Affiliation:
1Department of Medicine, Division of General Internal Medicine and Geriatrics, Indiana University School of Medicine, Indianapolis, IN 46202, USA
Email: fpperez@iu.edu
ORCID: https://orcid.org/0000-0002-0068-5252
Affiliation:
1Department of Medicine, Division of General Internal Medicine and Geriatrics, Indiana University School of Medicine, Indianapolis, IN 46202, USA
Affiliation:
2Department of Bioengineering, University of Illinois at Chicago, Chicago, IL 60607, USA
Affiliation:
3Department of Electrical and Computer Engineering, Purdue University, Indianapolis, IN 46202, USA
Affiliation:
3Department of Electrical and Computer Engineering, Purdue University, Indianapolis, IN 46202, USA
Explor Neurosci. 2025;4:100674 DOI: https://doi.org/10.37349/en.2025.100674
Received: December 01, 2024 Accepted: February 14, 2025 Published: February 25, 2025
Academic Editor: Ryszard Pluta, Medical University of Lublin, Poland
The article belongs to the special issue Alzheimer's Disease
The use of neurostimulation devices for the treatment of Alzheimer’s disease (AD) is a growing field. In this review, we examine the mechanism of action and therapeutic indications of these neurostimulation devices in the AD process. Rapid advancements in neurostimulation technologies are providing non-pharmacological relief to patients affected by AD pathology. Neurostimulation therapies include electrical stimulation that targets the circuitry-level connection in important brain areas such as the hippocampus to induce therapeutic neuromodulation of dysfunctional neural circuitry and electromagnetic field (EMF) stimulation that targets anti-amyloid molecular pathways to promote the degradation of beta-amyloid (Aβ). These devices target specific or diffuse cortical and subcortical brain areas to modulate neuronal activity at the electrophysiological or molecular pathway level, providing therapeutic effects for AD. This review attempts to determine the most effective and safe neurostimulation device for AD and provides an overview of potential and current clinical indications. Several EMF devices have shown a beneficial or harmful effect in cell cultures and animal models but not in AD human studies. These contradictory results may be related to the stimulation parameters of these devices, such as frequency, penetration depth, power deposition measured by specific absorption rate, time of exposure, type of cell, and tissue dielectric properties. Based on this, determining the optimal stimulation parameters for EMF devices in AD and understanding their mechanism of action is essential to promote their clinical application, our review suggests that repeated EMF stimulation (REMFS) is the most appropriate device for human AD treatments. Before its clinical application, it is necessary to consider the complicated and interconnected genetic and epigenetic effects of REMFS-biological system interaction. This will move forward the urgently needed therapy of EMF in human AD.
As of 2022, an estimated 6.5 million Americans lived with Alzheimer’s disease (AD), and as the population ages, this number is expected to rise to 14 million by 2060. AD costs the nation $321 billion in 2022, and unless we develop an effective treatment, costs will continue to escalate, reaching $1 trillion in 2050 [1]. The standard of care for AD treatment includes cholinesterase inhibitors, memantine hydrochloride (N-methyl-D-aspartate receptor antagonist), and monoclonal antibodies (mAbs). However, these methods are unable to lower the toxic Aβ levels without causing brain swelling or microhemorrhages [2, 3], unable to stop AD progression [4], unable to reach multiple targets [5, 6], unable to easily cross the blood-brain-barrier (BBB) [7], and unable to link the aging process to AD pathology [8].
AD is described by beta-amyloid (Aβ) plaques and tangles of neurofibrillary tau proteins in the brain [9]. The amyloid hypothesis states that increases in Aβ levels cause the disease [9], specifically, the binding of Aβ oligomers to multiple cellular receptors is the probable cause of neuronal toxicity [10]. This induces mitochondrial dysfunction and oxidative stress in AD neurons [11]. A human study with 13C6-leucine demonstrated that the pathological factor in AD was a deficit in the clearance rather than the overproduction of Aβ from the central nervous system (CNS) to the periphery [12, 13]. This deficiency of plaque clearance progresses into neuronal degeneration, causing memory impairment and cognitive decline. Therefore, any therapy that has anti-amyloid properties that lower Aβ levels would likely prevent neurodegeneration [14] and cognitive impairment if it does not cause brain inflammation or bleeds. Preclinical studies showed that lowering Aβ levels by repeated electromagnetic field stimulation (REMFS) stops and reverses AD. Even though mAbs lower Aβ levels, they have not stopped AD progression due to serious side effects and the fact that they target extracellular Aβ [2, 3], instead of enhancing intracellular proteostasis or autophagy.
Numerous EMF devices have recently investigated the effects of exposure to EMFs on the underlying pathomolecular pathways of AD [15], leading to innovative therapeutic strategies into the mechanisms through which EMF modulates AD-related symptoms. Depending on the type of system, it can be classified as electric current (direct or alternating), magnetic stimulation (causes induction of electric field to depolarized neurons), and REMFS, which is based on the frequency used. EMFs can be classified in extremely low frequency (ELF) (3–30 Hz), super LF (SLF) (30–300 Hz), ultra LF (ULF) 300 Hz–3 kHz, very LF (VLF) 3–30 kHz, LF 30–300 kHz, medium frequency (MF) 3 kHz–3 MHz, high frequency (HF) 3–300 MHz, very HF (VHF) 30–300 MHz, ultra HF (UHF) 300 MHz–3 GHz, super HF (SHF) 3–30 GHz, extremely HF 30–300 GHz, and terahertz radiation 0.3–3 THz [16–18]. Electromagnetic pulses (EMPs) vs. continuous waves are another type of EMF and can be distinguished by their unique properties. Pulse EMF can affect alpha activity [16] and impair cognitive function [17, 18], whereas continuous EMF does not affect alpha activity or impair cognition. This review focuses on continuous wave REMFS. Depending on the amplitude, time of exposure, intensity, tissue characteristics, and EM frequency range, EMFs can produce no beneficial or harmful health effects within the human body.
