Affiliation:
1Division of Neuroscience and Experimental Psychology, Faculty of Biology, Medicine and Health, Manchester Academic Health Science Centre, School of Biological Sciences, University of Manchester, M13 9PL Manchester, UK
2Current address: Department of Health and Sport Sciences, Neuromuscular Diagnostics, TUM School of Medicine and Health, Technical University of Munich, 80992 Munich, Germany
Email: Iain.hunter@tum.de
ORCID: https://orcid.org/0000-0002-4506-9349
Explor Neurosci. 2024;3:27–38 DOI: https://doi.org/10.37349/en.2024.00034
Received: August 17, 2023 Accepted: December 24, 2023 Published: February 21, 2024
Academic Editor: Dirk M. Hermann, University of Duisburg-Essen, Germany
Proprioception provides important sensory feedback regarding the position of an animal’s body and limbs in space. This interacts with a central pattern generator responsible for rhythmic movement, to adapt locomotion to the demands that an animal’s environment places on it. The mechanisms by which this feedback is enabled are poorly understood, which belies its importance: dysfunctional proprioception is associated with movement disorder and improving it can help reduce the severity of symptoms. Similarly, proprioception is important for guiding accurate robotic movement and for understanding how sensory systems capture and process information to guide action selection. It is therefore important to interpret research that investigates mechanisms of proprioception, to ask: what type of information do proprioceptive sensors capture, and how do they capture it? Work in mammalian models has made important progress towards answering this question. So too, has research conducted Drosophila. Fruit fly proprioceptors are more accessible than mammalian equivalents and can be manipulated using a unique genetic toolkit, so experiments conducted in the invertebrate can make a significant contribution to overall understanding. It can be difficult, however, to relate work conducted in different models, to draw general conclusions about proprioception. This review, therefore, explores what research in the fruit fly has revealed about proprioceptor function, to highlight its potential translation to mammals. Specifically, the present text presents evidence that differential expression of mechanoelectrical transducers contributes to tuning of fly proprioceptors and suggests that the same mechanism may play a role in tuning mammalian proprioceptors.
Mechanosensation is a broad term that encapsulates mechanotransduction (the conversion of a mechanical signal into an electrical one), and the neural information relayed downstream of that transduction. It enables hearing, light touch, stretch, vibration and load sensation, and is important for health and disease [1–3]. This review focusses on mechanosensory systems for muscle stretch and vibration sensitivity, which is relayed by muscle spindle afferents in mammals. It is important to first state that separating stretch and vibration is an oversimplification. Both exist on a spectrum of information that is sensed by mechanosensors that is explored below, and the artificial separation is only made to make communicating some of the following discussion simpler. With that said, spindle afferents relay stretch and vibration-related information to support proprioception, which enables an animal to sense its body’s position in space. This sensation is necessary for normal movement, as is exemplified by proprioceptive regulation of central pattern generators (CPGs). CPGs are groups of neurons that generate rhythmic outputs, and particular CPGs produce the repetitive movement patterns associated with locomotion (e.g., walking [4, 5], swimming [6] and flying [7]). Proprioceptive input modulates CPG activity, so that an animal can adapt its locomotor pattern to the demands of its environment ([8], Figure 1). For example, soleus muscle spindle afferent input is interpreted by the central nervous system to modify stepping gait, to navigate uneven ground while walking [9].
