Affiliation:
1Department of Infectious Diseases, San Carlo Hospital, 85100 Potenza, Italy
Email: carlo.acierno@ospedalesancarlo.it
ORCID: https://orcid.org/0000-0002-1239-7012
Affiliation:
2Liver Unit, Agostino Landolfi Hospital, Azienda Ospedaliera di Rilievo Nazionale San Giuseppe Moscati, 83029 Solofra, Italy
ORCID: https://orcid.org/0000-0003-3320-3878
Affiliation:
3Department of Anesthesiology and Intensive Care, San Carlo Hospital, 85100 Potenza, Italy
ORCID: https://orcid.org/0000-0002-1860-6188
Affiliation:
4Scanmed Medical Center in Krakow, 30150 Krakow, Poland
ORCID: https://orcid.org/0000-0003-3858-6804
Affiliation:
5Department of Medicine and Health Science “Vincenzo Tiberio”, Università Degli Studi del Molise, 86100 Campobasso, Italy
ORCID: https://orcid.org/0000-0002-6541-3821
Affiliation:
6Department of Advanced Medical and Surgical Sciences, University of Campania “Luigi Vanvitelli”, 80138 Napoli, Italy
ORCID: https://orcid.org/0000-0002-9142-7848
Affiliation:
7Department of Human Sciences and Promotion of the Quality of Life, San Raffaele Roma Open University, 00166 Rome, Italy
8Department of Endocrinology, Nutrition and Metabolic Diseases, IRCCS MultiMedica, 20099 Milan, Italy
ORCID: https://orcid.org/0000-0001-7066-5292
Affiliation:
6Department of Advanced Medical and Surgical Sciences, University of Campania “Luigi Vanvitelli”, 80138 Napoli, Italy
ORCID: https://orcid.org/0000-0001-8453-4912
Affiliation:
7Department of Human Sciences and Promotion of the Quality of Life, San Raffaele Roma Open University, 00166 Rome, Italy
ORCID: https://orcid.org/0000-0001-7761-7533
Explor Med. 2025;6:1001365 DOI: https://doi.org/10.37349/emed.2025.1001365
Received: May 12, 2025 Accepted: July 24, 2025 Published: October 16, 2025
Academic Editor: Amedeo Lonardo, Azienda Ospedaliero-Universitaria di Modena (–2023), Italy
The transition from non-alcoholic fatty liver disease (NAFLD) to metabolic dysfunction-associated steatotic liver disease (MASLD) reflects a paradigm shift in hepatology and highlights the need for a more pathophysiologically based classification. The aim of this review is to critically examine the conceptual evolution from NAFLD to MASLD, highlighting the implications for pathogenesis, diagnosis, risk stratification, and therapeutic strategies within the broader context of systemic metabolic dysfunction. Unlike the exclusion-based NAFLD definition, MASLD is grounded in positive diagnostic criteria and recognizes hepatic steatosis as a manifestation of metabolic disease. This reclassification improves clinical risk assessment and aligns hepatic care with cardiometabolic management. MASLD is closely linked to insulin resistance, lipotoxicity, chronic inflammation, and gut dysbiosis, which contribute to cardiovascular disease, chronic kidney disease, type 2 diabetes, and hepatocellular carcinoma. Non-invasive tools (e.g., FIB-4, elastography, ELF score) and emerging biomarkers (e.g., miR-122, CK-18, FGF21) support early diagnosis and personalized approaches. Therapeutically, MASLD management includes lifestyle modification, antidiabetic agents (GLP-1 receptor agonists, SGLT2 inhibitors), PPAR agonists, and novel drugs such as resmetirom. This evolving framework demands integrated, multidisciplinary strategies to address the systemic burden and clinical heterogeneity of MASLD.
In recent decades, the rising prevalence of liver diseases associated with metabolic dysfunction has necessitated a substantial revision of traditional diagnostic paradigms. The term non-alcoholic fatty liver disease (NAFLD), introduced in the 1980s to describe hepatic steatosis in the absence of significant alcohol consumption, initially enabled the identification of a new clinical entity [1].
Today, NAFLD is recognized as the leading cause of chronic liver disease worldwide, with an estimated prevalence of 25–30% in the general population and even higher rates among individuals with type 2 diabetes mellitus [2].
However, the conventional definition of NAFLD, based on exclusion criteria, has proven inadequate in reflecting the true pathophysiology of the disease, as it fails to acknowledge the central role of systemic metabolic dysfunction [3].
The lack of positive diagnostic criteria, phenotypic heterogeneity, and limited prognostic utility of the NAFLD definition have fueled growing dissatisfaction within the scientific community [4].
In 2020, a new nosological framework was proposed: metabolic dysfunction-associated fatty liver disease (MAFLD), defined by the presence of hepatic steatosis in conjunction with documented metabolic dysfunction [3].
Nevertheless, the term MAFLD met with criticism and was only partially adopted, in part due to terminological overlap with NAFLD and the resulting regulatory implications [5].
To address these issues, in 2023, the definition of MASLD was introduced, identifying hepatic steatosis in the presence of positive criteria for metabolic dysfunction, without necessarily excluding moderate alcohol consumption [6].
The increasing dissatisfaction with an exclusionary, non-pathophysiology-based definition led in 2020 to the proposal of the term MAFLD, which characterizes steatosis in the context of documented metabolic dysfunction [7].
However, the terminological coexistence with NAFLD and associated regulatory concerns limited its widespread adoption. Consequently, in 2023, the term MASLD was introduced. MASLD defines hepatic steatosis in the presence of at least one cardiometabolic risk criterion, even in the absence of complete abstinence from alcohol. MASLD represents a paradigm shift: It not only recognizes the liver as a target organ but places it at the core of systemic metabolic dysfunction, within a continuum that includes obesity, insulin resistance, dyslipidemia, hypertension, and low-grade chronic inflammation [8].
Insulin resistance remains the principal pathogenic driver, promoting intrahepatic lipid accumulation, lipotoxicity, and the activation of inflammatory and fibrogenic pathways [9].
The transition to MASLD enables the use of inclusive and proactive diagnostic criteria, improving prognostic stratification and fostering the integration of the hepatic phenotype into cardiometabolic risk management pathways [10].
This review offers an in-depth analysis of the conceptual shift from NAFLD to MASLD, examining its pathophysiological foundations, diagnostic and therapeutic implications, and future perspectives within the broader context of systemic metabolic diseases.
The term NAFLD was introduced in the 1980s to describe hepatic steatosis in individuals without significant alcohol consumption [1, 11].
Although initially useful in identifying a novel clinical entity, NAFLD was based on exclusion criteria and did not reflect the underlying pathophysiology of the disease [11].
Over time, accumulating scientific evidence has highlighted that metabolic dysfunction—rather than alcohol abstinence—is the principal causal factor in hepatic fat accumulation and disease progression [12].
The NAFLD paradigm has demonstrated significant limitations: reliance on exclusionary criteria, limited prognostic utility, and an inability to capture the clinical complexity of affected patients [13].
In response to these limitations, in 2020, an international panel of experts proposed a new designation: MAFLD, defined by the presence of hepatic steatosis in association with overweight/obesity, type 2 diabetes mellitus, or specific metabolic abnormalities [3].
Although MAFLD represented a step forward in aligning the nomenclature with disease pathophysiology, its adoption was only partial, hindered by overlap with pre-existing classifications and concerns regarding patients with concurrent alcohol use [4].
To address these issues, the term metabolic MASLD was introduced in 2023. While maintaining a focus on metabolic dysfunction, MASLD allows for the inclusion of individuals with moderate alcohol consumption [6].
According to MASLD criteria, the diagnosis of hepatic steatosis—documented via imaging, histology, or biomarkers—must be accompanied by at least one cardiometabolic risk factor, including obesity, prediabetes or diabetes, dyslipidemia, or arterial hypertension [8].