A new anti-amyloid strategy by repeated REMFS not only stops cognitive impairment but also reverses it [19, 20]. In AD mice, REMFS at 915–2,000 MHz and power deposition with a specific absorption rate (SAR) of 0.25 to 5 W/kg [21, 22] stopped AD progression [23] by lowering Aβ levels [24] in numerous AD rodent studies [19–21, 25–35] without causing brain edema or hemorrhages [2, 3]. These exposures did not cause any cancer after two years of treatment [20], the main side effect was a body temperature rise (TR) of 1.3°C in the AD mice [20]. Since radiofrequency heat can cause tissue injury, it must be maintained at a safe level of less than 0.5°C, according to regulatory agencies [36]. These circumstances raised a primary question: Can a combination of variables (frequency, power, and antenna type) generate the required penetration depth with a safe and effective SAR to lower Aβ levels with a TR < 0.5°C in the human brain? Here, we will examine possible answers to develop an appropriate device for human exposure.
Few neurostimulation studies have used cell cultures with direct current (DC) stimulation techniques like those used in clinical trials. A recent review [37] concluded that the DC stimulation effects are non-synaptic membrane polarization, are driven by glutamate, are gated by gamma-aminobutyric acid (GABA) activity reduction, require brain-derived neurotrophic factor (BDNF) expression, and require protein synthesis. In an in vitro model study, electrical stimulation did not affect the proliferation or survival of the examined cell lines but upregulated C-X-C motif chemokine 12 (CXCL12) in the astrocyte SVGA cell line and IL-1β in SH-SY5Y neuronal line [38]. Other studies found [39] that the response of the neurons to DC depended on the position and orientation of the axon and the electric parameters.
Deep brain stimulation (DBS) in animals is performed by stimulating electrodes implanted in the head, and the stimulator is connected externally, like in human DBS. Different techniques are used to apply DBS; one type is implanting the stimulators on the backs of the mice [40], and then the stimulating electrode is implanted at the target position and fixed to the skull with adhesive material. In other studies, screws are implanted in the skull to fix the electrodes [41]. In some studies, X-ray imaging or tissue staining confirms that the electrode is implanted in the target position [42]. DBS was shown to reduce Aβ plaques in the hippocampus and cortex of 6-week-old TgCRND8 mice [43] and amyloid precursor protein (APP) levels in 3xTg mice. Also, DBS modulates glial cell activity [44] in the AD rat model.
Magnetic stimulation was applied to cell cultures derived from the frontal cortex of murine embryos; the cultures were exposed to sinus-shaped HF magnetic fields [45] to examine the effects of repetitive magnetic stimulation on gene expression. Researchers found ten significant changes in gene expression out of 171 genes using an AD-related quantitative reverse transcription-polymerase chain reaction (qRT-PCR) array. Another study applied an LF pulsed magnetic field in peripheral blood mononuclear cells obtained from AD patients. They found that magnetic field modulates the expression of proteins involved in AD, including miR-107, miR-335, miR-26b, and β-site APP-cleaving enzyme 1 (BACE1) mRNA, which would improve AD pathology. In a low-intensity static magnetic fields (SMFs) study, primary cortical and hippocampal neurons were exposed to SMF (50 G) for 7 days, and they showed a 57.1 ± 6.3% decrease in the percentage of cells experiencing etoposide-induced apoptosis, accompanied by a marked reduction in the expression of the pro-apoptotic markers [46].
Repetitive transcranial magnetic stimulation (rTMS) with a round coil at 1 Hz with 100% output reversed memory deficits in a rat model of AD. rTMS was started after 14 days post-Aβ1–42 injections in the DG area of the dorsal hippocampus bilaterally and continued for 2 weeks. rTMS reversed the decrease in BDNF and up-regulated hippocampal N-methyl-D-aspartate receptor expression, improving long-term potentiation (LTP) and spatial memory [47].
In another study, a mouse AD model was exposed to rTMS at 1 Hz or 10 Hz (30% max. output at 1.26 T) after 1 day of Aβ1–42 injections, rTMS inhibited neuronal apoptosis, activated β-catenin signaling, and increased BDNF, nerve growth factor (NGF), and doublecortin levels [48]. These positive effects were established in genetically modified rodent model studies. An APP23/PS45 mouse model of AD-like disease [49] at 1.5 months of age was exposed to LF treatment for two weeks; the treatment reversed cognitive, synaptic deficits, and LTP impairment in the hippocampal CA1 region. The underlying mechanisms likely involve reductions in BACE1 and APP processing [49]. TMS also improved spatial learning deficits and enhanced hippocampal LTP in a frequency-dependent manner in 3xTg mice. TMS enhanced the conductance of calcium-activated potassium channels associated with cortical excitability [50].
A recent literature review [51] investigates the relationship between EMF and AD at the cellular level. Some studies show the beneficial relationship between EMF exposure and AD manifestations at the cellular level. On the contrary, some studies found no relationship with AD. For example, when IMR-32 neuroblastoma cells were exposed to 60 Hz at 50, 100, and 200 μT for four hours [52] there were no changes in the expression of APP695, an isoform of APP. In a study to assess the expression of proteins involved in the AD pathology (α3, α5, and α7 nicotinic acetylcholine receptors) in SH-SY5Y human neuroblastoma cells exposed to EMF, there was no change in expression after exposure to ELF EMF [53].
In addition, 50 Hz at 3.1 mT for 18 hours induced Aβ1–42 secretion [54], and another study showed increased production of prostaglandin E2 and decreased phagocytosis of fibrillary Aβ42 [55]. All of this demonstrates the possible harmful effects of the EMF during prolonged exposures (> 2 hours). On the other hand, short-term EMF exposures activated the Aβ clearance pathway, such as the chaperone-mediated autophagy pathway in human neuroblastoma SH-SY5Y cells, with maximum effect between 30–60 minutes of EMF exposure [56]. The ubiquitin-proteasome pathway was activated in primary hippocampal rat neurons exposed at 100 mT for 15 minutes [57]. Interestingly, when HT22 mouse hippocampal neuronal cells and SH-SY5Y human neuroblastoma cells were exposed to 1,950 MHz [58] with a high-power SAR of 6 W/kg for two hours per day for 3 days, the levels of APP, Aβ precursor protein cleaving enzyme 1, disintegrin metalloproteinase 10, and presenilin-1 were not significantly different between EMF exposed culture and controls exposures. Remarkably, this researcher previously found that a SAR of 5 W/kg was beneficial for AD pathology in rats, suggesting an upper limit for the beneficial effects on AD pathology not higher than a SAR of 5 W/kg [35].
