Affiliation:
1Neurosurgical Department, Policlínica Juaneda Miramar, 07011 Palma de Mallorca, Spain
2Neurosurgical Department, Policlínica Nuestra Señora del Rosario, 07800 Ibiza, Spain
Email: jg.machado@hotmail.com
ORCID: https://orcid.org/0000-0003-2884-6422
Explor Neuroprot Ther. 2022;2:174–181 DOI: https://doi.org/10.37349/ent.2022.00027
Received: April 11, 2022 Accepted: June 17, 2022 Published: August 30, 2022
Academic Editor: Brandon Lucke-Wold, University of Florida, USA
The article belongs to the special issue Emerging Concepts in Subarachnoid Hemorrhage
The global prevalence of intracranial aneurysms (IA) ranges from 5–10%, with a demographic variation. Large and giant aneurysms typically involve cavernous and paraclinoid segments of the internal carotid artery (ICA), and represent 5% of IA. Typically, these lesions involve segments of the ICA, especially the cavernous and paraclinoid segments. The remaining cases affect the vertebrobasilar region, middle cerebral artery (MCA), and anterior cerebral artery (ACA). From the morphological point of view, they are divided into saccular and fusiform. In cases of rupture, the subarachnoid hemorrhage (SAH) is the most common presentation followed by intracerebral hemorrhage (ICH), or both. Other manifestations can occur as occlusion of perforating vessels, embolic events, seizures, and mass effects. The management of unruptured intracranial aneurysms (UIA) is controversial, and the aim of treatment is to exclude the lesions and preserve neurological function. Endovascular techniques for the treatment of paraclinoid aneurysms, in particular, ICA reconstruction using flow-diverting stents, have become a valid option. However, surgery or endovascular treatment has a number of limitations and the choice of treatment is individual in each case. This type of lesion has an extremely poor natural history, and treatment is a challenge regardless of the technique used.
The report described a clinical case of a 55-year-old female, with a personal history of hypertension, hyperthyroidism, and depressive syndrome. The patient started complaints of moderate-intensity right frontal headache, progressively worsening with two months of evolution. She also reported blurred vision and diplopia. Brain computed tomography (CT) documented a partially calcified sellar and parasellar lesion. Subsequently, magnetic resonance imaging (MRI)/MRI angiographies were performed and showed a saccular aneurysm of the right ICA, cavernous segment. The patient underwent a diagnostic and therapeutic angiography with stent placement. Clinical and imaging improvements were documented by angiography and MRI angiography with progressive reduction of the aneurysm during the period of follow-up.
Intracranial aneurysms (IA) have an estimated incidence between 5% and 10%, with some demographic variation. Depending on the size, the internal carotid artery (ICA) aneurysms are defined as large (1.5–2.5 cm in diameter) and giant (> 2.5 cm in diameter) being considered among the most complex pathologies. The complexity is inherent in its size and location. In the case of surgical treatment, the complexity is associated with the dimension of the aneurysms, cohesion of their walls with the base, the proximity of functionally important structures, and the need for highly delicate dissection of the lesion in order to preserve vision and blood flow in the ICA branches and perforating arteries [1].
According to used references studies, detailed preoperative planning includes the following imaging techniques: magnetic resonance imaging (MRI), computed tomography angiography (CTA), and angiography (gold standard).
The treatment of these aneurysms must be defined taking into account the risks and benefits associated with the techniques used. The decision must be made on a case-by-case basis, as we will see throughout this work. Different strategies for the treatment of these injuries should be considered. Published studies demonstrate that surgical experience, as well as endovascular techniques, is a fundamental parameter to achieving better results in the treatment of this pathology [2].