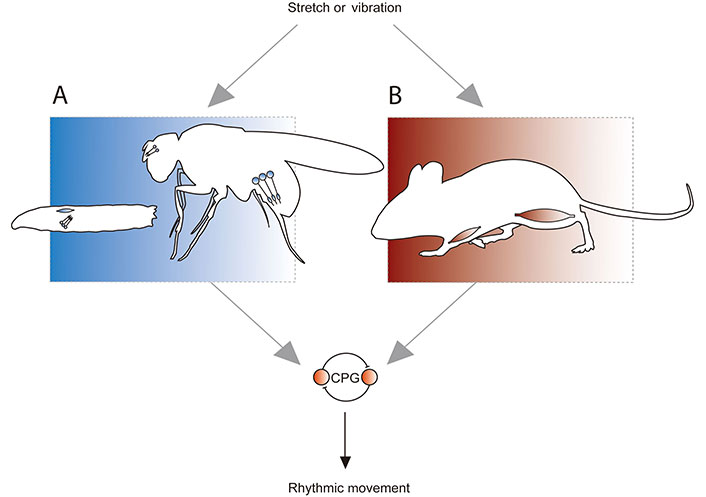
Proprioception in Drosophila and mammals. A. Stretch and vibration are detected by dorsal bipolar dendrite (DBD) neurons (blue oblong) in Drosophila larvae, and chordotonal (CH) neurons (circles with extensions that represent cilia, which facilitate mechanosensation) in larvae and adults. Positions shown approximate anatomy; DBD and CH neurons are present in the body wall of larvae, while CH are present in the antenna [Johnston’s organ neurons (JONs)] and femur of adults; B. stretch and vibration are detected by muscle spindle afferents in mammals. Spindles are comprised of intrafusal muscle fibres, and so are present throughout the mammalian muscular system. In both Drosophila and mammals, proprioceptive input regulates a CPG comprised of spinal cord neurons (or equivalents in fly nerve cord), which produces rhythmic movement. This allows the animal to adapt that movement to the demands of its environment
Given its role in movement, it is unsurprising that dysfunctional proprioception is implicated in several disorders. Improving understanding of how it works, should, therefore, lead to better treatments for them. Clinicians exploit the proprioceptive regulation of CPGs to train gait following spinal cord injury [10], and a better understanding of proprioception may help to optimise training strategy. Similarly, aberrant proprioceptive activity during development can contribute to genetic movement disorders [11]. This may be due to an increase or decrease in the normal range (set point) of activity present in locomotor circuits, which seems to be established during a critical period of development [12, 13]. Indeed, excessive activity in proprioceptors during a critical period of development increases sensitivity to induced seizure in mature Drosophila larvae [12]. In addition to this potential to contribute to the progress of medical science, understanding proprioception may inform robotics; accurate robot movement must account for the environment, as animal movement does [14]. Finally, proprioception can be abstracted and used as a general model of how information is gathered and processed to influence output. It therefore, represents a tool for investigating how biological systems make decisions, which contributes to study of action selection [15] and control theory [16].
Despite the potential utility of its comprehension, proprioception is poorly understood. This is in part due to spindle anatomy. Spindles are comprised of mechanosensory afferents associated with intrafusal fibres that are buried deep in muscles, which makes them difficult to access. Despite this difficulty, what is known is that intrafusal fibres run in parallel with the extrafusal fibres responsible for muscle contraction. This enables afferents to report static muscle length (Ia and II-type afferents), dynamic changes in length (Ia afferents only) and vibration [17]. Recently, two groups have made significant progress in developing our understanding of spindles. They reported genetic differences in subgroups of spindle afferents [18, 19], however, it will take time to fully understand the implications of these findings. For this reason, and several others explored below and elsewhere [20], there is considerable value in studying models of muscle spindles in invertebrates.
One invertebrate, Drosophila melanogaster, offers two excellent models of muscle spindles and several other cell types suitable for the study of mechanosensation. The first is the larval DBD neuron, which is anatomically similar to mammalian muscle spindles and sensitive to stretch [21, 22]. The second is the adult femoral CH neuron, which functions as part of a group of cells [a femoral CH organ (FECO)] that provide sensitivity to stretch and vibration of the fly leg [23–25]. The other fly mechanosensory neurons which may be used to help understand mechanisms of mechanosensation, include the much-studied JONs of the antenna and larval CH neurons (Figure 1). All are highly tractable, accessible, and form part of recently or nearly completed connectomes [15, 26]. These connectomes could be, and in some instances have already been, used to ask questions related to mechanisms and action selection associated with mechanosensation [15, 24, 27, 28]. This review, therefore, discusses research regarding proprioception in Drosophila and how it relates to what is known in mammals, to ask: “what type of information do proprioceptive sensors capture, and how do they capture it?”. It does so with particular focus on the CH neurons, but discusses results gleaned from other cell types when appropriate.
