Affiliation:
1Department of Cardiology, Ataturk University Medical Faculty, Erzurum 25240, Türkiye
ORCID: https://orcid.org/0000-0002-0667-2516
Affiliation:
2Department of Cardiovascular Surgery, Ataturk University Medical Faculty, Erzurum 25240, Türkiye
Email: eyupserhatcalik@hotmail.com
ORCID: https://orcid.org/0000-0001-7682-6229
Affiliation:
2Department of Cardiovascular Surgery, Ataturk University Medical Faculty, Erzurum 25240, Türkiye
ORCID: https://orcid.org/0000-0003-0695-5089
Affiliation:
1Department of Cardiology, Ataturk University Medical Faculty, Erzurum 25240, Türkiye
ORCID: https://orcid.org/0000-0002-2824-2701
Affiliation:
2Department of Cardiovascular Surgery, Ataturk University Medical Faculty, Erzurum 25240, Türkiye
ORCID: https://orcid.org/0000-0003-2000-6090
Affiliation:
2Department of Cardiovascular Surgery, Ataturk University Medical Faculty, Erzurum 25240, Türkiye
ORCID: https://orcid.org/0000-0002-1380-9779
Affiliation:
1Department of Cardiology, Ataturk University Medical Faculty, Erzurum 25240, Türkiye
ORCID: https://orcid.org/0000-0003-0009-4396
Explor Neurosci. 2023;2:264–275 DOI: https://doi.org/10.37349/en.2023.00027
Received: August 01, 2023 Accepted: November 21, 2023 Published: December 14, 2023
Academic Editor: Brandon Lucke-Wold, University of Florida, USA
Aim: One of the main risk factors for an ischemic stroke is significant carotid artery stenosis, and extracranial severe carotid artery stenosis accounts for 20% of ischemic strokes. Prior to the development of carotid artery stenting (CAS), the only effective and reliable treatment for carotid artery stenosis was carotid endarterectomy (CEA). This study compares the results of CAS and CEA in patients with significant carotid artery stenosis.
Methods: Between 2018 and 2022, hospital records of all patients who underwent carotid artery revascularization at the institution were retrospectively analyzed. Patients were divided into two groups depending on whether CEA or CAS was performed for carotid revascularization. Propensity score matching was performed to reduce bias by equating the baseline clinical characteristics of the groups. To compare 30-day, 1-year, and long-term outcomes, rates of transient ischemic attack (TIA), myocardial infarction, stroke, all-cause mortality, and composite endpoints were analyzed.
Results: After PSM, 76 patients each in the CEA and CAS groups were compared. The mean age was 69.80 years ± 11.35 years and 121 (80%) were male. The patients were followed up for a mean of 33 months ± 6 months. The incidence of TIA in the perioperative period [9 (12%) vs. 4 (5%); P < 0.05], TIA and composite endpoint at 1-year period [11 (15%) vs. 2 (3%); P < 0.05 and 27 (36%) vs. 16 (21%); P < 0.05, respectively] were significantly higher in the CAS group than in the CEA group. No difference was observed between the groups in the long-term.
Conclusions: There was no noticeable difference between the CEA and CAS groups in the examination of cases with severe carotid artery stenosis in terms of 1-month, and 1-year results (apart from TIA and composite endpoints), or long-term outcomes. Extracranial carotid artery stenosis can be treated safely and effectively also by CAS.
The second most common cause of mortality worldwide is stroke, which is also a major public health issue [1]. Significant carotid artery stenosis is one of the major risk factors for an ischemic stroke, and 20% of ischemic strokes are brought on by extracranial cerebrovascular diseases [2]. The major goal of treatment for carotid artery stenosis is to lower the risk of stroke and death from stroke. A significant moment in this field was the introduction of carotid endarterectomy (CEA) in the 1950s. When treating carotid stenosis in the 1980s, balloon angioplasty was carried out similarly safely to CEA. Carotid artery stenting (CAS) gained popularity in 1995 [3].