This approach, based on inclusive and positive criteria, improves risk stratification, facilitates recruitment into clinical trials, and aligns liver disease classification with the real-world clinical profiles of patients [14].
Most importantly, MASLD redefines hepatic involvement as an integral component of systemic metabolic dysfunction, promoting the integration of hepatology into cardiometabolic care pathways [7, 15].
Recent data suggest that this redefinition enhances predictive accuracy for both hepatic and extrahepatic outcomes [6, 16].
In parallel, the introduction of metabolic dysfunction and alcohol-related liver disease (MetALD) has enabled more accurate classification of patients with mixed etiologies [4].
The conceptual convergence between MASLD and MetALD has been formally adopted in the 2024 EASL–EASD–EASO Clinical Practice Guidelines, marking a critical step toward the standardization of terminology and clinical practice [8].
This chronological transition is visually summarized in a timeline that outlines key terminological milestones—from the exclusion-based definition of NAFLD to the inclusive and metabolically grounded frameworks of MAFLD and MASLD, culminating in their integration into international clinical guidelines (Figure 1).
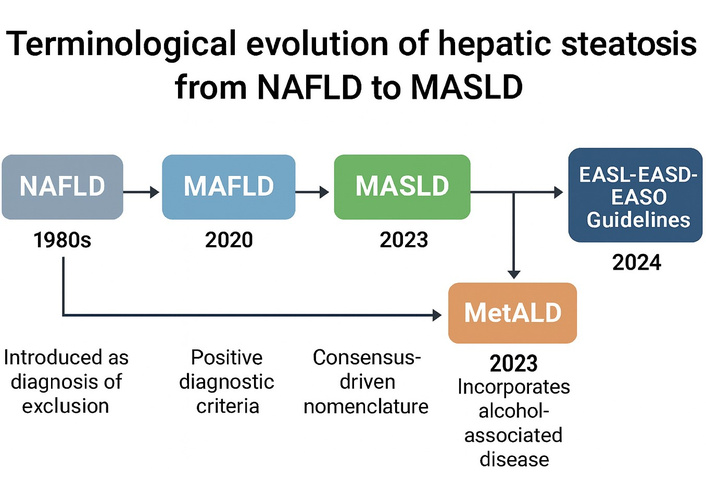
Evolution of fatty liver disease terminology: from NAFLD to MASLD and beyond. Timeline of the terminological evolution in hepatic steatosis from non-alcoholic fatty liver disease (NAFLD) to metabolic dysfunction-associated steatotic liver disease (MASLD) and its adoption into international clinical guidelines. NAFLD, introduced in the 1980s, was based on exclusion criteria. The MAFLD definition, proposed in 2020, introduced positive diagnostic parameters focused on metabolic dysfunction. In 2023, MASLD was established as a consensus-based term, allowing for more inclusive criteria. The same year, MetALD was introduced to classify cases involving moderate alcohol consumption. The EASL–EASD–EASO guidelines of 2024 formally adopted this nomenclature, consolidating clinical practice. Image created using GraphPad Prism version 10, and Microsoft PowerPoint 2021.
This evolution represents a turning point in modern hepatology, enhancing diagnostic precision and fostering multidisciplinary approaches to the growing global epidemic of metabolic liver disease [14].
The evolving definitions from NAFLD to MASLD reflect a progressive shift toward positive diagnostic criteria and improved clinical utility. A comparative overview of these nosological entities, including their respective diagnostic frameworks, limitations, and implications, is summarized in Table 1.
Nosological evolution of hepatic steatosis from NAFLD to MASLD: definition comparison.
| Definition | Year of introduction | Main diagnostic criteria | Main limitations | Clinical implications | References |
|---|---|---|---|---|---|
| NAFLD | 1980s | Hepatic steatosis in the absence of significant alcohol consumption | Based on exclusion criteria; does not account for metabolic dysfunction; phenotypic heterogeneity | Poor prognostic utility; exclusion of individuals with moderate alcohol intake | [1–4, 11] |
| MAFLD | 2020 | Hepatic steatosis + overweight/obesity; T2DM, or metabolic dysfunction | Terminological overlap with NAFLD; not universally accepted | Better alignment with metabolic pathophysiology; partial clinical adoption | [3–5, 7, 12] |
| MASLD | 2023 | Hepatic steatosis + ≥ 1 cardiometabolic risk factor; allows moderate alcohol consumption | Variable integration into healthcare systems; still undergoing formal codification | Inclusive diagnosis; promotes prognostic stratification and inclusion in clinical trials | [6, 8, 10, 14, 16, 18] |
MAFLD: metabolic dysfunction-associated fatty liver disease (intermediate definition not fully adopted); MASLD: metabolic dysfunction-associated steatotic liver disease (current definition endorsed by EASL–EASD–EASO 2024); T2DM: type 2 diabetes mellitus.
Although the term MASLD was formally adopted in June 2023 by an international expert panel, including the AASLD, its integration varies substantially across healthcare systems. Many institutions and professionals continue to use “NAFLD” or “MAFLD,” resulting in terminological heterogeneity in the scientific literature and interpretive confusion among clinicians, researchers, and patients [17].
Concurrently, updates to coding systems—particularly the implementation of MASLD within ICD-11—are still ongoing, undermining consistency in data collection, diagnostic coding, and therapeutic pathways. In addition, the transition faces regulatory obstacles, the need for structured training of healthcare professionals, and the revision of existing clinical and care protocols—factors that collectively hinder global adoption [18].
The conceptual transition from NAFLD to MASLD is grounded in a more precise understanding of the molecular and clinical mechanisms underlying hepatic steatosis, now recognized as a manifestation of complex systemic metabolic dysfunction [6].
The interplay between insulin resistance, lipotoxicity, chronic inflammation, mitochondrial dysfunction, and gut dysbiosis defines the core molecular framework of MASLD pathogenesis, as illustrated in a comprehensive mechanistic model (Figure 2).
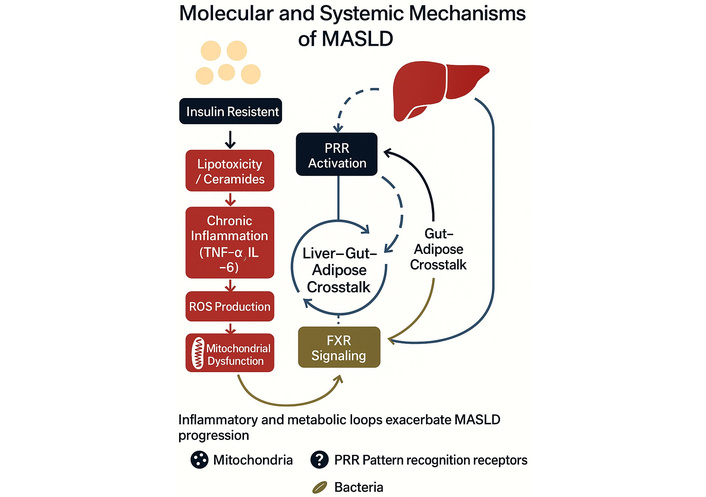
Illustration of the molecular and systemic pathomechanisms involved in MASLD. Graphical model illustrating the molecular and systemic mechanisms driving the progression of metabolic dysfunction-associated steatotic liver disease (MASLD). Insulin resistance initiates lipotoxicity through ceramide accumulation, promoting chronic inflammation mediated by tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). This cascade leads to reactive oxygen species (ROS) production and mitochondrial dysfunction. Pattern recognition receptor (PRR) activation and bile acid-related farnesoid X receptor (FXR) signaling modulate liver–gut–adipose crosstalk, reinforcing metabolic and inflammatory loops. These interorgan interactions perpetuate MASLD pathogenesis and systemic involvement. Image created using GraphPad Prism version 10, and Microsoft PowerPoint 2021.