Given that it is known proprioceptors capture mechanical stimuli, the first part of this question must really be: “what range(s) of frequency and amplitude can proprioceptors capture?”. With that in mind, it is interesting that muscle spindle afferents and fly proprioceptors sense stretch and vibration. This demonstrates that both possess a large dynamic range of mechanical sensitivity. Indeed, one indication of this range may be present in results from dose-response experiments, which show that mammals detect low frequency and up to ~240 Hz (humans) to ~1,000 Hz (mice) stimulation (however, these experiments captured sensitivity that reflects contributions from several types of mechanosensor, including, but not uniquely spindle afferents [29]). CH also respond to a large range of frequencies, from very low, to 500–1,000 Hz [30, 31]. In both systems, responses are a function of the frequency and amplitude of mechanical input. Specifically, fixing frequency and increasing amplitude of stimulation, increases activity recorded in both adult JON [32] and larval CH neurons [31]. Similarly, increasing vibration frequency or amplitude increases the strength of response to vibrotactile pitch (perception of how high or low a vibration’s frequency is) in mice and humans (which again, is a result of stimulating several different types of mechanosensor [29]). This is true until the degree of stimulation reaches a threshold at which both human and non-human animals can no longer discern one parameter from the other. Thus, some evidence suggests that the range of sensitivity present in invertebrate and vertebrate proprioceptors is broad and features the latter interesting quality. While, to the author’s knowledge, the significance of the particularly high frequency end of this range has yet to be linked neatly to behaviour, it is logical to assume that much of the breadth of sensitivity of mechanosensors (and by reasonable extension, proprioceptors) is functional in that it contributes to regulation of movement. It is therefore important to understand which mechanisms, enable it. One such mechanism is inherent in a multiple mechanoelectrical transducer (MET) model of mechanotransduction.
It is possible that one mechanism that contributes the range of sensitivity present in proprioceptors, is enabled by multiple MET channels and associated proteins. METs are the sensors that enable proprioception. They are non-selective cation channels present in cell membranes, which gate mechanotransduction by increasing open probability in response to mechanical stimulation. Many publications have tried to identify primary METs (i.e., those which act as the main transducer) present in a cell or organ. For example, debate regarding mechanisms of mechanosensation in JONs, revolves around the ideas of either nanchung-inactive (nan-iav) or no mechanoreceptor potential C (NompC) being the primary MET. The nan-iav model describes a nan-iav heteromer as the 1° MET, with NompC acting as an amplifier of its signal [33–36]. The NompC model describes the opposite-NompC as the 1° MET, with nan-iav amplifying its signal [37–40]. Similarly, more than one MET has been proposed to be the 1° MET in spindle afferents: evidence supports epithelial sodium channel/degenerin (ENaC/DEG) family channels [41] or Piezo2 [42] fulfilling this role. While it is notable that the consensus now favours nan-iav and Piezo2 as the 1° METs in CH and spindle afferents, respectively, it is equally important to highlight that normal transduction probably requires several transducers. Indeed, the difficulty in confidently identifying 1° METs in CH and spindle afferents, is surely due, in part, to the meaningful contributions made by several other proteins.
The evidence that multiple METs are necessary for normal mechanosensation in proprioceptors includes that for a dynamic range of sensitivity (prior section) and tuning which enables that range (following section). The idea is also supported by the expression and functional importance of multiple METs in proprioceptive cell types. In addition to nan-iav and NompC, CH express piezo-like (PZL, [43]), and fly orthologues of mammalian piezo and transmembrane-like channels 1 and 2, Drosophila melanogaster piezo (DmPiezo) [44] and TMC [45], respectively. Like nan-iav and NompC, PZL has been linked to behaviour; it provides the proprioception necessary for backwards crawling, turns and head-rearing [43]. Thus, PZL appears to contribute to similar behaviours as mammalian transducers.
Before attempting to conclude this section, it is necessary to mention two caveats with regards to the significance of the expression of certain METs across fly proprioceptors. First, PZL and TMC have not yet been confirmed as bona fide pore-forming transducers. Second, larval and adult CH neurons may be different, providing different degrees of ability to translate findings to mammalian proprioceptors. Specifically, while early activity in CH neurons clearly plays an important role in relaying muscle twitches during critical periods of development [12, 30, 46], and depolarising CH neurons instigates spontaneous rhythmic currents indicative of CPG activity in mature larvae (Iain Hunter, unpublished data), they have not yet been shown to make convincing acute contributions to locomotion. The contribution proposed by Cheng et al. [47] is controversial, as DBD and related dorsal multidendritic neuron 1 (DMD1) neurons which again, are good models of spindles [21], appear to provide the majority of proprioception necessary for normal crawling [22, 31, 48]. Similarly, while there is evidence that CH neurons promote rolling, this contribution to action selection is only additive to a much larger one provided by neighbouring multidendritic neuron (MD) neurons [15]. By contrast, FECOs (adult) are clearly proprioceptors necessary for normal locomotion [23–25]. However, despite the need for greater clarity regarding these caveats, the point made above still stands: research in Drosophila proposes that various METs may be expressed in single proprioceptors, which provides sensitivity to stimulation (Figure 2) that can be related to certain behaviours.