Although CEA has been deemed the “gold standard” for treating severe symptomatic carotid artery stenosis [4–6], many researchs have suggested that CAS may be an equally effective alternative in preventing ipsilateral stroke [7–9]. In a previous study conducted in the institution, we reported that CAS was as effective and safe as CEA in patients with symptomatic carotid artery stenosis [3]. In this study, we aimed to compare CEA and CAS applications in patients with severe carotid artery stenosis in the last five years in parallel with the increase in patient care quality, surgical, anaesthetic and interventional techniques and technological developments.
Between January 2018 and December 2022, patients with severe carotid artery stenosis who received CEA and CAS as elective operations were examined in this single-center retrospective cross-sectional study. Patients who had experienced a stroke, transient ischemic attack (TIA), or amaurosis fugax within the previous six months met the symptomatic criteria. If there was 50–99% carotid artery stenosis, CEA or CAS was performed. Asymptomatic patients who demonstrated 70–99% carotid artery stenosis were also included. Patients who underwent combined coronary artery bypass grafting (CABG) and CEA were not included in the study. The medical records’ clinical data were gathered and compiled for the study.
At our institution, experienced cardiologists and cardiovascular surgeons performed CAS and CEA procedures [10]. Prior to both procedures, duplex ultrasonography (DUS) was performed on all patients. Patients received diagnostic carotid angiograms and diffusion-weighted magnetic resonance imaging (MRI) or computed tomography (CT) to further assess their anatomy and eligibility for CAS or CEA after DUS revealed a hemodynamically severe stenosis (Figure 1A). According to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) guidelines, the degree of stenosis was assessed angiographically [4]. A multidisciplinary team (cardiologist, cardiovascular surgeon, neurologist, and radiologist) provided all patients with advice on the advantages and disadvantages of both therapy techniques. An independent neurologist conducted clinical examinations of all patients prior to and one day following surgery or other intervention.
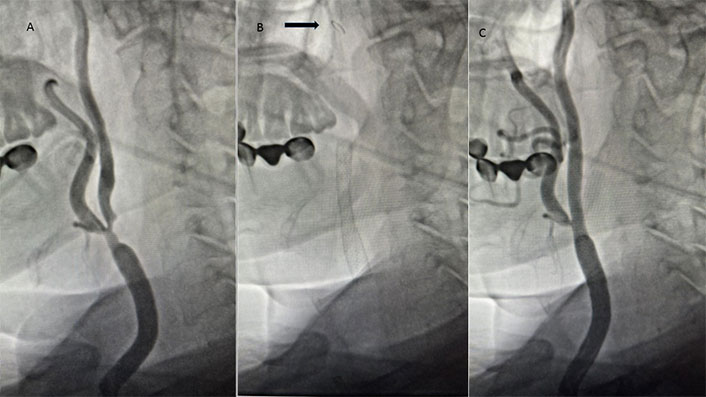
A patient with significant carotid artery stenosis. A. Severe stenosis from the common carotid artery to the internal carotid artery; B. filter placed in the internal carotid artery (arrow) and stent placed from the common carotid to the internal carotid artery; C. final imaging after stenting procedure
Patients were disqualified from the CAS if they had unfavourable aortic arch morphology, significantly calcified carotid lesions, lesions with a new thrombus, or poor femoral arterial access on both their right and left sides. Patients whose ICA was completely stenosed were not eligible for the CAS or CEA operations. Patients who were at high risk for traditional CEA underwent CAS. Patients who met the high-risk criteria had to have experienced one or more medical comorbidities in the previous three months, such as a myocardial infarction (MI) or ipsilateral stroke. Additionally, CAS was the recommended method for revascularization in patients with tracheostomies, ipsilateral radical neck dissection, ipsilateral neck irradiation, or CEA. Based on whether CEA or CAS was used for the carotid revascularization, the patients were split into two groups. Preoperative risk factors, 30-day and 1, 2, and 3-year outcomes of TIA, MI, ipsilateral stroke, and all-cause death rates were compared between the groups.