The principal pathophysiological driver is insulin resistance, which leads to increased lipolysis in visceral adipose tissue and a consequent rise in hepatic influx of free fatty acids (FFAs) [9, 19].
The excess of intrahepatic lipid substrates activates lipotoxic and pro-inflammatory signaling pathways, promoting oxidative stress, mitochondrial dysfunction, and hepatocellular death [20, 21].
Concurrently, chronic hyperinsulinemia-induced de novo lipogenesis further exacerbates hepatic lipid overload, with accumulation of triglycerides and toxic lipid species [22, 23].
A key event is the disruption of mitochondrial homeostasis: impaired oxidative capacity and increased production of reactive oxygen species (ROS) trigger a vicious cycle of hepatocellular injury [20, 24].
Low-grade chronic inflammation also plays a central role in pathogenesis [25].
Pattern recognition receptors (PRRs) activate innate immune responses, promoting the release of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β [25, 26].
An additional contributor is intestinal dysbiosis, which facilitates microbial translocation and increased hepatic exposure to lipopolysaccharides (LPS), further activating innate immunity [27, 28].
Progression to metabolic dysfunction-associated steatohepatitis (MASH) occurs through hepatocellular injury, hepatocyte ballooning, and fibrosis—processes amplified by hepatic stellate cell activation and extracellular matrix deposition [29, 30].
Recent studies have identified a role for glucagon resistance in the MASLD context: impaired amino acid clearance and secondary hyperglucagonemia exacerbate metabolic dysfunction [31–33].
Intracellular lipid metabolism is profoundly disrupted, with accumulation of DAG and ceramides—bioactive molecules that promote insulin resistance and inflammation [33].
Another abnormality involves lipoprotein metabolism, characterized by inefficient very-low-density lipoprotein (VLDL) production and the accumulation of toxic lipids [34].
Bile acid metabolism is also implicated: Reduced activity of the FXR promotes dyslipidemia, steatosis, and hepatic inflammation [35].
Immunometabolic dysregulation further leads to neutrophil activation and infiltration of immune cells into hepatic lobules [30].
In advanced stages of MASLD, oncologic risk increases: persistent oxidative stress, chronic inflammation, and epigenetic alterations promote the development of hepatocellular carcinoma (HCC) [36].
Emerging evidence also indicates an increased incidence of intrahepatic cholangiocarcinoma (iCCA) in patients with severe MASLD [7, 37].
Cardiovascular involvement is likewise significant: MASLD is associated with endothelial dysfunction, early atherosclerosis, and arterial stiffness [7, 38, 39].
Additionally, MASLD adversely impacts renal risk, accelerating progression to chronic kidney disease (CKD) through shared inflammatory mechanisms [40, 41].
These findings underscore the urgent need for an integrated and personalized clinical and therapeutic approach [42].
The central pathophysiological mechanisms involved in MASLD—including insulin resistance, lipotoxicity, chronic inflammation, and gut dysbiosis—culminate in progressive liver damage and systemic complications, as illustrated in the mechanistic diagram (Figure 3).
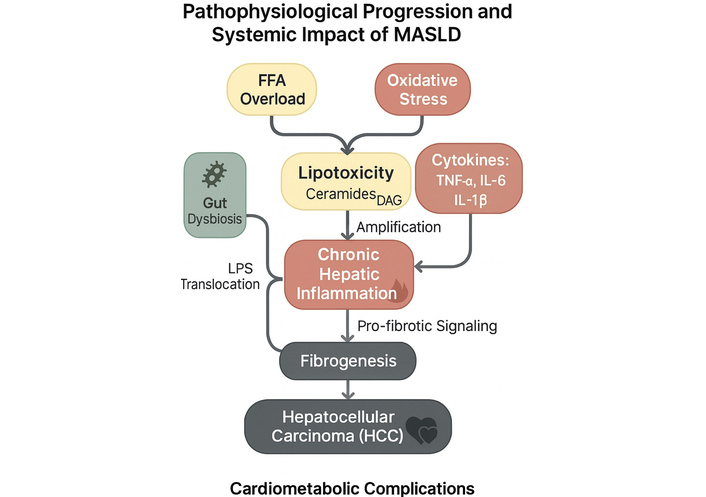
Pathophysiological mechanisms underlying the progression and systemic impact of MASLD. Mechanistic representation of the pathophysiological progression and systemic impact of metabolic dysfunction-associated steatotic liver disease (MASLD). Free fatty acid (FFA) overload, oxidative stress, and gut dysbiosis contribute to hepatic lipotoxicity, driven by bioactive lipids such as ceramides and diacylglycerols (DAG). This induces chronic hepatic inflammation mediated by pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β). The translocation of lipopolysaccharides (LPS) from the gut amplifies immune activation, reinforcing inflammation. Persistent inflammatory and lipotoxic stimuli promote fibrogenesis, which may progress to hepatocellular carcinoma (HCC) and is associated with systemic cardiometabolic complications. Image created using GraphPad Prism version 10, and Microsoft PowerPoint 2021.
While hepatocentric mechanisms remain relevant, endothelial dysfunction is emerging as a critical systemic process in the pathogenesis of MASLD [43].
In the early stages, a reduction in NO-mediated vasodilation is observed, accompanied by a pro-inflammatory and pro-thrombotic state that promotes arterial stiffness, impaired renal perfusion, and the onset of subclinical atherosclerosis [44].
These alterations contribute to subclinical atherosclerosis, vascular stiffening, and diminished renal perfusion [45].
The interplay between hepatic steatosis, insulin resistance, and endothelial dysfunction—mediated by impaired eNOS activity and reduced NO availability—creates a pathophysiological feedback loop that promotes subclinical atherosclerosis and systemic metabolic injury (Figure 4).
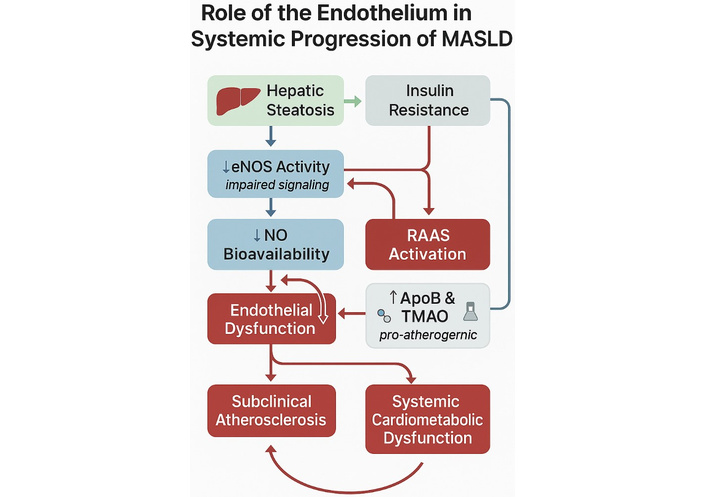
Mechanistic illustration of the role of endothelial dysfunction in the systemic progression of MASLD. Diagram illustrating the central role of endothelial dysfunction in the systemic progression of metabolic dysfunction-associated steatotic liver disease (MASLD). Hepatic steatosis and insulin resistance contribute to impaired endothelial nitric oxide synthase (eNOS) activity, resulting in reduced nitric oxide (NO) bioavailability. This triggers endothelial dysfunction, which is further exacerbated by renin–angiotensin–aldosterone system (RAAS) activation and elevated levels of pro-atherogenic factors such as apolipoprotein B (ApoB) and trimethylamine N-oxide (TMAO). These processes promote subclinical atherosclerosis and systemic cardiometabolic dysfunction, establishing a self-reinforcing pathophysiological loop. Image created using GraphPad Prism version 10, and Microsoft PowerPoint 2021.