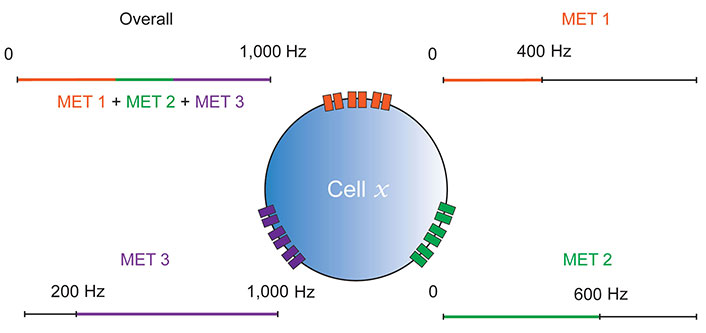
Multiple MET model of mechanosensation. Cell x is a representative proprioceptive or otherwise mechanosensitive cell, so could be a Drosophila CH/DBD neuron, or a mammalian spindle afferent. Three distinct MET channel types (MET 1, MET 2 and MET 3) are shown contributing different ranges to the cell’s overall mechanosensitivity. MET biophysical properties may likely contribute to both frequency and amplitude sensitivity in this way, however, frequency alone is shown for simplicity
For now, it is difficult to link differential expression of METs in spindle afferents to behaviour in mammals, as neatly as it is possible to link expression in CH with behaviour in flies. Indeed, there is no direct evidence for differential expression of METs in afferent endings. There is, however, strong, recent evidence for genetic differences in their cell bodies [18, 19]. Perhaps interrogation of afferent endings could reveal similar differences that are related to function, and corresponding roles in behaviour. This, in addition to the considerable similarities in sensitivity outlined above, suggest that it is reasonable to hypothesize that a multiple MET model of mechanosensation, such as the one in Figure 2, applies to spindle afferents as well as CH.
Some research related to proprioceptors makes conclusions based on observations of many cells acting together. For example, work on CH neurons often records the activity of populations of larval neurons [30, 33]. Similarly, mammalian proprioceptor activity is often measured as the sum of several sensors [49, 50]. This approach is, of course, useful for generating results that document population-level responses to stimulation but cannot offer the insight that other work has provided regarding subpopulations of proprioceptors. This research shows that groups, and even individual cells, are tuned to provide peak response(s) to different stimuli: the larval CH neuron lateral CH neuron 1 (LCH1), exhibits higher levels of Ca2+ activity in response to a given stimulation, than the 4 other cells which comprise the same group [31]. Subgroups of CH neurons in the adult Johnston’s Organ (i.e., JONs) and femur also demonstrate specialization. JONs are organised into subsets A–E [51], where A and B are sensitive to ≥ 50 nm vibration [38] and respond to sound, so are required for hearing [52]. C and E are sensitive to ≥ 250 nm vibration and respond to maintained antennal deflections for gravity and wind detection [52]. D respond to vibration and deflection of the antenna, which may support wind and wing beat sound sensation [53]. FECOs consist of club, claw and hook-type neurons that detect directional movement, position or directional movement, respectively [23].
It seems logical to explain the phenomenon that groups of CH neurons can respond to “distinc” stimuli such as vibration or movement, but that individual cells are tuned to provide peak response to a specific frequency and amplitude of stimulation, at least in part by them expressing different METs in particular ratios. This refines the basic idea proposed earlier (Figure 2), to suggest that CH may express several channels with distinct biophysical properties in different ratios, to provide tuning (Figure 3). For example, those tuned for peak response to the high-frequency and low amplitude stimuli associated with vibration, might express relatively more nan-iav than the other two METs. Likewise, those tuned for peak response to the lower frequency, higher amplitude stimulation associated with movement may express more PZL, than nan-iav or NompC. Given that NompC has been implicated in both hearing [37–39] and proprioception [48], it could act as a transducer for “mid-range” stimuli, ora transducer-amplifier for a wide range of stimulation.
