1 anti-αSyn antibodies [76]. The different experimental immunotherapies based in the attenuation of T-cell driven neuroinflammation as treatment for Parkinson’s disease are summarized in the Table 1.
Experimental T-cell-based immunotherapies tested in pre-clinical models of Parkinson’s disease
| Treatment | Effect on T-cells | Effect on Parkinson’s disease | Model | Reference |
|---|---|---|---|---|
| FK506 | Inhibits T-cell activation | Attenuates motor impairment and neurodegeneration | AAV-A53T-hαSyn in rat | [22] |
| FTY720 | Arrest T-cells in lymph nodes | Attenuates neuroinflammation | AAV-hαSyn in mouse | [17] |
| FTY720 | Arrest T-cells in lymph nodes | Attenuates motor impairment, neuroinflammation and neurodegeneration | 6-OHDA in mouse | [55] |
| FTY720 | Arrest T-cells in lymph nodes | Attenuates motor impairment and neurodegeneration | 6-OHDA in mouse | [56] |
| FTY720 | Arrest T-cells in lymph nodes | Attenuates motor impairment and neurodegeneration | Rotenone in mouse | [56] |
| FTY720 | Arrest T-cells in lymph nodes | Attenuates motor impairment and neurodegeneration | MPTP in mouse | [57] |
| Rapamicyn + αSyn | Increases Treg differentiation | Attenuates neuroinflammation, neurodegeneration and synuclein pathology | Transgenic mice overexpressing hαSyn | [58] |
| PNU-282987 | Increases Treg response | Attenuates motor impairment, neuroinflammation and neurodegeneration | 6-OHDA in rat | [59] |
| VIP | Increases Treg number and activity | Attenuates neuroinflammation and neurodegeneration | MPTP in mouse | [11] |
| VIP (LBT-3627) | Increases Treg number and activity | Attenuates neuroinflammation and neurodegeneration | AAV-hαSyn in rat | [64] |
| VIP (LBT-3627) | Increases Treg number and activity | Attenuates neuroinflammation and neurodegeneration | 6-OHDA in rat | [64] |
| VIP (LBT-3627) | Increases Treg number and activity | Attenuates neuroinflammation and neurodegeneration | MPTP in mouse | [65] |
| GM-CSF | Favours Treg generation | Attenuates neuroinflammation and neurodegeneration | MPTP in mouse | [69] |
| GM-CSF | Favours Treg generation | Attenuates neuroinflammation and neurodegeneration | AAV-hαSyn in rat | [69] |
| PG01037 | Inhibits Th1/Th17 and increases Treg activity | Attenuates motor impairment, neuroinflammation and neurodegeneration | MPTP in mouse | [75] |
| PG01037 | Inhibits Th1/Th17 and increases Treg activity | Attenuates neurodegeneration | 6-OHDA in mouse | [75] |
| Grp94 + αSyn | Promotes Treg and Th1 response | Attenuates neuroinflammation | MPTP in mouse | [76] |
Finally, it is important to mention that most of the current therapeutic approaches attempting to manipulate the T-cells response as a treatment for Parkinson’s disease are directed to unmodified αSyn, but not specific to pathogenic forms of αSyn. Thus, most of these therapies would attenuate the T-cell-mediated inflammatory response to unmodified αSyn even in healthy conditions. This fact might be potentially dangerous, as an inflammatory T-cell response specific to unmodified αSyn may be beneficial under some particular circumstances, such as in neuronal cancer (i.e. neuroblastoma). Accordingly, to improve the safety of T-cell-based immunotherapies for Parkinson’s disease, it is important to direct the Treg response specifically to pathogenic forms of αSyn, but not to unmodified αSyn.
Current evidence suggests an important role of T-cell-driven inflammation in early stages of Parkinson disease development in human individuals. In addition, recent studies in animals and human individuals indicate that both CD4+ and CD8+ T-cells play important roles in the development of neurodegeneration in Parkinson’s disease. These studies encourage the design of immunotherapies for Parkinson’s disease based in the generation of Treg responses specific to pathogenic forms of the αSyn or other antigens relevant for this disorder (i.e. mitochondrial autoantigens). A number of studies that have analysed the T-cell epitopes from αSyn indicate that, compared with unmodified αSyn, pathogenic forms of αSyn contains different T-cell epitopes, an important point to be considered at the moment of developing T-cell mediated immunotherapies geared to target selectively only pathogenic forms of αSyn, but not healthy αSyn. Interestingly, pathogenic forms of αSyn contain epitopes recognized by autoreactive T-cells, some of them strongly restricted by HLA alleles and others just poorly dependent of HLA alleles. Moreover, some studies suggest the possibility that autoreactive T-cell responses might be triggered by HSV1 infection due to molecular mimicry of viral components with αSyn. Furthermore, a number of researchers have designed experimental immunotherapies directed to attenuate the T-cell driven neuroinflammation associated to Parkinson’s disease in preclinical models. Some studies have sought to decrease the inflammatory response induced by Th1 and Th17 cells, others have attempted to potentiate the immunosuppressive response of Treg, and other researchers have tried to do both together. However, most of these experimental immunotherapies are not specific for pathogenic forms of αSyn, or even not specific for unmodified αSyn. Further efforts should be done to design T-cell based immunosuppressive immunotherapies specific only for pathogenic forms of αSyn and with no effect on healthy unmodified αSyn.
αSyn: alpha-synuclein
AAV: adeno-associated viral vectors
CFA: complete Freund’s adjuvant
CNS: central nervous system
DCs: dendritic cells
DRD3: dopamine receptor D3
GM-CSF: granulocyte-monocyte colony-stimulating factor
hαSyn: human αSyn
HLA: histocompatibility leukocyte antigens
HSV1: herpes simplex virus 1
MHC: major histocompatibility complex
MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
NαSyn: αSyn containing 3-nitrotyrosines
NSG: NOD SCID gamma-chain deficient
PINK1: PTEN-induced kinase 1
ROS: reactive oxygen species
SN: substantia nigra
TCR: T-cell receptor
Th: T helper
TNFα: tumour necrosis factor α
Treg: regulatory T cells
VIP: vasoactive intestinal peptide
6-OHDA: 6-hydroxydopamine
The author contributed solely to the work.
The author declares that he has no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work was supported by Programa de Apoyo a Centros con Financiamiento Basal [AFB-170004] from “Comisión Nacional de Investigación Científica y Tecnológica de Chile (CONICYT)”, by grants [FONDECYT-1210013] from “Fondo Nacional de Desarrollo Científico y Tecnológico de Chile”, and [MJFF-15076] from the Michael J. Fox Foundation for Parkinson’s research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2021.
Copyright: © The Author(s) 2021. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