Endothelial dysfunction represents a key pathogenetic node linking MASLD to cardiovascular risk. It acts both by amplifying traditional risk factors (dyslipidemia, hypertension, hyperglycemia) and by directly inducing vascular injury through chronic inflammation, oxidative stress, and dysregulation of the renin–angiotensin–endothelin system [46].
Castillo-Núñez Y et al. [47] further elucidate the molecular and cellular mechanisms connecting MASLD to atherosclerosis, including atherogenic dyslipidemia, low-grade vascular inflammation, foam cell formation, proliferation of vascular smooth muscle cells, gut dysbiosis, hypercoagulability, and impaired fibrinolysis. Emerging data also suggest a causal relationship between hepatic lipid accumulation driven by genetic variants and coronary artery disease, mediated by ApoB-containing lipoproteins [47].
In essence, endothelial dysfunction promotes systemic immune activation, contributing to multiorgan damage. In the setting of kidney transplantation, as shown by Prabhahar A et al. [44], it is associated with declining glomerular function, worse cardiovascular outcomes, and reduced graft survival. Despite initial improvement post-transplant, endothelial homeostasis remains only partially restored due to the influence of uremic toxins, immunosuppression, and persistent inflammation [44].
Concurrently, endothelial dysfunction intensifies systemic immune activation, exacerbating multiorgan injury. Recent studies—including cohorts with advanced hepatic fibrosis and kidney transplant populations—have confirmed its central role, reinforcing the need for therapeutic strategies targeting endothelial health in MASLD patients [48].
In summary, endothelial dysfunction not only precedes but also integrates and amplifies MASLD progression toward cardiovascular, renal, and systemic complications. This underscores the need for endothelial screening strategies and targeted therapies to preserve vascular function from the earliest stages of the disease.
The multifactorial pathogenesis of MASLD involves complex molecular and systemic mechanisms that promote both hepatic injury and extrahepatic complications. An integrated summary of these key pathways, including their mediators and systemic consequences, is provided in Table 2.
Key pathogenetic mechanisms of MASLD: an integrated overview.
| Mechanism | Description | Main mediators/pathways | Systemic implications | References |
|---|---|---|---|---|
| Insulin resistance | Increases lipolysis and FFA flux to the liver | IRS-1/2, Akt, hormone-sensitive lipase | Promotes steatosis, endothelial dysfunction | [19, 22, 33] |
| Lipotoxicity and oxidative stress | Accumulation of ceramides and DAG, mitochondrial dysfunction | ROS, impaired mitophagy | Fibrosis, progressive liver damage | [20, 23, 24] |
| Chronic low-grade inflammation | Activation of innate immunity and cytokine production | TNF-α, IL-6, IL-1β, PRRs | Promotes hepatic and systemic injury | [25, 26, 30] |
| Gut dysbiosis | LPS translocation, TLR4 activation | LPS, TLR4, intestinal permeability | Hepatic fibrosis, systemic inflammation | [27, 28] |
| Hepatic stellate cell activation and fibrogenesis | ECM deposition and fibrosis progression | TGF-β, TIMP-1 | Advanced stages: cirrhosis, carcinoma | [29, 30] |
| Glucagon resistance | Altered amino acid clearance, hyperglucagonemia | Glucagon receptor, urea cycle | Worsens metabolic dysfunction | [31–33] |
| Lipoprotein dysmetabolism | Reduced VLDL secretion, toxic lipid accumulation | ApoB, inefficient VLDL | Accelerated atherosclerosis | [33, 34] |
| FXR receptor dysfunction | Impaired bile acid homeostasis | FXR, SHP | Dyslipidemia, steatosis, inflammation | [35] |
| Endothelial dysfunction | Reduced NO, pro-inflammatory/vasoconstrictive state | eNOS, RAAS, vascular inflammation | Cardiovascular and renal complications | [40, 41] |
DAG: diacylglycerols; ECM: extracellular matrix; FFA: free fatty acids; FXR: farnesoid X receptor; NO: nitric oxide; PRRs: pattern recognition receptors; RAAS: renin–angiotensin–aldosterone system; ROS: reactive oxygen species; SHP: small heterodimer partner.
The redefinition of hepatic steatosis as MASLD has formally established the liver’s role as a key organ in the systemic pathophysiology of metabolic diseases [19, 32].
MASLD is not an isolated hepatic condition; rather, it represents the fulcrum of a complex network of pathological interactions involving obesity, insulin resistance, type 2 diabetes mellitus, atherogenic dyslipidemia, and arterial hypertension [9, 49, 50].
Numerous epidemiological studies have demonstrated that MASLD is associated with a significantly increased cardiovascular risk, independent of traditional risk factors [51–55].
Vascular alterations include endothelial dysfunction, arterial stiffness, and subclinical atherosclerosis, driven by systemic chronic inflammation and disruptions in lipid metabolism [51, 55].
MASLD is also associated with an elevated risk of developing CKD, through mechanisms involving insulin resistance, systemic inflammation, and endothelial dysfunction [40, 56].
The intersection with type 2 diabetes mellitus is particularly noteworthy: In diabetic individuals, the presence of MASLD is linked to more rapid fibrosis progression, a heightened risk of cirrhosis, and a greater incidence of HCC [57, 58].
The coexistence of MASLD and diabetes defines a high-risk clinical phenotype that necessitates a targeted, multidisciplinary management approach [32, 39, 59].
MASLD is likewise associated with other metabolic and endocrine conditions, including obstructive sleep apnea syndrome (OSAS), polycystic ovary syndrome (PCOS), and sarcopenia [60–62].
From an oncological perspective, MASLD is considered a major risk factor not only for HCC but also for iCCA, underscoring the disease’s potential to drive carcinogenesis beyond the hepatic compartment [36, 37].
Key mechanisms underlying this transition include chronic inflammation, lipotoxicity, and immune dysfunction [29, 63].
In parallel, patients with MASLD have been shown to face an increased risk of severe infections, as highlighted in a recent meta-analysis by Mantovani A et al. [64].
MASLD is also linked to a higher prevalence of cognitive impairment and dementia, suggesting systemic involvement that extends into neurodegenerative processes [65].
The contribution of gut dysbiosis further reinforces the systemic nature of MASLD: Alterations in the intestinal microbiota promote both metabolic dysfunction and hepatic fibrosis progression [27, 28, 66].
Within the framework of precision medicine, MASLD offers a unique opportunity to develop novel risk stratification models based on metabolic, inflammatory, and genomic biomarkers [67].
The prognostic implications of MASLD have been confirmed by multiple longitudinal studies, which have documented increased all-cause mortality among patients with metabolic hepatic steatosis compared to the general population [2, 68].
Ultimately, MASLD acts as a true “dysmetabolic hub,” capable of amplifying and accelerating the progression of non-communicable chronic diseases across multiple organ systems. This underscores the need for an integrated, predictive, and multidisciplinary management approach [15, 49].
The multisystemic nature of MASLD is reflected in its broad spectrum of extrahepatic complications, ranging from cardiovascular and renal dysfunction to endocrine, neurological, and oncologic disorders. These associations are detailed in Table 3, highlighting the systemic burden and clinical heterogeneity of the disease.