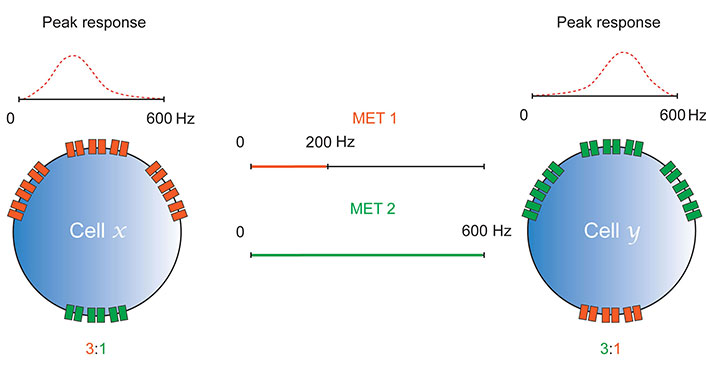
Differential expression of METs enables tuning. Cells x and y express MET channels, MET 1 and MET 2. These channels have different biophysical properties that provide them with different sensitivities to mechanical stimulation (MET 1 responds to 0–200 Hz, MET 2 to 0–600 Hz). The ratios in which they express each of these channels determines the frequency of mechanical stimulation at which they elicit a peak response. As in Figure 2, tuning is likely enabled by MET sensitivity to frequency and amplitude; only frequency is shown here for simplicity
Finally, it is also important to note that the METs expressed in CH, which have not yet been directly linked to specific behaviours (DmPiezo and TMC), could provide more support for the notion that differential expression of channels supports tuning. Given the relationship between mammalian piezos, fly PZL and proprioception [42, 43], DmPiezo may transduce low frequency, high amplitude mechanical stimulation of CH. This would agree with evidence that it contributes to stretch perception in the DBD neuron [21]. Moreover, the link between TMC-1 and 2 and mammalian hearing [54] suggests that the channels may act similarly to nan-iav and transduce high-frequency, low amplitude stimulation in CH neurons.
Again, given the functional similarities between CH and spindles, plus possible genetic differences in spindle afferent somas (that could imply similar differences in endings), it is possible that mammalian proprioceptors feature similar tuning to CH. Indeed, it may be that tuning differentiates not only the information relayed by different spindle afferents, but also in other mechanosensors (such as Merkel cells). Thus, it is possible both fly and mammalian proprioceptors are tuned at least partly, by a mechanism such as the one depicted in Figure 3.
Given that the present text focusses on METs, it is necessary to acknowledge that recent work published since this article was written, suggests FECOs do not feature differential expression of METs. Rather, it demonstrates that biomechanical specialization is a key determinant of proprioceptor tuning [55]. Furthermore, if there is a role for differential expression of METs in proprioceptors, there are other cellular and circuit-level mechanisms for tuning sensors to stimuli. For example, in flies, mechanosensory signals are amplified by dynein motor proteins. This drives phase locking of antennal JONs to high frequency stimulation [56] and is necessary for larval CH neuron response to vibration [57] and muscle contraction [31]. Similarly, opponency supported by filtering/gamma-aminobutyric acid (GABA) ergic regulation of the excitability in aPN3 cells downstream of JONs further refines their communication with the rest of the nervous system [27]. In mice, differential expression of voltage-gated potassium channels in spindle afferent subtypes may play a role in tuning [18, 19] and in both flies and mice, specific neural circuit motifs likely contribute, too [58]. While further detail of these mechanisms is beyond the scope of this text, it is important to highlight the fact that the ideas presented here do not ignore these other contributing factors. Rather, they describe a phenomenon produced by several mechanisms while aiming to clarify the contribution of one.