All CAS procedures were performed in the catheter lab with local anesthesia and mild sedation, and utilised either the MoMa® ultraproximal protection device (Medtronic Invatec, Roncadelle, Italy) or the Angioguard® filter (Cordis, Johnson & Johnson Interventional Systems, New Jersey, USA) as cerebral protection devices (Figure 1B). Carotid stenting was performed using the 7–10 mm/30 mm Cristallo Ideale Carotid Self-Expanding Stent-System (Medtronic, Invatec, Roncadelle, Italy) (Figure 1B and C). Expert operators carried out CAS as previously described in detail. An evaluation of the intracerebral circulation was done to decide the selection of the emboli protection device. In the absence of sufficient cerebral collateralization, the use of a filter protection device was advised [10]. Patients who have a high degree of stenosis, especially 90–99% stenosis, present a challenge for CAS intervention. The lesion needs to be predilatated before placement of the emboli protection device, and the risk of embolism is also high in this procedure.
The method of anaesthesia for CEA was decided by alertness tests. Regional anaesthesia was administered if the patients were well coherent, and general anaesthesia was administered in patients who were not coherent, anxious and had carotid lesion at C2 level. Based on measured stump pressures of less than 40 mmHg (5.3 kPa), selective shunting was employed. All patients got pericardial patch grafts for patch closure. In addition, near-infrared spectroscopy (NIRS) was used to monitor cerebral oxygenation in selected patients with inadequate cerebral collateral network [11].
All patients undergoing both CEA and CAS continued to receive antiplatelet therapy before the procedures. Asymptomatic patients generally received single antiplatelet therapy with clopidogrel and symptomatic patients received dual antiplatelet therapy with aspirin and clopidogrel up to 5 days before their procedure, and clopidogrel was given to all patients until both CAS and CEA procedures. All patients were anticoagulated with intravenous heparin during both procedures. Patients undergoing CAS received aspirin and clopidogrel for at least one month, after which they usually continued with aspirin. Patients undergoing CEA were discharged on aspirin and/or clopidogrel.
TIA, MI, stroke, and all-cause mortality rates were designated as the study’s primary objectives. The total of TIA, MI, stroke, and all-cause fatalities was determined to be a composite outcome. Death was deemed to be passing away for any reason. MI was identified using new pathogenic Q waves in two or more nearby electrocardiograph lines. Any contralateral side of body neurologic dysfunction that lasts longer than 24 h is considered a ipsilateral stroke. Contralateral neurologic events that end within 24 h of commencement are referred to as ipsilateral TIAs.
The data was analysed using IBM SPSS Statistics for Windows version 21.0 (IBM Corp., Armonk, NY, USA). The Chi square (χ2) or Fisher’s exact test was used to compare categorical variables, while the Student’s t test or Mann-Whitney U test was used to compare continuous variables. Propensity score matching (PSM) was done to reduce potential bias in the comparison of CEA and CAS group patients. The comparison was performed using Kaplan-Meier survival analysis with log-rank test for the composite endpoint (stroke, MI, and all-cause death). The threshold for statistical significance was a P ≤ 0.05.
A total of 270 patients were analysed, of which 122 underwent CEA and 148 underwent CAS procedures. Patients who underwent CEA had a mean age of 68.36 ± 12.53, and 80% of them were male. Patients who underwent CAS had an average age of 73.42 ± 11.75, and 74% of them were male. Among the patients with symptomatic carotid artery occlusive disease treated with CEA or CAS, 138 had TIA, 38 had amaurosis fugax, 22 had a stroke, and 72 of them for asymptomatic carotid artery disease with ≥ 70% stenotic lesion.
The baseline features of the two treatment groups were demonstrated in Table 1. The CAS patient group was significantly older (73.42 ± 11.75 vs. 68.36 ± 12.53; P < 0.05). Asymptomatic patients significantly preferred CEA (36% vs. 19%; P < 0.05). Patients in the CAS group were noticeably more likely to have comorbid conditions including chronic obstructive pulmonary disease (COPD; 22% vs. 7%; P < 0.05). The mean baseline serum low-density lipoprotein (LDL) levels in the CEA group were considerably higher than those in the CAS group (138.48 mg/dL ± 29.86 mg/dL vs. 119.56 mg/dL ± 28.84 mg/dL, respectively; P < 0.05). The preoperative triglyceride, creatinine, and glucose levels in the CAS and CEA groups were comparable (Table 1). PSM including age, asymptomatic patients, COPD and LDL levels was performed to equalise baseline demographic characteristics to eliminate bias in the comparison of the treatment groups. After PSM, 76 patients for each group with similar demographic characteristics were obtained (Table 2.)