Overall, studies investigating the effect of EMF in vitro have shown mixed results regarding the expression of genes or the level of proteins related to AD, with one study showing a decreased mRNA level of genes involved in AD, two studies showing no relationship at all, and two other studies showing an increased level of proteins involved in AD. These discrepancies in the results may be due to differences in the frequency, power deposition, or SAR, exposure period of EMF, differences in the animal model, and cell type mentioned in detail in our previous study [59]. Regulatory agencies recommend using SAR measurements for safety and radiofrequency biological effects [60].
In our previous study, our lab utilized primary culture because it is directly extracted from human tissue and grown in a laboratory, maintaining its natural characteristics. On the other hand, an immortalized cell line is a genetically modified cell population that can divide indefinitely in culture, often derived from a tumor and therefore different from the natural cell function [61, 62]. Previously, we found that repeated REFMS at 50 MHz, exposure times of 5, 15, 30, 60, and 120 minutes, power of 0.5 W, and a SAR of 0.6 W/kg activated the HSF [63] (master regulator of proteostasis [62] and the autophagy proteins ATG5 and ATG12 [56, 64]) in primary human fibroblasts. Given that the age-related attenuation of HSF1 [65, 66] plays a central role in the process of abnormal autophagy [67] that occurs during aging, it suggested that EMF interventions to push HSF1 toward its activated state are essential for the activation of autophagy and the clearance of abnormal proteins such as Aβ in age-related diseases [68, 69]. This prompted us to examine REMFS effects on the Aβ levels in primary human brain (PHB) cultures.
To find an appropriate EMF dose (dosimetry) [70], we reviewed the negative or positive actions of REMFS treatments on memory and AD pathology in multiple studies. For this purpose, we used the inverted U-shaped dose-effect curve (IUSDEC) [71]. Initially, we reviewed the literature [72–74] from cell culture [56, 63, 75–80], animal [19–21, 25–34, 81–83], and human [84–88] studies before we performed our human brain culture studies. We found that a radiofrequency power deposition that results in a SAR between 0.25–5 W/kg improves AD pathology and memory. On the contrary, when the SAR was lower than 0.25 W/kg [89–103] or higher than 5 W/kg [52, 55, 58, 104–115] it had no effects or was detrimental to AD pathology and cognition, suggesting an IUSDEC. Similarly, two human studies support an optimal SAR range. One study found impaired speed in cognitive tasks [18] at radiofrequency field radiation with a SAR of < 0.2 and > 5 W/kg, in contrast to a SAR of 1 W/kg where accuracy increased [17]. In addition, longer exposures caused demyelination in mice neurons (5 h) [116], and shorter exposures (< 30 minutes) did not have any effects [56, 59], suggesting a time and dose-dependent effect [117]. Then, following the recommendations from the International Commission on Non-Ionizing Radiation Protection (ICNIRP) [60] and IEEE [118] Standard for Safety Levels (2 W/kg local head), and considering the differences in size, geometry, tissue dielectric properties, long exposure time, thermal physiology of animals, and that neurons can be damaged even if the global SAR is within the safety limits [60], we adjusted the SAR upper limit to 0.9 W/kg and lower limit to 0.4 W/kg for our human brain cultures experiments. Therefore, we exposed PHB with different EMF frequencies, times of exposure, daily schedules, and SARs [75] to determine if REMFS was effective and safe in human brain neurons. REMFS treatment decreased Aβ40 and Aβ42 levels without evidence of toxicity. After 14 days of REMFS, we determined levels of Aβ40 peptide; treatment started on day 7 in vitro (DIV 7). Initially, we applied a frequency of 64 MHz with a SAR of 0.6 W/Kg for one hour daily for 14 days; this treatment achieved a 46% reduction in Aβ40 levels compared to the non-treated cultures [75]. Subsequently, we demonstrated that REMFS at 64 with a SAR of 0.4 W/kg for 14 days achieved a comparable reduction in Aβ40 and Aβ42 levels. Then, when we increased the exposure time from 1 to 2 hours, there was a similar reduction in the Aβ levels, so this project established the upper time limits of REMFS efficacy. We also found that a SAR of 0.4 W/kg was the minimal SAR required to lower Aβ levels, suggesting that the SAR range of 0.4–0.9 W/kg was potentially an effective and safe framework for human studies. Figure 1 demonstrates the REMFS device that was used in our previous published experiments.
