Affiliation:
Clinical Academic Center of Trás-os-Montes and Alto Douro (CACTMAD), University of Trás-os-Montes and Alto Douro, 5000-801 Vila Real, Portugal
Email: sgoncalves@utad.pt
ORCID: https://orcid.org/0000-0002-8287-1357
Explor Med. 2025;6:1001355 DOI: https://doi.org/10.37349/emed.2025.1001355
Received: June 29, 2025 Accepted: August 26, 2025 Published: September 11, 2025
Academic Editor: Patricia Tai, University of Saskatchewan, Canada
The article belongs to the special issue Practical Tips for Cancer Care: Guidance for Patients, Caregivers, and Healthcare Professionals
Cancer patients and their caregivers often face a high burden of physical and psychological symptoms, such as pain, fatigue, anxiety, insomnia, and emotional distress, which significantly impact quality of life. While pharmacological treatments remain central to oncology care, they may not fully address these complex needs and can introduce risks such as polypharmacy. Non-pharmacological interventions offer complementary strategies that are generally safe, cost-effective, and adaptable to various clinical and home settings. This review explores practical, evidence-based non-pharmacological interventions that can be integrated into supportive cancer care. These include aromatherapy, massage therapy, mindfulness practices, guided imagery, music and art therapy, and sleep hygiene techniques. Each approach is examined in terms of its mechanisms of action, symptom targets, delivery methods, and implementation considerations. For example, lavender and citrus essential oils have been shown to have anxiolytic and sedative effects through olfactory-limbic pathways. Massage therapy has been associated with reductions in pain, stress, and cortisol levels. Mindfulness-based practices and guided imagery can help regulate emotional distress and may have a positive influence on immune and inflammatory markers. Music and art therapy support emotional expression and coping, while sleep hygiene strategies improve sleep quality and reduce fatigue. These interventions can benefit both patients and caregivers by enhancing resilience, fostering emotional connection, and supporting holistic well-being. Healthcare professionals play a crucial role in guiding the safe and appropriate use of these non-pharmacological interventions. Integrating these approaches into routine care requires individualized planning, clinician training, and institutional support. As evidence grows, non-pharmacological interventions should be recognized as valuable components of comprehensive cancer care.
Cancer remains a leading cause of morbidity and mortality worldwide, with more than 10 million cancer-related deaths reported annually, according to the World Health Organization [1–3]. Alongside the physiological toll of the disease and its therapies, patients frequently experience high levels of symptom burden—including pain, fatigue, insomnia, anxiety, and emotional distress—that significantly impair quality of life. These challenges extend beyond the patient to caregivers, who often face physical, emotional, and psychological strain that can lead to burnout and diminished well-being [4, 5].
While pharmacological treatments remain central to cancer management, they are not always sufficient or appropriate for addressing the full spectrum of symptoms, particularly those related to emotional and psychosocial health. In this context, non-pharmacological interventions have emerged as vital components of supportive oncology care [6]. Supportive care in oncology encompasses the prevention and management of adverse effects associated with cancer and its treatment, addressing symptoms, psychosocial issues, and quality of life throughout the disease trajectory [7]. Integrative care, by contrast, combines conventional medical treatments with evidence-based complementary therapies—such as massage, aromatherapy, or mindfulness—to support holistic well-being, emphasizing patient-centered, whole-person care. Techniques such as aromatherapy, massage, mindfulness, guided imagery, music and art therapy, and sleep hygiene strategies offer low-risk, accessible, and often self-administered tools that can enhance resilience, reduce suffering, and foster a greater sense of control for both patients and caregivers [8–10]. When integrated thoughtfully, these approaches aim to reduce suffering, improve outcomes, and empower both patients and caregivers in the cancer journey. Notably, many of these practices have demonstrated biological plausibility, with evidence pointing to their effects on neuroendocrine pathways, autonomic balance, and inflammatory processes. This growing body of research highlights the need to bridge clinical applications with mechanistic insights [11, 12].