Baseline patient features for CEA and CAS
| Variables | Total (n = 270) | CAS (n = 148) | CEA (n = 122) | P value |
|---|---|---|---|---|
| Age, mean ± SD | 70.89 ± 12.14 | 73.42 ± 11.75 | 68.36 ± 12.53 | < 0.05 |
| Male gender | 208 (77%) | 110 (74%) | 98 (80%) | 0.092 |
| Qualifying events | ||||
| Amaurosis fugax | 38 (14%) | 23 (16%) | 15 (12%) | 0.586 |
| TIA | 138 (51%) | 64 (43%) | 74 (61%) | 0.058 |
| Stroke | 22 (8%) | 10 (7%) | 12 (10%) | 0.078 |
| Asymptomatic | 72 (27%) | 28(19%) | 44 (36%) | < 0.05 |
| Diabetes mellitus | 124 (46%) | 66 (46%) | 58 (48%) | 0.896 |
| Hypertension | 186 (69%) | 112 (76%) | 74 (61%) | 0.095 |
| Smoker | 179 (66%) | 93 (63%) | 86 (71%) | 0.084 |
| CAD | 168 (62%) | 102 (69%) | 66 (54%) | 0.243 |
| Previous MI | 94 (35%) | 62 (42%) | 32 (26%) | 0.128 |
| Previous CABG | 38 (14%) | 22 (15%) | 16 (13%) | 0.765 |
| Previous PTCA | 129 (48%) | 72 (49%) | 57 (47%) | 0.923 |
| Atrial fibrillation | 46 (17%) | 27 (18%) | 19 (16%) | 0.856 |
| PAD | 38 (14%) | 26 (18%) | 12 (10%) | 0.085 |
| COPD | 40 (15%) | 32(22%) | 8 (7%) | < 0.05 |
| HDL, mean ± SD (mg/dL) | 36.68 ± 7.84 | 36.28 ± 7.35 | 37.08 ± 8.33 | 0.062 |
| LDL, mean ± SD (mg/dL) | 129.02 ± 29.35 | 119.56 ± 28.84 | 138.48 ± 29.86 | < 0.05 |
| Triglycerides, mean ± SD | 208.32 ± 103.59 | 204.36 ± 84.42 | 212.28 ± 122.76 | 0.278 |
| Creatinine, mean ± SD (mg/dL) | 1.14 ± 0.39 | 1.12 ± 0.46 | 1.16 ± 0.32 | 0.153 |
| Glucose, mean ± SD (mg/dL) | 145.38 ± 64.31 | 142.53 ± 62.24 | 148.23 ± 66.38 | 0.385 |
| Preprocedural medication | ||||
| Aspirin | 227 (84%) | 115 (78%) | 112 (92%) | 0.238 |
| Clopidogrel | 178 (66%) | 108 (73%) | 70 (57%) | 0.285 |
| Antihypertensive | 186 (69%) | 112 (76%) | 74 (61%) | 0.095 |
| Statin | 270 (100%) | 148 (100%) | 122 (100%) | 0.994 |
Significant values are in bold. SD: standard deviation; CAD: coronary artery disease; PTCA: percutaneous transluminal coronary angioplasty; PAD: peripheral artery disease; HDL: high-density lipoprotein
Baseline patient features for CEA and CAS after PSM
| Variables | Total (n = 152) | CAS (n = 76) | CEA (n = 76) | P value |
|---|---|---|---|---|
| Age, mean ± SD | 69.80 ± 11.35 | 70.14 ± 11.43 | 69.46 ± 11.27 | 0.383 |
| Male gender | 121 (80%) | 60 (79%) | 61 (80%) | 0.972 |
| Qualifying events | ||||
| Amaurosis fugax | 19 (13%) | 10 (13%) | 9 (12%) | 0.854 |
| TIA | 80 (53%) | 39 (51%) | 41 (54%) | 0.795 |
| Stroke | 12 (8%) | 5 (7%) | 7 (9%) | 0.855 |
| Asymptomatic | 46 (30%) | 22(29%) | 24 (32%) | 0.598 |