After the publication of the International Subarachnoid Aneurysm Trial, the approach to IA has changed, and more cases are treated using endovascular techniques. As mentioned, the giant and large aneurysms treatment remains a challenge, even in centers with high volume and experience. Paraclinoid aneurysms are defined as those that have their origin in the ICA between the distal dural ring and the posterior communicating artery. As previously mentioned, the different treatment modalities have associated advantages and disadvantages. Direct clipping methods have been developed during the last decades for treatment of all aneurysms and particularly of ICA paraclininoid aneurysms. The decompression by aspiration of giant aneurysms (GA) to drain their contents, is part of the procedure, thus allowing a better dissection. In addition, intraoperative monitoring, electrophysiology, and blood flow study are recommended to prevent complications. Different forms of electrophysiology monitoring can be used, such as somatosensory, motor, and visual evoked potentials; blood flow can be monitored using Doppler, intraoperative angiography, or intraoperative video angiography with indocyanine green. Actually the best treatment recommended is the combination of all these techniques and devices, if necessary, adjusted for each case if they are available [3]. In the field of endovascular treatment, several approaches are also described. Definitely, and according to published articles, we think that the choice of treatment must be individualized in each case, achieving aneurysm exclusion without causing new neurological deficits.
It described a clinical case of a 55-year-old female with a personal history of hypertension, hyperthyroidism, and depressive syndrome. The patient started complaints of moderate-intensity right frontal headache, progressively worsening in the two months prior to the evaluation at the emergency room. She also reported blurred vision and diplopia. The patient did not report any other complaints. In ophthalmological evaluation, there was no change in visual fields. The initial laboratory analysis excluded hematological, renal, and hepatic changes. The hormonal study did not show relevant alterations, and this point is important in the differential diagnosis, when considering the presence of sellar masses.
A cranial computed tomography (CT) revealed the presence of a partial calcified sellar and parasellar mass with a 27 mm diameter, which was confirmed by MRI (Figure 1) and MRI angiography showing saccular aneurysm of the right ICA, cavernous segment (Figure 2).
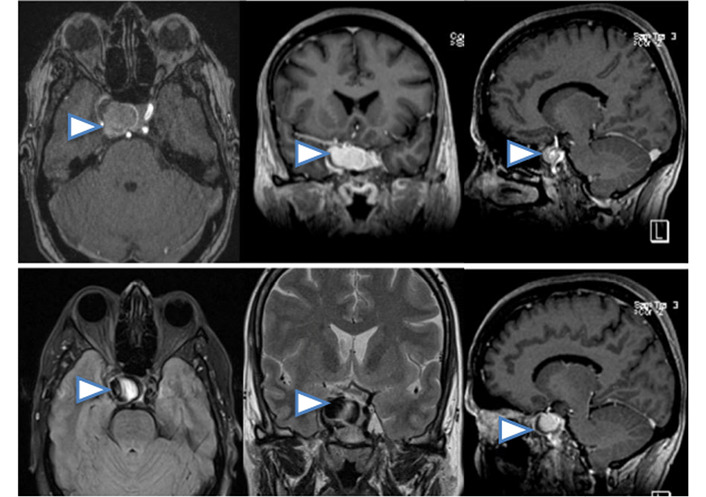
MRI scan presenting axial, coronal and sagittal slices in which can be seen sellar and parasellar lesion, lateralized to the right side, partially calcified, arrowheads. No subarachnoid hemorrhage (SAH) associated
The angiography is the gold standard technique for confirming the presence of intracranial aneurysmal lesions. Based on the results obtained on CT and MRI, we decided to perform an angiography that confirmed a saccular aneurysm (Figure 3).
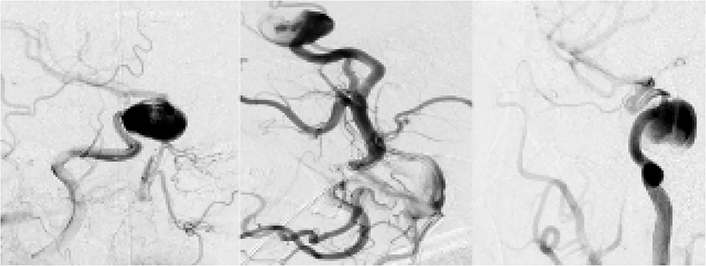
Diagnostic angiography: identified a giant saccular aneurysm, right ICA in the cavernous segment
After multidisciplinary discussion, an endovascular treatment was decided. The patient underwent a therapeutic angiography, 10 days before the intervention, dual antiplatelet therapy was started with clopidogrel 75 mg/day and aspirin 100 mg/day. It was decided to perform the procedure under general anesthesia. Heparin was administered at the beginning and intermittently during the procedure. In our center we used for this case a right femoral approach. A 6 Fr, 0.070 inch inside diameter (ID) guide catheter was used to access the right ICA. Through a 3.2 Fr, 0.027 inch ID microcatheter, flow diverters (FDs) (4 mm × 25 mm, 4 mm × 16 mm) were implanted into the neck of the aneurysm. The procedure was performed without complications (Figure 4).