This review synthesizes current evidence on effective non-pharmacological interventions in cancer care, with a focus on practical applications for patients, caregivers, and healthcare professionals. By highlighting both clinical utility and underlying mechanisms, the article aims to offer a scientifically grounded, user-friendly guide to integrating these therapies into routine care.
This article presents a narrative review that aims to synthesize practical, evidence-based non-pharmacological interventions used in supportive cancer care. A comprehensive literature search was conducted using PubMed, Scopus, Web of Science, and Google Scholar. The search encompassed English-language publications from January 2000 to May 2025, focusing on clinical trials, systematic reviews, meta-analyses, and relevant guidelines that addressed non-pharmacological therapies for cancer patients and their caregivers. The search strategy utilized a combination of Medical Subject Headings (MeSH), keywords, and Boolean operators to ensure a comprehensive retrieval of studies. Key search terms included, but were not limited to: “cancer”, “integrative oncology”, “supportive care”, “non-pharmacological interventions”, “aromatherapy”, “massage therapy”, “mindfulness”, “guided imagery”, “music therapy”, “art therapy”, “sleep hygiene”, and “caregiver burden”. Additional sources were identified by manually reviewing reference lists from key articles.
This is a narrative review, not a systematic review. Studies were selected based on their relevance to clinical application, methodological quality, and practical implications for patient and caregiver support in oncology settings.
Cancer imposes a multidimensional burden that extends well beyond tumor biology or pharmacological treatment [13]. Patients undergoing active cancer therapy, as well as those living with advanced disease or survivorship, frequently experience a constellation of debilitating symptoms that impair quality of life. Simultaneously, informal caregivers, often family members, shoulder substantial emotional and physical stress, placing them at elevated risk for psychological morbidity and burnout [4, 14]. Understanding the breadth and depth of symptom burden in both populations is essential for guiding supportive care interventions.
Cancer-related fatigue (CRF) is among the most prevalent and distressing symptoms reported by patients, affecting up to 80% of individuals undergoing treatment [15]. Unlike typical fatigue, CRF is persistent, not relieved by rest, and significantly interferes with daily functioning. The pathophysiology is multifactorial, involving inflammatory cytokines, hypothalamic-pituitary-adrenal (HPA) axis dysregulation, anemia, and mitochondrial dysfunction [16]. CRF can persist long after treatment has ended, particularly in survivors of breast, lung, and colorectal cancers.
Despite the advent of advanced antiemetic regimens, nausea and vomiting remain common side effects of chemotherapy, radiotherapy, and some forms of immunotherapy. Acute nausea typically occurs within 24 h of treatment, while delayed nausea can persist for several days, impairing nutritional intake and adherence to treatment [17]. The anticipatory component—triggered by psychological conditioning—further complicates symptom control. Nausea is also reported in advanced disease due to gastrointestinal obstruction, opioid use, or tumor-related factors.
Sleep disturbances, including insomnia, fragmented sleep, and circadian rhythm disruption, affect approximately 30–70% of cancer patients [18]. Contributing factors include corticosteroid therapy, pain, anxiety, and environmental conditions during hospitalization. Poor sleep exacerbates fatigue, cognitive impairment, and emotional distress. Moreover, persistent sleep dysfunction during survivorship is linked to increased risk of depression and reduced immune surveillance [19].
Emotional distress is a frequent and underdiagnosed issue among oncology patients. Up to 20% experience clinically significant anxiety and/or depression during their illness trajectory [20]. Anxiety may be heightened during diagnosis, treatment initiation, and recurrence, while depression often emerges in the context of physical decline, social isolation, or existential distress. Both conditions are associated with poorer treatment adherence, diminished immune function, and increased mortality risk [21].
Cancer-related pain is reported by approximately 59% of patients receiving active treatment and 64% of those with advanced disease [22]. It may stem from tumor invasion of tissues, treatment-induced neuropathy, or musculoskeletal complications due to immobility or surgery. While opioids are a cornerstone of pain management, they are not always practical or well-tolerated, prompting interest in integrative modalities that may provide adjunctive benefit.