| Diabetes mellitus | 70 (46%) | 36 (47%) | 34 (45%) | 0.927 |
| Hypertension | 105 (69%) | 54 (71%) | 51 (67%) | 0.759 |
| Smoker | 107 (70%) | 52 (68%) | 55 (72%) | 0.095 |
| CAD | 89 (59%) | 47 (62%) | 42 (55%) | 0.067 |
| Previous MI | 49 (32%) | 27 (36%) | 22 (29%) | 0.079 |
| Previous CABG | 19 (13%) | 10 (13%) | 9 (12%) | 0.912 |
| Previous PTCA | 82 (54%) | 42 (55%) | 40 (53%) | 0.897 |
| Atrial fibrillation | 25 (16%) | 13 (17%) | 12 (16%) | 0.983 |
| PAD | 21 (14%) | 12 (16%) | 9 (12%) | 0.498 |
| COPD | 21 (14%) | 13(17%) | 8 (11%) | 0.068 |
| HDL, mean ± SD (mg/dL) | 37.97 ± 7.21 | 37.49 ± 6.57 | 38.45 ± 7.84 | 0.278 |
| LDL, mean ± SD (mg/dL) | 122.86 ± 26.31 | 121.46 ± 27.19 | 124.26 ± 25.43 | 0.629 |
| Triglycerides, mean ± SD | 209.67 ± 85.30 | 208.86 ± 68.24 | 210.47 ± 102.36 | 0.462 |
| Creatinine, mean ± SD (mg/dL) | 1.10 ± 0.58 | 1.09 ± 0.54 | 1.11 ± 0.62 | 0.241 |
| Glucose, mean ± SD (mg/dL) | 142.27 ± 63.46 | 140.86 ± 54.48 | 143.67 ± 72.44 | 0.428 |
| Preprocedural medication | ||||
| Aspirin | 137 (90%) | 67 (88%) | 70 (92%) | 0.786 |
| Clopidogrel | 102 (67%) | 53 (70%) | 49 (65%) | 0.617 |
| Antihypertensive | 92 (61%) | 48 (63%) | 44 (58%) | 0.083 |
| Statin | 152 (100%) | 76 (100%) | 76(100%) | 0.988 |
The patients with stenosis degree of 70–79% were significantly higher in the CAS group than the CEA group (36% vs. 8%; P < 0.05). On the contrary, patients with a stenosis grade of 90–99% were significantly higher in the CEA group (37% vs. 10%; P < 0.05). In both groups, the modified Rankin Scale scores were similar which these demonstrated in Table 3.
Patients’ angiographic lesion severity and their modified Rankin Scale scores in both treatment groups (after PSM)
| Variables | CAS, n (%) | CEA, n (%) | P value |
|---|---|---|---|
| Angiographic lesion severity | |||
| 60–69% | 5 (7) | 4 (5) | 0.852 |
| 70–79% | 27 (36) | 6 (8) | < 0.05 |
| 80–89% | 36 (47) | 38 (50) | 0.916 |
| 90–99% | 8 (10) | 28 (37) | < 0.05 |
| Modified Rankin Scale score | |||
| 0–1 | 47 (62) | 53 (70) | 0.759 |
| 2–3 | 29 (38) | 23 (30) | 0.078 |
Significant value is in bold
According to Table 4, TIA in the periprocedural period was statistically significantly higher in CAS group patients (12% vs. 5%; P < 0.05). There was no periprocedural MI in either group and stroke rates were comparable (4% vs. 3%; P = 0.527). The hospital mortality rate was 3% (n = 2) in the CAS group and 4% (n = 3) in the CEA group, and the rates were similar (P = 0.694).