Multisystem complications associated with MASLD: organs involved, mechanisms, and clinical implications.
| System/organ | Main complication | Pathogenetic mechanism | Clinical impact | References |
|---|---|---|---|---|
| Cardiovascular | Early atherosclerosis, heart failure | Endothelial dysfunction, chronic inflammation, lipotoxicity | Increased MACE, CV mortality | [43, 46, 47, 52] |
| Kidney | CKD | Insulin resistance, systemic inflammation, vascular dysfunction | Progression to ESRD, worse outcomes | [40, 41, 45, 56] |
| Liver | HCC, iCCA | Advanced fibrosis, oxidative stress, epigenetic alterations | Cancers even without cirrhosis | [36, 37, 63] |
| Central nervous system | Cognitive decline, dementia | Systemic inflammation, cerebral endothelial dysfunction | Increased risk of cognitive impairment | [65] |
| Immune system | Severe infections | Immune dysregulation, chronic inflammatory state | Hospitalizations, increased mortality | [64] |
| Endocrine/metabolic system | PCOS, OSAS, sarcopenia | Hormonal dysfunction, adiposopathy, inflammation | Worsening of metabolic dysfunction | [60–62] |
| Postmenopausal women | Increased cardiovascular and MASLD risk | Loss of estrogen protection, visceral obesity | Worsened metabolic phenotype | [66, 72, 73] |
CKD: chronic kidney disease; CV: cardiovascular; ESRD: end-stage renal disease; HCC: hepatocellular carcinoma; iCCA: intrahepatic cholangiocarcinoma; MACE: major adverse cardiovascular events; OSAS: obstructive sleep apnea syndrome; PCOS: polycystic ovary syndrome.
Recent evidence highlights gender-specific cardiovascular risk profiles in patients with MASLD. Male individuals exhibit a higher prevalence of hepatic steatosis, a greater incidence of early atherosclerosis, and increased cardiovascular mortality [69, 70].
Hao WR et al. [71] further emphasized the importance of an integrated and timely clinical approach to mitigate this mortality risk.
Women, protected by estrogen during reproductive years, lose this advantage after menopause, leading to worsening metabolic profiles, increased insulin resistance, and visceral adiposity. These changes contribute to a higher prevalence of MASLD and chronic cardiovascular disease in postmenopausal women [66, 72].
A prospective study by Yang C et al. [73] involving over 4,300 women demonstrated that menopause increases the risk of MASLD (HR 1.219, 95% CI: 1.088–1.365), partially mediated by visceral adipose tissue accumulation.
A Korean population-based cohort study (n = 5,666,728; ages 20–39 years) found that MASLD was significantly associated with increased risk of major adverse cardiovascular events (MACE), with adjusted hazard ratios of 1.23 for myocardial infarction [95% CI: 1.18–1.27], 1.12 for ischemic stroke [95% CI: 1.07–1.17], and 1.18 for heart failure [95% CI: 1.15–1.21], compared to individuals without steatosis. Subgroup analysis revealed that cardiovascular risk was especially pronounced in obese women with MASLD [74].
These findings underscore the need to stratify cardiovascular risk by sex and reproductive stage, and to tailor therapeutic and preventive interventions accordingly.
MASLD, frequently associated with diabetes and obesity, is increasingly recognized as an independent determinant of heart failure, even at a young age [75, 76].
Heart failure in individuals under 40 years of age now accounts for more than 3% of all cases, with rising prevalence. Parizad R et al. [77] report a puzzling increase driven by obesity, insulin resistance, and metabolic syndrome—now emerging as key risk factors for early-onset heart failure.
In a national Korean cohort of over 7.2 million adults aged 20 to 79, MASLD was significantly associated with an increased risk of cardiovascular events (HR per risk factor increment: 1.18; 95% CI: 1.18–1.19). Incident MASLD further amplified this risk (HR 1.28), whereas regression of MASLD was linked to a reduction in cardiovascular events (HR 0.84) [78].
These data underscore the importance of proactively incorporating MASLD diagnosis into cardiovascular risk prediction models, including for younger individuals and those with apparently normal body weight.
The multisystemic impact of MASLD, involving cardiovascular, renal, endocrine, pancreatic, immune, and neurological domains, is summarized in a schematic representation that underscores its role as a systemic metabolic disease (Figure 5).
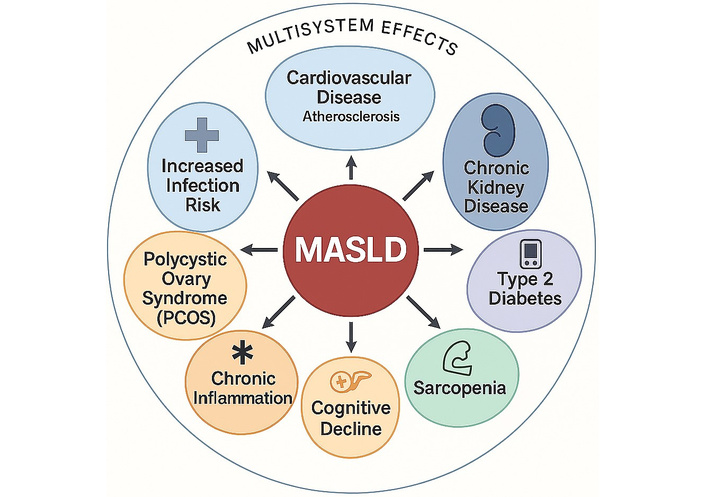
Multisystem complications of MASLD. Overview of the main multisystem comorbidities associated with metabolic dysfunction-associated steatotic liver disease (MASLD). Beyond hepatic involvement, MASLD is implicated in a wide range of extrahepatic conditions, including cardiovascular disease (e.g., atherosclerosis, endothelial dysfunction), chronic kidney disease (CKD), type 2 diabetes mellitus, sarcopenia, cognitive decline, chronic inflammation, polycystic ovary syndrome (PCOS), and increased susceptibility to infections. These associations reflect the systemic impact of MASLD and underscore the need for integrated, multidisciplinary management approaches. Image created using GraphPad Prism version 10, and Microsoft PowerPoint 2021.
The diagnosis of MASLD is based on the identification of hepatic steatosis in the presence of positive criteria for metabolic dysfunction, thereby moving beyond the exclusion-based approach characteristic of NAFLD [6, 8, 13].
According to the EASL–EASD–EASO Clinical Practice Guidelines, diagnosis requires demonstration of steatosis by imaging, histological examination, or biomarkers, in combination with at least one of the following factors: obesity, prediabetes or type 2 diabetes mellitus, atherogenic dyslipidemia, or arterial hypertension [8].
The adoption of positive diagnostic criteria enables greater inclusivity, encompassing patients with moderate alcohol consumption or with so-called “cryptogenic” steatosis [10, 79].
This paradigmatic shift also facilitates prognostic stratification, patient selection for clinical trials, and the integration of hepatic management within cardiometabolic care pathways [2].
Hepatic steatosis can be detected through various non-invasive modalities [80, 81].
Abdominal ultrasonography is the most commonly used tool due to its low cost and wide availability, though its sensitivity declines in cases of steatosis < 20% and in obese patients [82, 83].
Transient elastography (FibroScan®), combined with the controlled attenuation parameter (CAP), allows for semiquantitative assessment of steatosis and estimation of liver fibrosis [84–86].
Magnetic resonance imaging with proton density fat fraction (MRI-PDFF) is currently the gold standard for quantifying steatosis, offering high sensitivity even in patients with severe obesity; however, its use is limited by high cost and limited accessibility [87, 88].
For the evaluation of hepatic fibrosis, non-invasive scores such as FIB-4 and the non-alcoholic fatty liver disease fibrosis score (NFS) are widely used in clinical practice [89, 90].
A FIB-4 value below 1.3 has a high negative predictive value (NPV) for ruling out advanced fibrosis, whereas values above 2.67 indicate a high likelihood of its presence [80, 83].
Despite being an invasive procedure, liver biopsy remains the diagnostic gold standard for confirming MASH and for accurate staging of fibrosis [91, 92].