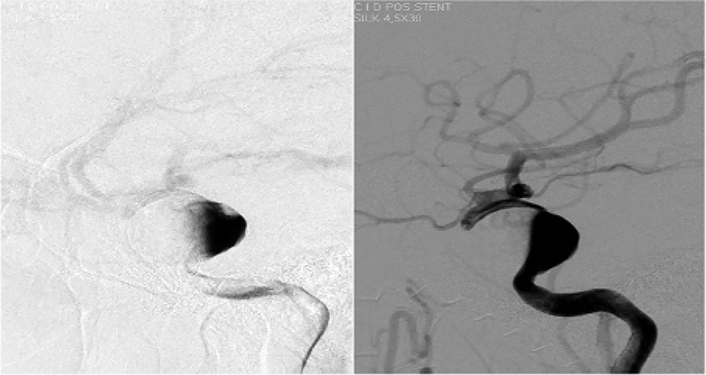
Therapeutic angiography placed stent, flow-diverter, without complications and good distal perfusion
The patient was discharged from the hospital with a clinical and image improvement documented by angiography with progressive reduction of the aneurysm dimension. Clinical and imaging follow-up was maintained. The patient did not report again blurred vision or diplopia and referred improvement in headache. At 6 months of follow-up, there performed an MRI with angiographic effect with shows a reduction of aneurysm dimensions (Figure 5).
In conclusion, here reported a case of a patient with moderate-intensity right frontal headache, progressively worsening in the two months prior to arrival at the emergency room. She also reported blurred vision and diplopia caused by ICA giant aneurysm.
An endovascular treatment was decided and successfully performed as described above. In cases treated with endovascular approach, there are many described forms with single stent or multiples stents especially in large aneurysms to reduce the flow inside the aneurysm dome and promote progressive thrombosis. We expose our single case and only want to describe one approach more, but don’t forget that cases are very complex, and a multidisciplinary approach is necessary in many cases. We kept clinical and imaging follow-ups for exclusion of new signs of revascularization, one of the long-term complications associated with endovascular treatment, according to studies carried out to date.
GA are considered rare, with an incidence of around 5% with some demographic variance. The pediatric population verified a higher proportion of this type of aneurysm. Frequently, these lesions develop in the cavernous and paraclinoid segments of the ICA. The remaining cases mainly affect middle cerebral artery (MCA) and anterior cerebral artery (ACA) [1].
The natural history of untreated giant brain aneurysms is poor. Associated complications may be caused by the mass effect on the surrounding brain, embolic events and the high risk of rupture [2].
GA can be classified into saccular, fusiform, and serpentine, according to their appearance and pathogenesis. Saccular GA are round or oval vascular malformations, with a neck that connects them to the vessel. They are thought to arise from small saccular (berry) aneurysms through repeated tearing and scaring of the aneurysmal wall. These processes lead to an intra-aneurysmal turbulent flow, which maintains the progressive growth of the aneurysm. Partially or complete thrombosed aneurysms are thought to appear due to inflammatory mechanisms triggered by chronic dissections and hematomas of the aneurysmal wall and by the concomitant proliferation of the vasa vasorum. ICA aneurysms can become symptomatic in three different ways: by rupture, (and so, causing SAH), by acting as a tumor which exerts a mass effect on the adjacent cerebral structures, and by compressing an adjacent vessel, thus determining cerebral ischemic lesions [3]. As noted in the International Study for Unbroken Intracranial Aneurysms, the aneurysmal pathology is common in general population and the detection increasing with noninvasive imaging release for other causes.