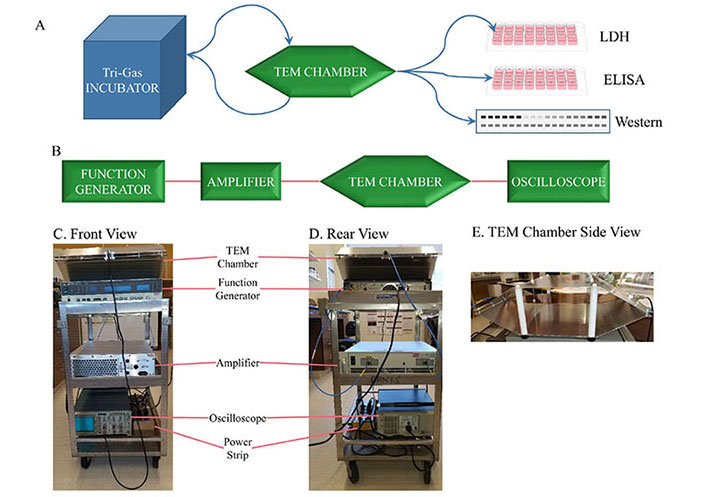
Previous REMFS experiment workflow and apparatus. (A) Culturing cells in a tri-gas incubator, alternating with treatments in a TEM chamber. After final treatments, cells were processed, and extracts were used for analysis. (B) Source of the EMF (function generator). The signal is then sent to an amplifier, then to the TEM chamber. The signal is monitored through the TEM chamber with an oscilloscope. (C) Front view photograph of a compact and convenient equipment system. (D) Rear view of the compact cart setup. (E) A side view of the TEM chamber shows a shelf across the middle for cell cultures. ELISA: enzyme-linked immunosorbent assay; EMF: electromagnetic field; LDH: lactate dehydrogenase; REMFS: repeated electromagnetic field stimulation; TEM: transverse electromagnetic. Reprinted from [75] (CC BY 4.0)
Numerous studies investigated the relationship between EMF and AD in animal models; most of these are listed in recent reviews [72, 73, 119]. These take into account the various molecular biological mechanisms of AD that have been studied in various animal models, including transgenic AD mouse models and C. elegans, these models are explained in detail in Ribeiro et al. [120]. For example, an AD mouse model exposed to REMFS at 918 MHz with a SAR of 0.25 W/kg for two hours a day for 6 months of treatment showed an improvement in Aβ deposition and cognitive function [20]. Also, REMFS at 918 MHz in the AD mouse model produces an improvement in brain mitochondrial function and an increase in soluble Aβ1–40 following a daily two-hour exposure for a month [19]. An EMF study at 50 Hz, 10 mT for 14 days in AD rats showed improved learning and memory performance [33]. A REMFS at 1,950 MHz and SAR of 5W/kg for 2 h/day in 5xFAD mice and controls showed a significant reduction in Aβ plaques, APP, and CTFs in the hippocampus and entorhinal cortex [35]. A long-term REMFS study reduced hyperactivity and anxiety symptoms while improving memory and increased glucose metabolism [121]. Another REMFS at 918 MHz study in human and primary rat astrocytes decreased Aβ levels, reactive oxygen species (ROS), H2O2-induced phosphorylation of p38 mitogen-activated protein kinase (p38MAPK) and extracellular signal-regulated kinases 1 and 2 (ERK1/2), and mitochondrial ROS, while increasing mitochondrial membrane potential (MMP) [76]. REMFS at 1,950 MHz [22] and a SAR of 5 W/kg repressed microgliosis genes (Csf1r, CD68, and Ccl6), pro-inflammatory cytokine IL-1β. And microglial function genes, including Trem2, Fcgr1a, Ctss, and Spi1 in 5xFAD mice, suggest that REMFS has beneficial effects in AD pathology and cognition in AD models. Several more recent REMFS studies [122–129] and systematic reviews [130–132] in rodents confirmed the beneficial effects in AD.
On the contrary, prolonged exposure to continuous EMP at 100 Hz and a very high electric field (50 kV/m), for 8 months in Sprague Dawley led to cognitive and memory impairment, increased Aβ level, increased expression of Aβ oligomer and APP, and increased expression of tau, suggesting a continuous high electric field exposure can cause harmful effects and increase AD pathology [106].
Also, another study at 100 Hz and a very high electric field (50 kV/m), for 8 months in Sprague Dawley, showed an increase in Aβ, BACE1, tau, and APP in the hippocampus, and cognitive impairment [133]. Another study at 50 Hz and low magnetic field of 100 μT in Sprague Dawley rats [134] for 12 weeks produced no effects on cognitive function and Aβ level changes, suggesting that low magnetic field does not have biological effects. Another 15-minutes REMFS exposure at 900 MHz and a higher SAR of 6 W/kg exposure showed that radiofrequency-EMF did not affect cognition in AD or control rats [135].
REMFS at 1,950 MHz and SAR 5 W/kg 2 h/day for 3 months did not improve cognition in AD mice [136], suggesting that a treatment course of 3 months does not affect AD pathology, but longer courses improve cognition and AD pathology [22, 30].
Invasive electrical stimulation systems induce neuromodulation of dysfunctional neural circuitry [137]. These implantable neurostimulation systems target specific deep subcortical and cortical areas. These devices regulate neuron activity by using internal pulse generators to electrodes in target areas of the brain for AD, including the fornix [138], nucleus basalis of Meynert [139], and ventral capsule/ventral striatum [140].
DBS has been shown to play a role in the modulation of neural networks in AD such as the fornix that subserves memory function, specifically the Papez circuit [141].
Some alternative techniques include the ventral capsule/ventral striatum [140], which improves executive dysfunction and behavior but not memory, and the nucleus basalis of Meynert [142], which stabilized the mini-mental state exam (MMSE) and Alzheimer’s disease assessment scale-memory (ADAS-mem) but showed a decline in the ADAS-cognition (ADAS-cog). Therefore, the fornix is the most common target structure for DBS treatments, with more than one hundred patients taking this procedure [143].
In general, the results from randomized clinical trials have shown that cognitive function improved in some patients but deteriorated in others [144]. DBS takes the risk of major surgery and its complications, including bleeding, infection, pain, and hardware failure [145]. Age is an important factor in the treatment outcome. In participants below the age of 65, the ADAS-cog 13 significantly declined compared to older participants.
The proposed mechanisms of action [146] include regulation of neural networks [147], promotion of nerve oscillation [148], and reduction of Aβ [43], tau [149], and neuroinflammation [150]. It also potentially causes an increase in acetylcholine [40] and NGF in the hippocampus [151].
Although bilateral DBS appears safe for AD, evidence shows more severe complications and higher mortality events than unilateral DBS [143].
The surgical technique for invasive vagus nerve stimulation (iVNS) implantation is made in the cervical area 2 mm from the trachea [152]. The stimulation frequencies are between 0.5 Hz to 100 Hz and intensities from 0.6 mA to 4.5 mA. An iVNS study in AD patients showed improvement in ADAS-cog and MMSE after 24 weeks of treatment [152]. The iVNS was well tolerated, and its side effects were mild and transient.