It is particularly indicated in cases where discrepancies exist between imaging results and non-invasive scores, in patients at high risk of disease progression, or when precise staging is required for prognostic purposes or enrollment in clinical trials [86, 93].
However, procedural risks, interobserver variability, and associated costs limit its routine use in current clinical practice [94].
A variety of non-invasive techniques and biomarkers are available to assess hepatic steatosis and fibrosis in MASLD, each with specific diagnostic strengths and limitations. A comparative summary of these tools is provided in Table 4, supporting their complementary use in clinical decision-making.
Non-invasive diagnostic tools and emerging biomarkers in MASLD.
| Method/biomarker | Principle | Advantages | Limitations | References |
|---|---|---|---|---|
| Abdominal ultrasound | Assesses hepatic echogenicity | Inexpensive, widely available | Low sensitivity in obesity and < 20% steatosis | [82, 83] |
| CAP (FibroScan®) | Ultrasonographic liver attenuation | Semi-quantitative; simultaneous fibrosis assessment | Operator-dependent, affected by high BMI | [84–86] |
| MRI-PDFF | Quantifies hepatic fat fraction | Gold standard for steatosis; high sensitivity | Expensive, limited access | [87, 88] |
| FIB-4/NFS | Composite scores from clinical and lab data | Fibrosis risk stratification; suitable for outpatient use | Limited accuracy in intermediate-risk cases | [89, 90] |
| ELF score | Direct measure of fibrogenesis | High accuracy for fibrosis | Costly, limited availability | [91] |
| CK-18 fragments | A marker of hepatocyte apoptosis | Differentiates MASH from simple steatosis | Variable cut-offs, low standardization | [95, 96] |
| miR-122/miR-34a | Liver-specific microRNAs | High potential for early diagnosis | Not yet in routine clinical use | [67] |
BMI: body mass index; CAP: controlled attenuation parameter; CK-18: cytokeratin 18; ELF: enhanced liver fibrosis; FIB-4: fibrosis-4 index; MASH: metabolic dysfunction-associated steatohepatitis; MRI-PDFF: magnetic resonance imaging with proton density fat fraction; NFS: non-alcoholic fatty liver disease fibrosis score.
Growing interest in precision medicine has driven the development of novel biomarkers for non-invasive diagnosis and prognostic stratification in MASLD [93].
Liver-specific microRNAs, such as miR-122 and miR-34a, have shown promising diagnostic potential [67].
Other protein biomarkers—including cytokeratin-18 (CK-18) fragments, fibroblast growth factor 21 (FGF21), and soluble Fas (sFas)—have demonstrated utility in distinguishing simple steatosis from MASH [95, 96].
Omics technologies, such as metabolomics and lipidomics, are enabling increasingly refined molecular phenotyping, supporting a more integrated and personalized diagnostic approach [41, 67].
The 2024 EASL–EASD–EASO Clinical Practice Guidelines define MASLD as the presence of hepatic steatosis (documented via imaging, histology, or biomarkers) in conjunction with at least one of the following cardiometabolic dysfunction criteria:
Overweight or obesity (BMI ≥ 25 kg/m2, or ≥ 23 kg/m2 for Asian individuals);
Prediabetes or type 2 diabetes mellitus;
Atherogenic dyslipidemia (triglycerides ≥ 150 mg/dL and/or HDL < 40 mg/dL in men or < 50 mg/dL in women, or treatment for dyslipidemia);
Arterial hypertension (≥ 130/85 mmHg or use of antihypertensive medication) [8].
Importantly, according to the updated guidelines, moderate alcohol consumption does not exclude a diagnosis of MASLD. Patients consuming up to 30 g/day of alcohol for men and 20 g/day for women can be classified within the MASLD phenotype, thereby moving beyond the restrictive binary model of NAFLD [8].
These criteria support broader and earlier identification of at-risk individuals, aligning with real-world clinical profiles. Risk stratification can further be refined through the combination of non-invasive tests and composite scoring systems.
One of the key challenges in diagnosing MASLD lies in the so-called “lean” form—lean MASLD—which occurs in individuals with a normal BMI but significant metabolic dysfunction [97].
Although particularly prevalent in Asian populations, the lean phenotype is increasingly documented in European and Mediterranean settings as well [98].
Lean MASLD patients are often underdiagnosed, as they do not meet BMI-based criteria. Therefore, the use of alternative indicators such as the triglyceride-glucose (TyG) index and waist-to-hip ratio has been proposed, as these are more sensitive in detecting hidden dysmetabolic phenotypes [99, 100].
Lean MASLD is associated with a risk of progression to fibrosis and systemic complications that is comparable to, or even greater than, that observed in obese MASLD patients [101].
Early identification is thus critical and lays the groundwork for an ethnically and phenotypically tailored redefinition of diagnostic cut-offs.
The treatment of MASLD requires an integrated, multidimensional approach targeting multiple pathogenic drivers simultaneously: intrahepatic lipid accumulation, chronic inflammation, insulin dysfunction, and fibrosis progression [21, 31].
The shift from the NAFLD to MASLD definition has entailed a conceptual reorientation of therapeutic strategies, redirecting focus from strictly hepatologic endpoints to broader systemic, cardiometabolic, and inflammatory targets [7, 102].
The cornerstone of therapy remains intensive lifestyle modification [8].
Hypocaloric diets rich in fiber and low in simple sugars, combined with regular physical activity, can induce significant regression of steatosis with ≥ 5% weight loss and reduction of fibrosis with ≥ 10% weight loss [103, 104].
However, long-term adherence to these lifestyle changes remains a critical challenge in clinical practice [105].
Among pharmacological treatments, glucagon-like peptide-1 receptor agonists (GLP-1 RAs) currently represent the most promising therapeutic option [106, 107].
Semaglutide and liraglutide have shown efficacy in reducing steatosis and MASH, improving inflammatory profiles, and in some cases, stabilizing fibrosis [99, 108].
Recent evidence confirms that GLP-1 RAs produce concrete histologic and metabolic benefits in MASLD, with particularly pronounced effects in patients with type 2 diabetes.
In phase 3 randomized trials, weekly semaglutide 2.4 mg led to reductions in hepatic steatosis, inflammation, and fibrosis after 72 weeks of treatment [109, 110].
Similarly, real-world observational data have shown significant reductions in mortality, cardiovascular events, and complications from portal hypertension in patients treated with GLP-1 RAs [111].
Emerging clinical evidence also supports the use of tirzepatide, a once-weekly dual GIP/GLP-1 receptor agonist, for significant histological improvement in advanced MASLD/MASH (fibrosis stages F2–F3) [112].
In a 52-week trial, tirzepatide achieved MASH resolution without fibrosis worsening in over 44–62% of patients (vs. 10% with placebo) and ≥ 1-stage fibrosis improvement in 51–55% of cases [103].
This effect was accompanied by substantial weight loss (up to −15.6%) and marked reductions in hepatic enzymes and inflammatory markers [103].
Compared with other treatments, tirzepatide stands out as one of the most promising pharmacologic strategies for histologic and fibrotic improvement in this setting. Its superior efficacy over semaglutide in promoting weight loss—recently confirmed in non-diabetic obese individuals in the SURMOUNT-5 trial [113]—reinforces the pathophysiologic rationale for its use in MASLD, where adipose burden and insulin resistance are key disease drivers.
These therapeutic effects are mediated by the pleiotropic actions of this class, including appetite regulation, weight reduction, improved glucose and lipid metabolism, and attenuation of systemic inflammation. As such, GLP-1 RAs—and even more so GIP/GLP-1 RAs—emerge as promising tools for a multimodal therapeutic approach to MASLD in patients with and without type 2 diabetes.