Finally, it is conceivable that some researchers may criticize the comparison of multiple METs in fly and mammalian proprioceptors, on the basis that the less complex nervous system of Drosophila necessitates a multimodality that is not present in mammalian sense organs. For example, evidence for thermal and mechanical nociception in MD neurons [59] may be contrasted with the traditional association of mammalian sensors with a single sense. It is, therefore, important to highlight that the latter association is likely an oversimplification. While there is no doubt that most mammalian sense organs are predominantly associated with response to a particular type of stimulus, it is also clear that some possess “overlapping” sensitivity. For example, Meissner’s corpuscles, Pacinian corpuscles and muscle spindle afferents all respond to vibration [17, 60]. Furthermore, recent data that suggests widespread expression of voltage-gated potassium channels (Kv) in murine Ia afferents, may mean that spindles can relay muscle inflammatory pain as well as stretch [61] in a manner that is remarkably similar to the way that DBD neurons may detect stretch and nociception [21, 22, 31]. Thus, some mammalian sensors function with a similar multimodality to those present in Drosophila. It seems accurate to consider them, and perhaps the majority of mechanosensors, as broad-spectrum receivers. They will capture any mechanical information that exists within a given range of frequency and amplitude—that is, within the range they are tuned to detect.
Combining the concepts above suggests that one answer to the question: “what information do proprioceptors capture and how do they capture it?”, is informed by observations made in Drosophila mechanosensors, which may translate to mammalian muscle spindle afferents. Specifically, this answer is that proprioceptors detect mechanical stimulation within a given range of frequency and amplitude, by tuning supported by ratiometric expression of METs. This tuning enables groups or individual proprioceptors, and/or combinations of them, to regulate behaviour differently (Figure 4).
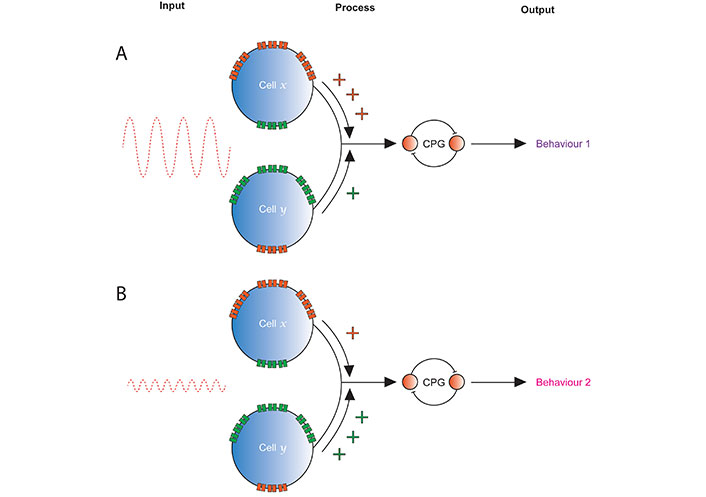
Strength of inputs from differently tuned proprioceptors may alter behaviour. Cells x and y represent two in a single sensory organ, e.g., Drosophila CH neurons or spindle afferents. A. A large amplitude, low frequency stimulus is transduced by both cells, but x expresses multiple METs in a ratio that tunes it to provide a larger response, and so stronger input to the rest of the nervous system, than y. The resulting regulation of the output produced by a CPG leads to behaviour 1; B. a low amplitude, high frequency stimulation stimulus is transduced by both cells, but y expresses multiple METs in ratio that tunes it to provide a larger response and stronger input the rest of the nervous system. This leads to a different regulation of the CPG than was present in the top panel, so produces a different behaviour (or degree of expression of the same behaviour), behaviour 2
CH: chordotonal
CPGs: central pattern generators
DBD: dorsal bipolar dendrite
DmPiezo: Drosophila melanogaster piezo
FECO: femoral chordotonal organ
JONs: Johnston’s organ neurons
MET: mechanoelectrical transducer
nan-iav: nanchung-inactive
NompC: no mechanoreceptor potential C
PZL: piezo-like
The author would like to thank Ellie S Heckscher (University of Chicago) and Richard Baines (University of Manchester) for their valuable feedback (intellectual assistance) on a draft of this manuscript.
IH: Writing—original draft, Writing—review & editing, Visualization.
The author declares that there is no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This project was supported by funding from the Wellcome Trust [217099/Z/19/Z] awarded to Prof Richard Baines (the author’s previous supervisor); Work on this project also benefitted from the Manchester Fly Facility, established through funds from the University of Manchester and the Wellcome Trust [087742/Z/08/Z]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3253
Download: 40
Times Cited: 0