Another iVNS study [153] found improvement in the ADAS-cog and MMSE after 6 months of treatment. Also, it showed decreased CSF-tau levels by 7.7% and cognitive improvement. In a follow-up study [154] from the previous pilot study, iVNS improved or showed no decline in the ADAS-cog in 41% of patients and the MMSE in 70% of patients after one year of treatment. Although iVNS is well tolerated, it has side effects in 10–30% of patients [155], including hematoma, superficial or deep infection, and vocal cord palsy. Post-implantation side effects are hoarseness, paresthesias, headache, and shortness of breath. In addition, technical complications [156] include lead fracture, disconnection, spontaneous turn-off, stimulator malfunction, battery or electrode failure, and lead breakage. All these complications have impeded the use of iVNS in frail patients.
Transcranial DC stimulation (tDCS) modulates the excitability thresholds of neuronal membrane potentials [157] and showed improved memory function in AD patients. A recent systematic review [158] of tDCS therapy provided a numerical evaluation of its effectiveness in improving cognitive function in AD patients. They evaluate various cognitive functions, such as memory, attention, and global cognitive function. The meta-analysis showed no effects on attention but beneficial effects on global cognitive measures and memory impairment in AD patients. They pointed out that the scarcity of trials and more high-quality studies with optimal parameters should be considered before we consider tDCS for AD treatment.
Transcranial Alternating Current Stimulation (tACS) on the other hand, provides electric current that oscillates between positive and negative values or peak-to-peak amplitude at a particular frequency [159], synchronizing the oscillations of the brain networks [160]. For instance, tACS treatments [161] on the temporal lobe for 30 days of 20 minutes per day for 6 weeks improved MMSE and ADAS-cog scores in mild to moderate AD patients.
In another study, gamma band tACS improved cognitive function MCI, but not in AD patients [162]. Follow-up after 2 years showed that non-responder MCI developed clinical disease [162]. In a recent study of combined Gamma-tACS with sound stimulation, a single patient [163] was exposed to two electrodes placed in the dorsolateral prefrontal cortex (DLPFC) and the contralateral supraorbital area, and along with sound stimulation 5 times a week for 3 weeks, there was improved global cognition.
Electroconvulsive Treatment (ECT) administers an electric current by a pair of electrodes, which induces a controlled seizure. A possible mechanism of action is inducing proliferative changes in the brain [164]. An ECT study showed improvement in the hippocampus after treatments [165]. Also, many studies showed improvement in depression in AD patients [166]. Another ECT study on depressed dementia, MCI group, and no cognitive impairment groups showed that MMSE scores increased after six months of treatments [167].
A retrospective cohort study of major depression patients treated with ECT reported that MMSE scores increased significantly from baseline after 6 months of therapy [168]. Moreover, AD patients have low levels of BDNF and several ECT studies showed increased levels of BDNF in depressed patients [169].
Cranial electrotherapy stimulation (CES) devices deliver low-intensity electrical current by electrodes attached to bilateral areas of the head to modulate central and peripheral nervous system activity [170].
A CES trial stimulation with asymmetric biphasic square impulses in bursts of trains for 30 minutes per day showed significant improvement in face recognition, picture recognition, and recognition subtest of the 8-word test after 6 weeks of treatment [171] in mild AD patients. Later, a similar study compared CES with TENS found that CES improved more in the cognitive test results than TENS in mild AD patients. Moreover, a HF (100Hz) CES study showed no improvement in cognition after 6 weeks of treatment.
Transcutaneous vagus nerve stimulation (tVNS) [152] devices neurostimulate the vagus nerve by contacting the skin of the neck or the ear. tVNS was developed to circumvent the complications of the implantation of iVNS and is currently being tested in AD clinical trials. However, the tVNS has less effect due to the lack of direct stimulation of the vagus nerve. Up to now, 7 tVNS [172] trials have been tested in mild cognitive patients and not in AD patients. They have shown increased functional connectivity between the left medial prefrontal lobe and right lingual gyrus and improvement in cognition. Furthermore, connectivity from the hippocampus to several cortical and subcortical areas also demonstrated change with tVNS compared with ear lobe stimulation [173].
rTMS sends continuous magnetic pulses with the same intensity over a specific time, including LF (≤ 1 Hz) and HF (≥ 5 Hz). The LF protocol decreases excitation of the brain cortex, and the higher-frequency pulses can increase it [174]. A study of a HF (10 Hz) rTMS applied on the right inferior frontal gyrus and vertex in mild AD patients found that this stimulation led to significant improvements in attention and psychomotor speed in these patients [175]. Another study showed improvement in the UCLA auditory verbal learning test, MMSE, and the ADAS-cog after 30 sessions of HF (20 Hz) rTMS on the posterior temporal and parietal cortex for 6 weeks [176]. However, these improvements occur only when cognitive deficits are mild. Similar findings were found [177] in a study with rTMS to the bilateral DLPFC; treatment improved accuracy on action naming in 15 AD patients. Later, in a follow-up study with 24 AD patients, where researchers divided the groups according to the level of AD severity (mild to moderate and severe AD), who received rTMS over bilateral DLPFC [178] showed that both groups improved in action naming, consistent with previous findings. However, patients with early AD did not show improvement in object naming accuracy, while those with moderate to severe AD improved significantly. Furthermore, the same researchers performed an rTMS study [179] that showed higher rates of correct auditory sentence comprehension and a long-term improvement after 8 weeks of treatment.
A combined study with HF rTMS [180] with cognitive training to the bilateral DLPFC, Broca’s area, Wernicke’s area, and bilateral somatosensory cortices in mild and moderate AD patients showed that the combined approach improved the ADAS-cog and the Clinical Global Impression of Change (CGIC) scores. Another HF rTMS in early AD patients on the left DLPFC [181] for four weeks of treatment showed improved scores on the ADAS-cog in the word recall, MMSE, and Addenbrooke’s Cognitive Examination-III (ACE-III) in the attention and visual-spatial scores and these results continued for four weeks. A recent rTMS study where patients were divided into high vs. LF [182] showed that AD patients who received HF (20 Hz) rTMS on the DLPFC demonstrated a significantly higher rate of correct responses in the MMSE, and this improvement was maintained for 3 months. A HF rTMS study [136] involving bilateral DLPFC, Broca’s area, Wernicke’s area, and bilateral somatosensory cortices in patients with mild or moderate AD treated for six weeks showed improvements in ADAS-cog, MMSE, and CGIC scores, especially in the early AD patients.