Another therapeutic axis is represented by PPAR receptor agonists. Pioglitazone (PPAR-γ) has demonstrated beneficial effects on insulin resistance and inflammation, while lanifibranor, a pan-PPAR agonist currently in clinical trials, shows promise for fibrosis improvement [106].
SGLT2 inhibitors have also proven effective in improving hepatic biomarkers and reducing steatosis, with potential synergistic effects when combined with GLP-1 RAs or pioglitazone [59, 114].
Among emerging agents, resmetirom—a selective thyroid hormone receptor-β agonist—represents a breakthrough: It has shown histologic efficacy in patients with MASH and stage F2–F3 fibrosis, and is the first drug to receive specific regulatory approval for MASLD [115].
In parallel, FXR receptor agonists such as obeticholic acid are in advanced stages of development, though not without relevant side effects [35].
A particularly promising area involves the gut microbiota: Its modulation through prebiotics, probiotics, and synbiotics may influence systemic inflammation and the gut-liver axis [27].
Future clinical trials should adopt multiparametric endpoints. In addition to histologic improvement, they should evaluate cardiovascular risk parameters, inflammatory biomarkers, and quality of life. This broader orientation has also been incorporated into the most recent EASL–EASD–EASO guidelines [8].
Finally, the integration of artificial intelligence into clinical practice and the implementation of personalized molecular phenotyping may help identify high-risk subgroups and optimize therapeutic responses [20, 67].
In summary, the therapeutic vision for MASLD must transcend an organ-specific framework and evolve toward a systemic, multifactorial, and personalized model—one that comprehensively addresses cardiometabolic risk, liver disease progression, and patient quality of life.
A variety of pharmacological agents targeting different pathogenic pathways have demonstrated efficacy in MASLD management. Table 5 summarizes current and emerging treatments, including their mechanisms of action, clinical benefits, and limitations, guiding therapeutic decision-making in personalized care models.
Emerging pharmacological treatments for MASLD: targets, efficacy, and limitations.
| Drug class | Molecular target | Development stage | Clinical benefits | Limitations/adverse effects | References |
|---|---|---|---|---|---|
| GLP-1 RAs (e.g., semaglutide) | GLP-1 receptor | Phase 3/approved | Reduction in steatosis, inflammation, weight | Gastrointestinal effects, high cost | [109, 110] |
| PPAR agonists (e.g., pioglitazone, lanifibranor) | PPAR-γ, pan-PPAR | Pioglitazone approved, lanifibranor under study | Improvement in insulin resistance and fibrosis | Weight gain, fluid retention | [106, 121] |
| SGLT2 inhibitors (e.g., empagliflozin) | SGLT2 | Off-label | Reduction in liver enzymes and steatosis | Urogenital infections | [59, 125] |
| FXR agonists (e.g., obeticholic acid) | FXR | Advanced trials | Fibrosis improvement | Pruritus, dyslipidemia | [35] |
| Resmetirom | Thyroid hormone receptor β | Approved | Histological improvement in MASH | Long-term data pending | [115] |
| Tirzepatide | Dual GIP/GLP-1 receptor agonist | Phase 3 | MASH resolution, weight and fibrosis reduction | Long-term safety under evaluation | [103, 112] |
FXR: farnesoid X receptor; GIP: glucose-dependent insulinotropic polypeptide; GLP-1 RAs: glucagon-like peptide-1 receptor agonists; MASH: metabolic dysfunction-associated steatohepatitis; PPAR: peroxisome proliferator-activated receptor; SGLT2: sodium-glucose transporter 2.
Prognostic stratification in patients with MASLD represents a major clinical challenge due to the heterogeneous nature of the disease and its potential progression to hepatic, cardiovascular, renal, and oncologic complications [86, 116].
Disease evolution does not follow a linear course and depends on the interplay of metabolic, genetic, immune, and environmental factors [21, 25].
Among all available predictors, liver fibrosis stands as the most robust prognostic determinant: Numerous studies have shown that fibrosis stage ≥ F2 is significantly associated with increased all-cause mortality, regardless of the presence of steatosis or histologic inflammation [117, 118].
However, not all individuals with significant fibrosis present with overt clinical phenotypes. The concept of “silent progressors” refers to patients at high risk of histologic progression despite lacking evident clinical markers (e.g., normal BMI, normoglycemia, mildly elevated aminotransferases) [2, 10].
In such cases, the use of advanced tools—such as liver elastography, direct fibrosis biomarkers like ELF or Pro-C3, and molecular biomarkers—is essential for early diagnosis and accurate risk stratification [67, 86].
A key tool is the three-tiered risk stratification model (low, intermediate, high), proposed to facilitate outpatient clinical management. These models, based on simple variables (age, diabetes status, FIB-4 or NFS scores), help optimize resource allocation by directing high-risk patients toward advanced evaluation or clinical trial inclusion [80, 93].
A tiered approach to managing patients with suspected liver fibrosis includes:
Low Risk: FIB-4 < 1.30, absence of diabetes mellitus and other relevant metabolic risk factors. In this category, the NPV is approximately 90%, supporting periodic clinical and laboratory monitoring without invasive testing.
Intermediate Risk: FIB-4 between 1.30 and 2.67, particularly in the presence of metabolic comorbidities (e.g., diabetes, obesity). In these cases, second-tier testing (e.g., VCTE/elastography, ELF test) is indicated to differentiate advanced risk and guide specialist management.
High Risk: FIB-4 > 2.67 or confirmation of advanced fibrosis via second-tier testing. Here, the positive predictive value (PPV) ranges from 60–80%, and the risk of severe liver events (cirrhosis, HCC, transplantation) is significantly increased. Non-invasive testing with higher diagnostic accuracy and referral to a hepatology center are recommended [119, 120].
This model allows prioritization of resources, directing high-priority patients toward advanced evaluations or trial enrollment. The intermediate-risk category includes the majority of MASLD patients [121].
In these cases, second-level techniques such as ultrasound elastography, ELF score, and MRI-PDFF improve diagnostic accuracy and prevent underdiagnosis of advanced fibrosis [80, 87].
Additional emerging prognostic factors include advanced age, male sex, type 2 diabetes mellitus, arterial hypertension, mixed dyslipidemia, and increased waist circumference [48, 122].
The coexistence of MASLD and diabetes is particularly concerning, as it confers a markedly increased risk of progression to cirrhosis and HCC [57].
Cardiovascular risk stratification is equally critical, as most deaths in patients with MASLD are attributable to cardiovascular rather than hepatic causes [7, 38].
The use of composite scores integrating hepatic and cardiovascular parameters (e.g., SCORE2 or ASCVD Risk Calculator) is recommended [123].
Renal risk must not be overlooked: MASLD has been associated with increased incidence of albuminuria, reduced estimated glomerular filtration rate (eGFR), and progression to CKD [40, 41].
The oncologic implications of MASLD—particularly its association with HCC and iCCA—further complicate risk stratification. MASLD is now recognized as an independent risk factor for HCC, even in the absence of cirrhosis [37].
The increasing use of molecular biomarkers and “omics” approaches promises to revolutionize risk stratification by enabling more precise phenotyping [67].
Some algorithms already incorporate genetic, metabolic, and inflammatory variables, bringing metabolic hepatology closer to the principles of precision medicine [41].
Effective risk stratification in MASLD requires the integration of fibrosis scores, clinical variables, and emerging biomarkers. Table 6 provides a comprehensive overview of stratification models and prognostic factors to identify high-risk individuals and optimize surveillance strategies.