TIA, MI, stroke, and death rates within the 30-day, 1-year, and long-time frames following CEA or stenting procedures (after PSM)
| Variables | CAS, n (%) | CEA, n (%) | P value |
|---|---|---|---|
| Periprocedural (30-days) | |||
| TIA | 9 (12) | 4 (5) | < 0.05 |
| MI | 0 | 0 | - |
| Stroke | 3 (4) | 2 (3) | 0.527 |
| Death | 2 (3) | 3 (4) | 0.694 |
| Postprocedural (1-year) | |||
| TIA | 11 (15) | 2 (3) | < 0.05 |
| MI | 6 (8) | 6 (8) | 0.956 |
| Stroke | 3 (4) | 2 (3) | 0.486 |
| Death | 7 (9) | 6 (8) | 0.748 |
| Composite endpoint | 27 (36) | 16 (21) | < 0.05 |
| Long-term (after 1-year) | |||
| Follow-up time (M, mean ± SD) | 33 ± 6 | 34 ± 8 | 0.442 |
| TIA | 12 (16) | 10 (13) | 0.578 |
| MI | 10 (13) | 9 (12) | 0.634 |
| Stroke | 5 (7) | 3 (4) | 0.086 |
| Death | 10 (13) | 10 (13) | 0.954 |
| Composite endpoint | 37 (49) | 32 (42) | 0.064 |
Significant value is in bold. M: month; -: no data
MI, stroke and death rates were 8% (n = 6), 3% (n = 2), and 8% (n = 6), respectively, in the post-procedural 1-year follow-up in the CEA group and these rates were comparable to the CAS treatment group and were 8% (n = 6), 4% (n = 3) and 9% (n = 7), respectively (Table 4). Only TIA was substantially greater in the CAS group when both groups were analysed in this period [15% (n = 11) in the CAS vs. 3% (n = 2) in the CEA; P < 0.05)] (Table 4).
Patients were followed up for a mean of 33 months ± 6 months in CAS group and 34 months ± 8 months in CEA group. There was no significant difference between the treatment groups in terms of TIA, stroke, MI and mortality rate during long-term follow-up. Long-term (after the first year) mortality rates were 13% (n = 10) in both groups (P = 0.954) (Table 4).
In the CAS group, the incidence of periprocedural TIA and stroke was high in patients with high stenosis grade. Among 8 patients with a stenosis grade of 90–99% (Table 3), 3 developed periprocedural TIA and 2 developed ipsilateral stroke.
According to the calendar date of admission, the 1-year and 30-month composite endpoint rates in the Kaplan-Meier curves demonstrated that CEA was significantly superior in the one-year period which was related to increased TIA in the CAS group during this period (log-rank P < 0.05). However, the long term did not differ between the CEA and CAS groups (log-rank P = 0.064) (Figure 2).
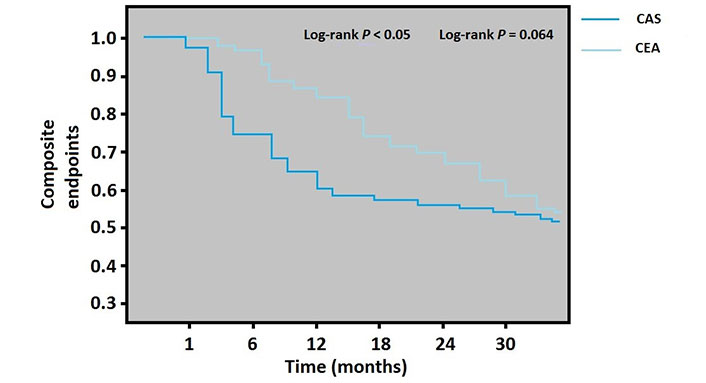
Kaplan-Meier analysis for the composite endpoint (the total of MI, stroke, TIA, and all-cause mortality) by CEA or stenting group
In the present study, we retrospectively analyzed results of patients with occlusive carotid artery disease which treated with either CEA or CAS for the last five years. We have demonstrated that there was no significant difference between the two procedures in the periprocedural (1-month) and postprocedural (1-year) death, stroke, and MI rates as in our previous study [3]. However, in our previous study, the rate of TIA occurrence in the postprocedural period was significantly higher in the CAS group, but the composite endpoint was similar. In this study, we observed that the incidence of TIA was significantly higher in the CAS group in both the periprocedural and one-year period in the comparison we performed after PSM analysis. We also found that composite endpoints were significantly higher in the CAS group compared to CEA in the 1-year period. There was no difference between the treatment groups in the long term periods.