On the contrary, a LF rTMS to the DLPFC in early AD demonstrated an increase in recognition memory at the end of the two-week treatment [183], and these outcomes continued for one month. In a more recent study [184], AD patients were divided into HF and LF bilateral DLPFC rTMS for two weeks; results showed lower scores on the BEHAVE-AD and ADL scores than baseline in both groups. The MMSE of HF TMS-treated patients increased from 14.22 ± 3.55 before treatment to 17.33 ± 3.11 points at 4 weeks of treatment but did not improve in the LF rTMS group.
rTMS has a beneficial on AD pathology and symptomatology. However, the main disadvantage is that the magnetic field created by the coil is transient and decays exponentially; it has a penetration depth of 2 cm under the focal region. To improve the problem of limited stimulation depth, one approach is the use of deep H-coils that allow a penetration depth of 3 cm, which is still not enough to reach important deep memory areas of the human brain [185].
Optogenetics uses proteins to produce membrane potential changes by the light effects on rhodopsins of retinal cells [186]. Opsin genetic material is delivered to brain tissues via viral vectors. Opsin proteins are activated by tissue infiltration with optical fiber or direct penetration with in-sight light sources [187]. Optogenetics can provoke gamma-band oscillations (GBOs) that are abnormal findings in AD. Studies that applied GBO in hippocampal AD mice decreased Aβ plaques and induced microglia reactivity [188]. The application challenges include a reliable gene delivery system and a light source that can reach deep brain neural networks without causing tissue damage [187]. A study examined the effects of 40 Hz sound stimulation vs. non-rhythmic visual stimulation [189] in AD patients; the study improved cognition in early AD patients, in contrast with the visual stimulation which involved nature pictures displayed on a television screen.
A study that provided constant white light and noise to the control group and synchronized audiovisual stimulation at 40 Hz to the active group [190] showed decreased loss of functional connectivity, improved memory performance, and ameliorated sleep markers in the treatment group. Also, the treatment group showed more brain volume and no decline in hippocampal volume. A more recent study applied audiovisual sensory stimulation over 4 or 8 weeks with early AD [191], the study showed improved functional connectivity, cytokine levels, and immune factors in the CSF.
Andel et al. [192] have investigated the relationship between repeated REMFS, specifically LF, and the risk of AD. The study showed that only high levels of EMF exposure were associated with increased dementia risk in late-onset AD. Another study [193] showed no significant association between AD and EMF. Similarly, a power-frequency electromagnetic fields study that followed 2,198 individuals [194] did not find any association between the two. Conversely, other studies [195, 196] showed that REMFS increased the risk of AD mortality and the risk of AD and dementia in men. Interestingly, a study showed that exposure to extremely low intensity improves visual memory and visuo-perceptive functions in AD patients [197].
Several studies have examined the effects of EMF exposure on AD patients. In a pilot study on AD patients with cognitive impairment [198], they applied emisymmetric bilateral stimulation (EBS) at carrier wave peaks at 10.5 GHz with powers in the range of 10–100 nW for 25 minutes (3/week for 5 weeks). The treatment improved immediate and delayed memory, executive function, and behavior.
Recently, a pilot human trial [84, 85] [transcranial electromagnetic treatment (TEMT)] at 915 MHz with a SAR of 0.25 to 1.6 W/kg in eight subjects with mild-moderate AD, of which five of the original eight AD subjects completed the 2.5 year extension protocol treatment, showed a decline in the ADAS-cog in EMF treated and not treated groups, about 6 and 10 point decline respectively. This suggested that EMF treatment did not stop AD progression in humans. The main explanation for these contradictory results from the animal studies is that the frequency of 915 MHz has a penetration depth of 3.9 cm [199], unable to reach deep human brain memory areas affected early as the hippocampus, posterior cingulate, and the locus coeruleus [200–202]. Also, considering that the Aβ pathology spreads to most brain areas [203] in later stages underlies the importance of achieving an appropriate penetration depth to reach deep memory areas in a homogeneous power and SAR to prevent untreated areas or hotspots. On the other hand, EMF exposures at 64 MHz have a penetration depth of 13.5 cm [199] with a radiofrequency power deposition suitable for a human head, able to reach all deep memory areas.
Our research group has devised a patented EMF-generating system that can be used in humans. Figure 2 demonstrates a perspective view of an EMF generation system with a head-mounted antenna. It includes a control system to generate one or more time-varying electrical signals and a coupling circuit between the signal generator and head-mounted antenna to produce at least one corresponding time-varying EMF directed into the patient’s head. There is a controller to determine the power of one or more time-varying electrical signals based on premeasured physical parameters so the EMF can reach a specified depth. Figure 3 demonstrates the view of the proposed REMFS head-mounted antenna unit seen in Figure 2. It includes a Birdcage coil in high-pass configuration includes 16 meander line coils and 32 tuning capacitors, with eight ports positioned at a 45° displacement from each other. The antenna will operate at a frequency of 64 MHz, similar to routine magnetic resonance imaging (MRI).