Many unruptured intracranial aneurysms (UIA) have a benign course, but carry a risk of rupture with SAH that could be potentially catastrophic [4]. Knowledge of the hemodynamic characteristics of each aneurysm is essential and plays an essential role in development, growth and potential rupture. The flow structure in this type of injury is highly complex. It’s important to determine the volumetry as well as the properties of intra-aneurysmal flow. In the study, an MRI with contrast was performed to assess fluid dynamics within a replica of a giant aneurysm printed in 3D. One of the main objectives of the study was to demonstrate the ability of in vitro 4D flow MRI to characterize hemodynamics. The study has several limitations; however, it allows obtaining several useful variants in the hemodynamic characterization present in the intracranial aneurysm. Application in the daily clinic can be challenging. New studies may allow greater precision and resolution. It is a non-invasive technique that may provide benefit for the diagnosis of these patients [5].
In cases of symptomatic patients with SAH, the prognosis was worse. As described in previous studies, the risk of rupture in UIA depends essentially on the size, location and shape of the aneurysm. Preventive treatment is justified when the benefits outweigh the anticipated risks of carrying out the treatment [4–7]. The decision to treat depends on risk of rupture, because the treatment in UIA is to prevent the SAH and the potentially devastating effects, as described in all studies evaluated. The ability to accurately estimate in each patient the risk of rupture is imperfect and should be one of the aspects to improve in future studies to characterize aneurysmal hemodynamics [8].
Each new diagnosis of unruptured aneurysm warrants a multidisciplinary evaluation that should reach a consensus on the risk of aneurysm rupture, risks of conservative treatment, risks of surgical treatment or endovascular treatment. It is important to consider the duration of the treatment, the patient’s age, comorbidities, and lifestyle [6].
The goal of the treatment of giant IA is to exclude the lesion from circulation. A multidisciplinary approach that includes neurosurgical and endovascular knowledge is often required to adequately treat these types of lesions. The complexity of these injuries makes their treatment more difficult, which is associated with a potentially high rate of complications.
Currently there are 2 effective methods for treatment as surgical clipping and endovascular management. Both treatments carry a risk of morbidity and mortality. In this context, it is suggested that the treatment of this pathology occur in centers with a high flow of cases and high experience in the management and choice of the most appropriate treatment [2, 4–8]. The treatment of this pathology is complex and requires strict, meticulous planning before treatment, as described previously in our paper and based on the articles. At our center, decision-making for the treatment of these injuries is individualized for each patient.
However, the decision may depend on the experience of each center as well as the resources available.
Traditional treatments of GA were direct surgical clipping or entrapment with or without bypass surgery. The surgical approach implies high complexity, being a great challenge and may be associated with significant morbidity. Currently, endovascular treatment is gaining ground and has largely replaced open surgery in the treatment of this pathology, in most locations [7]. This study shows that the preservation of the parental artery is feasible and effective. After performing the endovascular treatment, this technique globally presents acceptable morbidity and mortality rates, but its importance considers several factors such as characteristics of the aneurysm itself like size or aneurysm neck. We cannot forget that the experience of the team that performs the treatment also has an important value. As in the surgical approach is a challenge, but in endovascular treatments the problem of possible recanalization is higher and more common, especially in GA. The two major causes of recanalization are coil compaction due to thrombus resolution in the sac, and insufficient packing density [7]. Many recent endovascular technique advancements or tools are available for the treatment of GA, the advent and popularization of this technique increasing the paradigm shift in the treatment of cranial aneurysms, especially unruptured ones, in the last 20 years. As described previously, endovascular treatments are increasingly used, and several studies emphasized the advantages and main drawbacks of this technique as the long-term durability and increased recurrence rate. Recurrences after endovascular therapy are around 30%.
In fact, there is a lack of randomized controlled trials as well as a paucity of aggregated data on the risk of rupture for cases treated with endovascular techniques. Systematic analyzes and meta-analyses performed with the aim of determining the long-term risk of rupture in endovascularly treated patients proved to be extremely uncommon. This point is applicable especially to the small aneurysms. This study has many important limitations, such as the lack of systematization of the follow-up period between the different studies included, as well as a great diversity of imaging techniques used in the follow-up [9].