Informal caregivers—typically spouses, adult children, or close relatives—play a critical role in managing the physical, emotional, and logistical needs of cancer patients. However, this responsibility often comes at the cost of the caregiver’s well-being. Commonly reported challenges include chronic stress, emotional exhaustion, sleep deprivation, and social isolation [4, 23]. Caregiver burden is particularly pronounced in palliative and end-of-life care settings. Studies indicate that 30–50% of caregivers exhibit symptoms of clinical depression or anxiety, and many report poorer physical health outcomes compared to non-caregiving peers [24].
The bidirectional relationship between patient and caregiver distress underscores the need for supportive care strategies that address both populations simultaneously. Interventions focused on symptom control, emotional support, and caregiver education are crucial for maintaining quality of life and preventing long-term psychological sequelae.
Aromatherapy, the therapeutic use of plant-derived essential oils, has gained increasing recognition as a complementary approach in oncology care [25, 26]. Its non-invasive nature, ease of administration, and relatively low cost make it particularly suitable for cancer settings, where patients often experience multiple symptoms concurrently and may have limited tolerance for additional pharmacologic interventions. Aromatherapy has shown potential in alleviating a range of cancer-related symptoms, including anxiety, nausea, pain, and sleep disturbances [27–30].
The primary mechanism of aromatherapy involves olfactory stimulation, which activates the olfactory bulb and its direct projections to the limbic system, particularly the amygdala, hippocampus, and hypothalamus, brain regions involved in emotion, memory, and autonomic regulation [31]. This neuroanatomical pathway enables essential oil constituents to exert rapid effects on mood and stress responses. For instance, inhalation of specific volatile compounds can modulate sympathetic and parasympathetic nervous system activity, thereby influencing heart rate variability (HRV), respiratory rate, and cortisol levels [32].
In addition to olfactory pathways, some essential oils exhibit pharmacological properties when absorbed through the skin or mucous membranes. Constituents such as linalool, linalyl acetate, menthol, and limonene have demonstrated anti-inflammatory, antinociceptive, and anxiolytic effects in preclinical and clinical studies [33–35].
Several essential oils have been studied for their utility in managing cancer-related symptoms:
Lavandula angustifolia (Lavender): Rich in linalool and linalyl acetate, lavender oil is among the most extensively researched essential oils due to its anxiolytic, sedative, and mild analgesic properties. Clinical studies have shown that lavender aromatherapy can reduce pre-procedural anxiety and improve sleep quality in cancer patients [36–38]. Randomized controlled trials (RCTs) have reported significant reductions in anxiety levels among breast cancer patients undergoing chemotherapy when exposed to lavender essential oil via inhalation [39].
Citrus aurantium (Bitter orange): Containing limonene and linalool, this oil has shown promise in reducing anxiety and nausea. One study demonstrated its effectiveness in lowering perioperative anxiety in oncology patients, suggesting its potential as an adjunct in surgical and diagnostic settings [40].
Mentha piperita (Peppermint): Widely used for its antiemetic and analgesic effects, peppermint oil can help manage chemotherapy-induced nausea and vomiting (CINV) as well as tension-type headaches. Inhalation or topical application (diluted) to the temples has been associated with symptom relief in palliative care contexts [41].
Aromatherapy can be administered through various routes, each with its advantages and limitations:
Inhalation: The most common method, achieved through diffusers, cotton balls, personal inhalers, or humidifiers. It allows for rapid onset of action with minimal systemic absorption, making it particularly suitable for hospitalized or immunocompromised patients.
Topical application: This involves diluting essential oils in carrier oils (e.g., jojoba, sweet almond oil) and is often used in conjunction with massage therapy. This method enables localized effects, such as pain relief or skin support, but carries a higher risk of dermal irritation or sensitization.