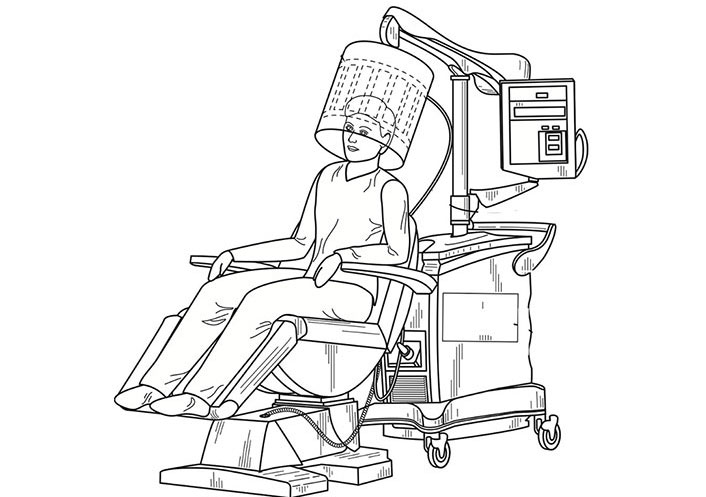
Perspective view of an REMFS with a head-mounted antenna, including control system and coupling unit. REMFS: repeated electromagnetic field stimulation. Modified with permission from “Electromagnetic Frequency Generation System and Method” by Perez, et.al. 2022. Copyright 2022 by Dr. Perez and Dr. Rizkalla (U.S. Patent Pending)
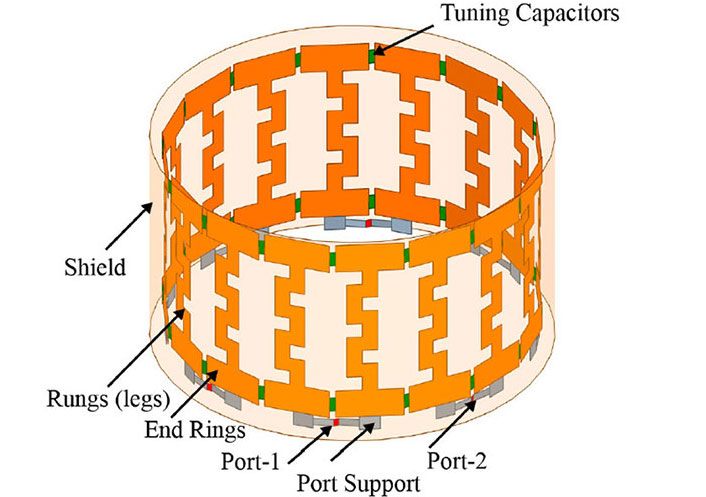
REMFS birdcage coil. The proposed REMFS birdcage coil in high-pass configuration includes 16 meander line coils and 32 tuning capacitors, with eight ports positioned at a 45° displacement from each other. REMFS: repeated electromagnetic field stimulation
The future direction of this work lies in taking the success seen in numerous cellular and AD animal models and applying it to human clinical scenarios. The challenge comes in translation. The translational success of animal-to-human EMF studies can be achieved by analyzing the biological effects on animal cells or tissues caused by the power absorbed (SAR) and then utilizing computer simulations to apply the same SAR values to human tissues, considering human dielectric properties and geometry. This review has shown the varying results seen in both preclinical and clinical trials, in particular EMF therapy. Part of the discrepancy in results lies in the various parameters and instruments used by individual researchers. There is also the problem of how to account for differences in patient physical characteristics such as head size, brain structure, and tissue thickness. This is an area of active investigation, and our lab is using artificial intelligence (AI), specifically a convolutional neural network, a type of deep learning algorithm designed to analyze visual data like MRI images that will help find personalized EMF parameters. Another area of interest lies in how to determine the real-time effectiveness of REMFS for patients. New blood biomarkers such as phosphorylated tau 217 (p-tau 217) and Aβ42 and Aβ40 hold promise as potential future markers that can be monitored pre and post-EMF treatment [200]. Also, we will obtain MRI, positron emission tomography (PET) amyloid, and PET tau pre and post-treatment. Additionally, we will perform neuropsychological tests pre and post-treatment. Temperature monitoring remains an utmost safety concern as well. Since radiofrequency heat can cause tissue injury, it must be kept to a safe level of less than 0.5°C per regulatory agencies [60]. There need to be clearly defined parameters for a safe SAR level that does not exceed this threshold in all patients before investigating in human trials. AI again holds potential in this regard for calculating the temperature effects of EMF therapy while accounting for unique patient characteristics. Preclinical studies suggest that one hour of daily REMFS would be an effective and safe treatment in humans. However, clinical trials will be performed to determine the optimal and effective treatment length. Given the circadian production and clearance of Aβ [204], especially early in the disease course, this might affect the duration and timing of treatment.
Here, we review all the EMF neurostimulation devices that can be used to treat AD. This review shows that the factors that prevent the development of neurostimulation as a routine include a lack of well-controlled studies, equivocal experimental results, and poor methodological standardization.
This review shows that the neurostimulation EMF devices have beneficial effects in preclinical studies. However, these treatments in humans have multiple difficulties, including differences in anatomy, geometry, tissue layers, and penetration depth. Also, some devices are invasive with the consequent risks, and others do not reach simultaneously several areas affected by AD pathology [202]. For example, rTMS is usually applied unilaterally to a localized area of the brain. When it is applied bilaterally, it causes more side effects [205], still not reaching all deep brain structures of the (2–6 cm) affected early by Aβ deposition.
In brief, the main shortcoming of the EMF experimental results regarding the biological effects of radiofrequency exposures is the consistency and inconsistencies between the results of the animal and human studies. This is explained by the difference in frequencies, penetration depth, tissue’s dielectric properties, mass density, and complex 3-D E-field distribution, all of which affect the energy absorbed or power deposition on the tissues [206, 207]. The SAR measures the energy absorbed or power deposition in the tissues relevant to specific biological effects. Therefore, it is a central consideration for EMF therapy simulations [208–211]. We cannot determine the SAR only via input power-based estimation due to its dependence on these factors. A crucial factor in the EMF and biological interaction in humans is penetration depth; the penetration depth decreases when frequency increases, which gives the skin depth within which 63% of the energy is deposited. Other important factors are the conductivity and permittivity of the tissues, as they determine how much electromagnetic radiation is absorbed by the body, with higher conductivity and permittivity leading to greater absorption; essentially, these properties dictate how readily electric fields can penetrate and interact with tissue at different frequencies, depending on the tissue type and its composition, particularly water content and ion concentration. For example, the cell phone frequency of 915 MHz has a penetration depth of 3.9 cm [199], unable to reach deep brain areas, on the other hand, the MRI frequency of 64 MHz has a penetration depth of 13.5 cm [199], able to get to important deep memory areas of the human brain. Therefore, we should consider the penetration depth of the EMF frequency before we apply it to a human brain because lower radiofrequency (10–200 MHz) have deeper penetration in a human head. Also, we should consider all the tissue layers of the human head that the EMF penetrates because most of the absorbed energy occurs in the superficial tissues. We chose 64 MHz for our studies for several reasons: A) ideal penetration depth (13.5 cm) [199] and homogeneous field distribution, B) previous studies with human cells and mouse cultures did not find toxicity [132], C) it is in the range 30–200 MHz [212] at whole human body resonance, so less power is needed to obtain SAR with a lower TR [213], and D) it has been used by MRI systems for 40 years, thus, providing an established and safe framework for human exposure.