Despite having a higher incidence of periprocedural TIA, and TIA and composite endpoint at 1-year, our current research has once again demonstrated that CAS can be an attractive alternative to CEA. We were unable to demonstrate a meaningful difference between CAS and CEA in the incidence of stroke or other serious adverse events in our high-risk patients over the long term period, according to composite endpoints.
Reducing the risk of stroke and stroke-related death is the primary goal of carotid stenosis treatment. Despite the fact that CEA has been deemed the “gold standard” for treating severe symptomatic carotid artery stenosis, prior research has revealed that CAS may be a substitute with similar efficacy in preventing ipsilateral stroke [4–9]. It has been debatable whether to utilise the CAS or CEA therapy approach to treat carotid artery stenosis [12, 13]. Because CAS was linked to a considerable increase in the likelihood of stroke or death at 30 days following the operation, several earlier studies suggested that it was less effective than CEA [14–16]. However, according to some research, CAS and CEA appear to be equal, particularly for elderly individuals who are under the age of 70 [17, 18]. In our study, we also found a high incidence of TIA, stroke and composite endpoint in the CAS group, especially periprocedural and 1-year periods.
The International Carotid Stenting Study (ICSS) randomised trial findings also failed to identify a more effective technique [19]. Stenting and endarterectomy are equally effective at preventing fatal or disabling strokes, according to the ICSS (6.4% and 6.5%, respectively). In the ICSS, there was no difference in the neurological outcome after carotid stenting, despite an increased short- and long-term risk of non-disabled stroke associated with the treatment. There were no appreciable differences in the occurrence of MI, cerebral infarction, in-hospital mortality, or follow-up procedures needed for re-stenosis within 30 days following surgery. Similarly, our results cannot definitively determine which method is more effective.
The study stroke, and mortality rates at 30 days for both treatment groups are comparable to those from previous major trials of CEA and CAS. Gray et al. [20] reported a combined 30-day stroke and death rate of 6.9% in the ARCHeR trial, which included high-risk patients. The incidence of stroke at 3 years did not significantly differ between the CEA and endovascular therapy methods, according to the results of the CAVATAS trial, which had 504 patients randomly assigned to either treatment. Additionally, almost three-quarters of the patients in this trial had balloon angioplasty as their only form of endovascular therapy because it offered little protection against emboli [21]. Our long-term results were similar. At a long-term follow-up of approximately 33 months, there was no difference between CAS and CEA in terms of TIA, stroke, MI, death and composite outcomes.
According to a NASCET study update, postoperative mortality among patients with stenosis of 70% or more was 0.6%, whereas the stroke and death rates were 5.8% and 1.2%, respectively, among patients with 50–60% symptomatic carotid artery stenosis [22]. The CREST trial found no appreciable difference between CAS and CEA in terms of risk of MI, stroke, or mortality. Contrary to the CEA group, the CAS group had a greater incidence of periprocedural stroke [23]. The CEA group experienced more periprocedural MI than the CAS group. In our study, the incidence of TIA was significantly higher in the CAS group in the periprocedural and 1-year period, but we did not observe significant differences in the incidence of stroke and MI in all follow-up periods.
In patients > 70 years of age, the CREST trial exhibited better CEA effectiveness [23]. The Carotid Stenting Trialists’ Collaboration examined the results from 4 clinical studies including 4,754 individuals. In these randomised controlled trials, patients between the ages of 70 and 74 received CEA rather than CAS [24]. The American Heart Association (AHA)/American Stroke Association Guideline for the prevention of stroke in individuals with stroke and TIA recommends CEA for symptomatic individuals aged > 70 years based on this [25]. In a meta-analysis of all randomised clinical trials comparing the two treatments for carotid artery stenosis, the risk of death or stroke within 30 days of treatment was greater with CAS than with CEA in the symptomatic patient group [26]. Particularly, in patients over 70 years of age, the probability of periprocedural mortality or stroke was substantially higher with CAS than with CEA.