Bathing and compresses: Less commonly used in clinical oncology settings, but may offer relaxation benefits in home care or palliative environments.
Safety is a critical consideration in oncology patients, who may have altered skin integrity, immunosuppression, or sensitivities due to treatment. Essential oils must be diluted appropriately (typically 1–3% for adults) and used with clinical-grade quality control. Certain oils (e.g., those high in phenols like clove or cinnamon) should be avoided in frail or pediatric populations due to potential irritant effects.
Clinicians must also be aware of potential drug-herb interactions, though these are minimal when oils are used via inhalation. Additionally, individual scent preferences and aversions should be respected, as olfactory memories can be emotionally potent, especially in patients with trauma histories or cognitive impairment. Table 1 outlines the essential oil, its primary therapeutic targets, mechanisms of action, evidence highlights, delivery methods, and safety considerations.
Summary of evidence-based essential oils for symptom management in oncology care.
| Essential oil | Primary symptoms targeted | Mechanism of action | Delivery methods | Safety considerations | References |
|---|---|---|---|---|---|
| Lavandula angustifolia (Lavender) | Anxiety, insomnia, mild pain | Olfactory modulation of the limbic system; autonomic nervous system regulation | Inhalation, diluted topical, and massage | Generally safe; dilute to 1–2%; avoid in patients with known linalool allergy | [42, 43] |
| Citrus aurantium (Bitter orange) | Preoperative anxiety, mild nausea | Limonene and linalool: anxiolytic and calming via GABAergic pathways | Inhalation | Photosensitizing: avoid direct skin exposure before UV/sunlight | [44, 45] |
| Mentha piperita (Peppermint) | Chemotherapy-induced nausea, headache, and tension pain | Menthol activates TRPM8 receptors; it has antiemetic and mild analgesic effects | Inhalation, diluted topical (temples, abdomen) | Use with caution in young children; may cause skin irritation if undiluted | [46, 47] |
GABA: gamma-aminobutyric acid.
Massage therapy is one of the most widely accepted and utilised complementary interventions in oncology, with a growing body of evidence supporting its role in enhancing quality of life, alleviating symptom burden, and promoting a sense of well-being. It involves the manual manipulation of soft tissues to promote relaxation, reduce tension, and relieve discomfort. In cancer care, massage therapy has been used to address both physical symptoms, such as pain and fatigue, and psychological concerns, including anxiety and depression.
Pain is a prevalent and often under-treated symptom among cancer patients. Massage therapy has demonstrated effectiveness in reducing both nociceptive and neuropathic pain associated with tumor growth, surgery, chemotherapy, or radiation [48]. Proposed mechanisms include enhanced blood flow, reduced muscular tension, and the activation of descending pain-inhibitory pathways via stimulation of pressure receptors, leading to the subsequent release of endogenous opioids [49].
A meta-analysis by Boyd et al. [50] (2016) found that massage therapy significantly reduced pain intensity in cancer patients compared to standard care or attention control conditions. The analgesic effects were most pronounced when massage was delivered consistently over multiple sessions.
Massage therapy has also been shown to significantly reduce anxiety, depression, and emotional tension in cancer patients. Through tactile and supportive human contact, massage can lower cortisol levels while promoting the release of serotonin and dopamine, which help modulate mood [51]. In a randomized trial of breast cancer patients undergoing chemotherapy, those receiving weekly massage sessions reported lower anxiety scores and improved mood compared to controls [52].
Massage has also been beneficial in palliative care, where it offers not only symptom relief but also a sense of human connection, which can be profoundly comforting at the end of life.
In hospitals or infusion clinics, massage therapy must be adapted to the patient’s medical condition, energy level, and treatment plan. Short, gentle sessions (15–30 min) focusing on the hands, feet, shoulders, or scalp are often well tolerated, especially when the patient is bedridden or receiving intravenous therapies. Oncology-trained massage therapists are essential, as they understand how to modify techniques to avoid areas of tumor involvement, lymphedema risk, surgical wounds, and fragile skin due to radiation or corticosteroid use.