Our review suggests that the most appropriate EMF strategy for human AD is the REMFS because it provides appropriate parameters for human treatments and addresses multiple AD issues. It can stop AD progression [4] due to anti-amyloid effects without causing bleeds or edema, and it has multitarget [5, 6] effects on beneficial molecular pathways involved in AD. Also, REMFS easily crosses the BBB [7], reaches all memory areas of the human brain, and links the aging process to AD pathology [8]. The REMFS’s initial hypothesis was that aging is the main risk factor for AD [214] and that the loss of the proteostasis [215, 216] is an early event [217] in the aging process. The loss of proteostasis is potentially the primary cause of Aβ accumulation in AD [218]. Additionally, since HSF1 has a central role in proteostasis [219], autophagy [67], Aβ clearance [220], and delaying the aging process [66, 116], this prompted us to use REMFS to activate it to lower Aβ levels. Moreover, multiple studies found that REMFS is a multitarget therapeutic strategy that activates several other beneficial AD pathways [6], including autophagy [221], the ubiquitin-proteasome system [57], oxidative stress [78], cytoprotection [79], inflammation [222], microglia activation [114], and mitochondrial and neuronal activity [19] to lower Aβ levels and potentially improve AD pathology and cognition in AD patients.
Another advantage of the REMFS technology is based on the science behind the EMF-biological systems interaction [59, 223] and the similarities with the MRI radiofrequency coil [224, 225] for its effectiveness and safety profile. The MRI coils [224, 225] apply radiofrequency pulses at similar penetration depth and SAR levels but for only a few minutes, and on the contrary, REMFS applies a longer radiofrequency continuous exposure for one hour, eventually increasing the temperature, so the device will monitor the temperature with a radiometer and an MRI-type control to adjust the power in the ports adjacent to the temperature increased. Also, the difference between MRI coils and REMFS coils is the smaller size of a portable REMFS device, which requires precise optimization. Moreover, REMFS uses 64 MHz, which provides an optimal penetration depth [226, 227] of 13.5 cm able to reach the hippocampus and other deep memory areas affected early in AD, such as the posterior cingulate and the locus coeruleus [200–202] that in later stages [228] spreads to most areas of the brain [203]. This underlies the importance of achieving an optimal penetration depth and a homogeneous power deposition with an optimal SAR to prevent untreated areas or hotspots [200, 202]. Also, the fact that REMFS uses the same frequency and power depositions with SAR values as the routine MRI that has been used for decades makes it a safe and effective strategy. Another important technical factor is that a birdcage antenna produces circular polarization necessary for biological effects [229]. Most animal studies used one antenna with polarized EMFs to activate pathways that lower Aβ and improve memory in AD mice [20] studies. EMF devices with multiple transmitters generate non-polarized EMFs with destructive or constructive interference, causing nil biological effects [229]. This polarization is an important factor in the EMF-biology interaction because radiofrequency-EMF oscillation on the H-bond causes proton tunneling [59] by increasing both vibration amplitude and the distance between the proton and acceptor at the quantum level [230], creating the protonation and conformational changes (tautomers) in RNA or other biomolecules that produce biological functions [59].
In this review, we examined the contribution of different lines of research; we used cell cultures, animal experiments, and clinical studies for the potential treatment of AD. We assessed the effectiveness and safety of REFMS and its clear implications for a broader understanding of pathophysiological mechanisms for future therapeutic interventions. Evidence demonstrated that cognitive functions affected by AD can be successfully modulated by REMFS. The lack of effective human studies makes the need for REMFS clinical trials highly significant in advancing this anti-amyloid strategy for future AD treatments.
AD: Alzheimer’s disease
ADAS-cog: Alzheimer’s disease assessment scale-cognition
AI: artificial intelligence
APP: amyloid precursor protein
Aβ: beta-amyloid
BACE1: β-site amyloid precursor protein-cleaving enzyme 1
BBB: blood-brain-barrier
BDNF: brain-derived neurotrophic factor
CGIC: Clinical Global Impression of Change
DBS: deep brain stimulation
DC: direct current
DLPFC: dorsolateral prefrontal cortex
ELF: extremely low frequency
EMF: electromagnetic field
EMPs: electromagnetic pulses
GBOs: gamma-band oscillations
HF: high frequency
IUSDEC: inverted U-shaped dose-effect curve
iVNS: invasive vagus nerve stimulation
LF: low frequency
LTP: long-term potentiation
mAbs: monoclonal antibodies
MMP: mitochondrial membrane potential
MMSE: mini-mental state exam
MRI: magnetic resonance imaging
NGF: nerve growth factor
PET: positron emission tomography
PHB: primary human brain
REMFS: repeated electromagnetic field stimulation
ROS: reactive oxygen species
rTMS: repetitive transcranial magnetic stimulation
SAR: specific absorption rate
SMFs: static magnetic fields
tDCS: transcranial direct current stimulation
TMS: transcranial magnetic stimulation
TR: temperature rise
tVNS: transcutaneous vagus nerve stimulation
FPP: Conceptualization, Supervision, Methodology, Writing—original draft. BW: Data curation, Writing—original draft, Writing—review & editing. JM: Writing—review & editing. HK: Supervision, Writing—review & editing. MR: Software, Writing—review & editing.
Figure 2 was modified the image from a patent application “Electromagnetic Frequency Generation System and Method”. The patent number is pending. The patent is of no financial interest to the subject matter and material of the manuscript.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Janusz Wiesław Błaszczyk
Tatsushi Yuri ... Hisashi Nojima
Priyanka Sengupta ... Debashis Mukhopadhyay
Danqing Xiao, Chen Zhang
Carlos Gutierrez-Merino
Julius Mulumba ... Yong Yang
Ezra C. Holston
Jorge Medeiros
Ryszard Pluta