The Stent-Protected Angioplasty vs. Carotid Endarterectomy (SPACE) and Endarterectomy vs. Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) studies revealed worse results with stenting compared with endarterectomy [27, 28]. Patients with symptomatic carotid artery stenosis were included in the EVA-3S and SPACE investigations, although there was a difference between their use of emboli protection devices and ours. In the EVA-3S trial, 92% of patients employed specific emboli protection devices, and 27% of patients in the SPACE experiment. In our investigation, we used emboli protection devices on every patient. Many studies comparing CEA and CAS have shown that early stroke rates are generally higher after CAS and MI rates are higher after CEA [23, 27–29]. We think that Transcarotid Artery Revascularization (TCAR) will be widely used as a highly effective and safe novel method to prevent these two complications. The TCAR quite attracts attention as a minimally invasive hybrid procedure which combination of CEA and CAS [29, 30]. Acute carotid stent thrombosis (ACST) is a rare but serious complication after CAS. Hypercoagulable states, inadequate antiplatelet therapy, plaque formation characteristics, and incorrect stent positioning are important risk factors for ACST. Methods such as thrombolysis, percutaneous mechanical thrombectomy, thromboaspiration and CEA can be applied in its treatment [31, 32].
Our study has several important limitations. Inadequate data reliability due to the retrospective nature of the study. The fact that it is a single-centre study and therefore the relatively the number of cases is low. The possibility of bias due to lack of randomisation which tried to decrease with PSM.
In conclusion, at 1-year follow-up, there was no discernible difference between patients treated with CAS and CEA in our trial in patients with severe carotid artery stenosis, with the exception of the frequency of TIA. In the management of severe carotid artery stenosis, CAS continues to be a secure and efficient choice. Our results are comparable to those of large studies showing that CEA is the “gold standard” in the treatment of carotid stenosis. In summary, our data support the views of many skilled interventionalists who believe that interventional therapy can be a useful tool when performed by experienced practitioners.
CABG: coronary artery bypass grafting
CAS: carotid artery stenting
CEA: carotid endarterectomy
COPD: chronic obstructive pulmonary disease
EVA-3S: Endarterectomy vs. Angioplasty in Patients with Symptomatic Severe Carotid Stenosis
ICSS: International Carotid Stenting Study
LDL: low-density lipoprotein
MI: myocardial infarction
PSM: propensity score matching
SPACE: Stent-Protected Angioplasty vs. Carotid Endarterectomy
TIA: transient ischemic attack
We thank Dr. Izatullah Jalalzai on language editing and improving the article.
OB: Conceptualization, Formal analysis, Data curation, Investigation, Resources, Project administration, Writing—original draft. ESÇ: Conceptualization, Formal analysis, Data curation, Investigation, Methodology, Writing—original draft, Supervision, Project administration. ÜA: Conceptualization, Data curation, Investigation, Resources, Supervision, Writing—original draft. YK: Conceptualization, Data curation, Investigation, Resources, Supervision, Writing—review & editing. UK: Conceptualization, Formal analysis, Data curation, Investigation, Methodology, Resources, Writing—review & editing. AÇ: Conceptualization, Investigation, Methodology, Resources, Supervision, Software, Validation. MHT: Conceptualization, Investigation, Supervision, Visualization, Project administration. All authors read and approved the final version of the manuscript.
The authors declare that they have no conflicts of interest.
The study was carried out according to the Declaration of Helsinki (as amended in 2013) and the approval of the Ethics Committee of the Atatürk University Faculty of Medicine was obtained for this study (B.30.2.ATA.0.01.00/714).
For surgical or interventional procedures, patients’ informed consend was acquired, however, since the study was retrospective and the data were anonymized, informed consent was waived.
Not applicable.
On reasonable request, the corresponding author will provide the data supporting the study’s conclusions.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 4903
Download: 32
Times Cited: 0