Risk stratification models and prognostic predictors in MASLD.
| Category | Model/factor | Description/cut-off | Prognostic implications | References |
|---|---|---|---|---|
| Three-tier models | FIB-4 < 1.3 | Low risk, NPV approximately 90% | Outpatient follow-up | [80, 119] |
| FIB-4 1.3–2.67 | Intermediate risk | Requires second-level testing (elastography, ELF) | [80, 120] | |
| FIB-4 > 2.67 | High risk | High probability of advanced fibrosis | [80, 119] | |
| Clinical factors | Fibrosis ≥ F2 | Associated with increased overall mortality | Indication for intensive surveillance | [117, 118] |
| T2DM + MASLD | High-risk phenotype | Accelerated progression to cirrhosis and HCC | [57, 121] | |
| Older age, male sex, mixed dyslipidemia | Independent aggravating factors | Increased CV and renal risk | [48] | |
| Integrative tools | SCORE2, ASCVD Risk Calculator | Cardiovascular risk integration | Greater predictive accuracy | [111] |
| Renal stratifiers | Albuminuria, reduced eGFR | Nephropathy risk assessment | CKD monitoring | [40, 41] |
| Emerging biomarkers | Pro-C3, “omics”, PNPLA3 | Personalized approaches | Towards precision medicine | [41, 67, 115] |
CKD: chronic kidney disease; CV: cardiovascular; eGFR: estimated glomerular filtration rate; ELF: enhanced liver fibrosis; HCC: hepatocellular carcinoma; NPV: negative predictive value; T2DM: type 2 diabetes mellituss.
Ethnic heterogeneity represents a critical factor in the identification and management of MASLD. Asian populations exhibit metabolic dysfunction, hepatic fibrosis, and “lean” clinical phenotypes at significantly lower BMIs compared to Western cohorts [124].
The 2024 EASL–EASD–EASO guidelines acknowledge this peculiarity by recommending a BMI ≥ 23 kg/m2 as the threshold for overweight in Asian individuals [8]. Nonetheless, genetic (e.g., PNPLA3 variants), cultural, and epigenetic differences persist, necessitating further regional adaptation of diagnostic thresholds and algorithms based on non-invasive tests (e.g., FIB-4, transient elastography).
This heterogeneity underscores the need for a “regionalization” of diagnostic scales and a personalized approach that integrates anthropometric, genetic, and cultural factors to enhance diagnostic accuracy, risk stratification, and global therapeutic strategies [115, 125].
Although the MASLD paradigm is grounded in robust biological evidence, its implementation in clinical practice faces significant limitations. Available real-world datasets—drawn from electronic health records and multicenter observational studies—exhibit methodological variability, non-standardized criteria, and potential selection bias [126].
Moreover, current literature lacks long-term follow-up data (beyond 5–10 years) to assess whether early diagnostic reclassification leads to a clinically meaningful reduction in liver disease progression, mortality, or cardiovascular outcomes. It remains uncertain whether the transition from NAFLD to MASLD promotes earlier adoption of “lean” or personalized therapeutic strategies and whether such approaches truly improve prognosis.
There is thus an urgent need for prospective, multicenter studies with extended follow-up capable of validating the effectiveness of the new diagnostic framework in terms of prevention, risk stratification, and overall clinical outcomes.
In conclusion, risk stratification in MASLD can no longer rely solely on histological assessment. An integrated approach is essential—combining clinical scoring systems, advanced imaging techniques, specific biomarkers, and systemic risk profiles—to enable early identification of high-risk patients and guide them toward personalized, multidisciplinary therapeutic pathways.
The transition from NAFLD to MASLD represents a paradigmatic shift in modern hepatology. It repositions the liver not merely as a passive target of metabolic dysfunction but as the epicenter of a systemic pathological network.
The new definition, grounded in positive diagnostic criteria, enables more accurate risk stratification, broader clinical inclusivity, and earlier identification of at-risk patients.
Nonetheless, several challenges remain: uneven adoption of the nomenclature, lack of standardized coding in international classification systems, and variability in clinical application across healthcare settings.
On the therapeutic front, available options are expanding, yet only a few have received formal regulatory approval. Variability in treatment response, high costs, and regulatory limitations advise caution in widespread clinical implementation.
Three key directions will shape future developments:
Precision medicine: The application of biomarkers derived from “omics” approaches and advanced phenotypic profiling will enable identification of clinically meaningful subgroups and more targeted therapeutic responses.
Predictive technologies: Algorithms based on artificial intelligence and big data will facilitate early diagnosis of “silent progressors” and enhance endpoints in clinical trials.
Integration into health systems: MASLD must be recognized as a systemic disease, necessitating targeted screening, multidisciplinary care pathways, and public health policies centered on prevention and equitable access.
The adoption of the MASLD definition carries significant clinical and societal implications. From the patient perspective, a definition based on positive criteria may enhance understanding and acceptance of the diagnosis, while reducing stigma associated with terms like “non-alcoholic” or “cryptogenic.” At the regulatory and insurance levels, MASLD allows for clearer and more appropriate categorization of patients eligible for screening and treatment—although full inclusion in reimbursement packages remains to be confirmed.
From a public health policy standpoint, this redefinition provides a valuable opportunity to revise eligibility criteria for hepatology and metabolic services.
MASLD also has substantial implications for general practice, diabetology, and community cardiology. Primary care physicians will need to update metabolic screening protocols, incorporating more precise algorithms that also capture normal-weight patients with metabolic dysfunction. Diabetology networks will be required to integrate liver assessment into care pathways for type 2 diabetes, while community cardiologists should include MASLD in cardiovascular risk assessment—particularly in younger patients or those with visceral obesity.
This integrated, interdisciplinary approach may help bridge the traditional divide between hepatology and internal medicine, advancing a systemic view of chronic disease management.
Moreover, the adoption of the MASLD terminology may reduce the social stigma associated with NAFLD, shifting the focus from notions of “blame” (e.g., lifestyle or presumed alcohol misuse) toward the biological and pathogenetic complexity of the disease.
To address these limitations, the term MASLD was introduced in 2023. This new definition maintains the emphasis on metabolic dysfunction while allowing for the inclusion of individuals with moderate alcohol consumption. According to MASLD criteria, hepatic steatosis—documented by imaging, histology, or biomarkers—must be accompanied by at least one cardiometabolic risk factor such as obesity, prediabetes or diabetes, dyslipidemia, or arterial hypertension [8]. By adopting positive and inclusive criteria, MASLD facilitates improved risk stratification, supports clinical trial enrollment, and better reflects the real-world heterogeneity of patients.
CKD: chronic kidney disease
DAG: diacylglycerols
FXR: farnesoid X receptor
GLP-1 RAs: glucagon-like peptide-1 receptor agonists
HCC: hepatocellular carcinoma
iCCA: intrahepatic cholangiocarcinoma
MAFLD: metabolic dysfunction-associated fatty liver disease
MASH: metabolic dysfunction-associated steatohepatitis
MASLD: metabolic dysfunction-associated steatotic liver disease
MetALD: metabolic dysfunction and alcohol-related liver disease
MRI-PDFF: magnetic resonance imaging with proton density fat fraction
NAFLD: non-alcoholic fatty liver disease
NFS: non-alcoholic fatty liver disease fibrosis score
NPV: negative predictive value
PCOS: polycystic ovary syndrome
PNPLA3: patatin-like phospholipase domain-containing protein 3
PRPs: pattern recognition receptors
ROS: reactive oxygen species
T2DM: type.2 diabetes mellitus
CA: Writing—original draft, Supervision. RN, FB, KZ, LR, FCS, CC, and LEA: Writing—review & editing. AC: Supervision. All authors read and approved the final version.
Ferdinando Carlo Sasso, who is the Editorial Board Member of Exploration of Medicine, had no involvement in the decision-making or the review process of this manuscript. The other authors declare no conflicts of interest.
Not applicable. This article is a review of previously published literature and does not include original studies involving human participants or animals performed by any of the authors.
Not applicable.
Not applicable.
Not applicable. No datasets were generated or analyzed during the current study.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