In the Study on Analysis of Recanalization After Endovascular Treatment of Intracranial Aneurysm (ARETA), the technique choice to treat UIA was observed to be a dependent on many aspects such as the sac size, neck diameter and location. The authors refer that 11.6% of UIA were treated by flow diversion, representing a significant proportion in this cohort. This device was especially used in wide-neck large and giant aneurysms. The results are in line with current recommendations [6].
FDs have changed the management of brain aneurysms; not only for complex aneurysms, but also for unruptured lesions especially in cases of large or giant wide-neck. This device presents low rates of complications and high rates of cure when compared with other therapeutic options [10]. FDs have a geometry similar to stents, however, as a difference, they have many more struts oriented to form a fine mesh, and have a higher pore density than stents, which reduces blood flow through the aneurysm neck, facilitating blood stagnation within the dome of the aneurysm [11–13]. The FDs devices are fundamentally based on two concepts: the placement of a high-density mesh device in the main vessel that causes interruption of blood flow to the aneurysm itself, resulting in progressive intra-aneurysmal thrombosis. The device provides a scaffold on which endothelial cells can grow to isolate the aneurysmal lumen from the circulation via recanalization. The FDs are suitable for both wide-necked and fusiform IA [14].
The American Stroke Association (ASA) guidelines recommend an interval of 6 months to 12 months for performing imagine control with CTA, MRI or digital subtraction angiography (DSA) can be used for at least 5 years after effective treatment of aneurysm [15].
The treatment option should be considered based on many factors such as age, medical history, comorbidities, location, morphology, size of the aneurysm, the patient’s preference, and the operator’s experience. At the beginning of the decision process, we must bear in mind that it must be dynamic and always guided by the best and most recent scientific evidence, as well as by new technological advances [16].
The mortality rate in endovascular treatment is lower than that described in cases of surgical clipping [17]. As described in the majority of evaluated studies, the treatment should be chosen after a multidisciplinary debate and with a joint approach if necessary. In cases treated with an endovascular approach, there are many described forms with a single stent or multiple stents especially in GA for reducing the flow inside the aneurysm dome and promoting progressive thrombosis. As described in some cases, forms are solved with a single stent, in many other cases are necessary multi stents or procedures due to coil and stent combination.
We expose our single case and only want to describe one approach more, but don’t forget that cases are very complex and a multidisciplinary approach is necessary in many cases. The clinical status of the patient at the time of diagnosis plays an important role in decision-making about the treatment to be adopted. This choice would be defined case by case. Therefore, in our opinion and as described in other studies, achieving complete occlusion is the goal to be overcome in the treatment of GA, and a strict clinical follow-up treatment with imaging techniques should be viewed as mandatory. In our case, the exclusion of the aneurysm was achieved only with the endovascular technique, however, in other cases, the use of other complementary techniques may be necessary.
The ideal form for treatment of this pathology is continuously uncertain but the aim of the treatment is to exclude the lesion of the circulation and preserve the function of the surrounding neurological structures. A multidisciplinary approach is recommended for the selection of the most appropriate therapeutic option in each case, microsurgical or endovascular techniques. The evolution of the microsurgical and endovascular techniques is fundamental to improving the therapeutic results of this pathology. In our case, the endovascular treatment allowed the exclusion of the aneurysm with good clinical results. Studies in a larger population size will define the ideal treatment for these injuries in the future.
FD: flow diverter
GA: giant aneurysms
IA: intracranial aneurysms
ICA: internal carotid artery
MRI: magnetic resonance image
SAH: subarachnoid hemorrhage
UIA: unruptured intracranial aneurysms
Thanks to João Reis (MD) Chairman of Neurorradiology Department, Centro Hospitalar Universitário Lisboa Central - Hospital de São José, Lisbon, Portugal.
The author contributed solely to this work.
The author declares that he has no conflicts of interest.
Not applicable.
Informed consent to participate was obtained from the relevant participant.
Informed consent to publication was obtained from the relevant participant.
Not applicable.
Not applicable.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 6179
Download: 53
Times Cited: 0
Eric J. Panther, Brandon Lucke-Wold
Stephan Quintin ... Brandon Lucke-Wold
Fettah Eren ... Sueda Ecem Yilmaz
Andrés Ricaurte-Fajardo ... Nathalia Melo Gonzalez
Mohammed A. Azab ... Brandon Lucke-Wold