Contraindications include severe thrombocytopenia, neutropenia, skin infections, or deep vein thrombosis. Light touch or manual lymphatic drainage may be appropriate alternatives in such cases, pending approval from a physician.
In-home care, such as massage, can serve as a practical and low-cost intervention, delivered either by trained professionals or family caregivers who receive basic instruction. Studies suggest that even non-professional massage—administered by partners or caregivers—can significantly reduce distress and enhance patient-caregiver bonding [53]. Simple techniques, such as hand and foot massage with neutral or calming oils (e.g., grapeseed oil or infused oils containing lavender), can be safe and soothing.
In palliative or hospice settings, massage is frequently used to ease existential distress, support sleep, and offer compassionate touch. Adaptability is key: techniques should be adjusted according to disease progression, patient preference, and functional capacity.
Professionals with oncology-specific training must deliver massage therapy to cancer patients. Such training ensures that therapists can appropriately modify techniques, adjust pressure, and avoid vulnerable areas such as tumor sites, surgical wounds, or regions affected by lymphedema [54, 55]. These adaptations are crucial due to the unique physical conditions and medical complexities commonly encountered in oncological care.
Effective communication between the massage therapist, patient, and healthcare team is essential [56]. This ensures that the intervention is safe and tailored to the patient’s current clinical status, particularly when medical devices such as ports or catheters are present or when the patient is experiencing clinical instability. Moreover, in hospital and institutional settings, massage therapy must be formally documented in the patient’s care plan. Informed consent should always be obtained before initiating treatment [57].
It is important to note that massage therapy is not intended to replace pharmacologic symptom management. Instead, it serves as a valuable complementary modality within a broader supportive or palliative care framework. Its ability to address both physical symptoms, such as pain and fatigue, and psychological concerns, such as anxiety and distress, highlights its relevance in delivering holistic, patient-centered care. A summary of massage therapy applications in oncology is provided in Table 2.
Summary of massage therapy applications in cancer care.
| Domain | Details |
|---|---|
| Primary benefits | • Reduction in pain intensity (nociceptive, neuropathic)• Decrease in anxiety and emotional distress• Improved sleep and relaxation |
| Mechanisms of action | • Activation of pressure receptors → parasympathetic response• Modulation of cortisol and endorphins• Increased circulation and lymphatic flow• Disruption of the pain-tension-anxiety cycle |
| Common techniques | • Gentle Swedish massage• Manual lymphatic drainage (for appropriate patients)• Hand, foot, back, and scalp massage |
| Settings for delivery | • Inpatient oncology units• Outpatient infusion clinics• Home care and hospice settings |
| Implementation considerations | • Oncology-specific training for therapists• Individualized protocols: avoid tumors, surgical wounds, devices (e.g., ports)• Adjust pressure, duration, and technique based on patient tolerance |
| Contraindications | • Severe thrombocytopenia or neutropenia• Active infections or open wounds• Risk of DVT or lymphedema (without proper clearance) |
| Safety and consent | • Document in clinical record• Obtain informed consent• Monitor for discomfort or adverse effects during and after the session |
Massage therapy should be used as a complementary, not a substitute, intervention. It is most effective when integrated into multidisciplinary supportive and palliative care plans. DVT: deep vein thrombosis.
Mindfulness-based interventions (MBIs) and guided imagery are cognitive-behavioral mind-body techniques that offer scalable, non-invasive tools to reduce psychological distress in both cancer patients and their caregivers. These interventions are especially relevant in oncology settings where individuals face emotional burden, treatment-related anxiety, uncertainty, and caregiver fatigue. Unlike some complementary therapies, mindfulness and guided imagery require no physical equipment or pharmacological agents. They can be easily delivered through brief, structured sessions or mobile applications, making them accessible in clinical, home, or virtual environments.
Mindfulness refers to the self-regulation of attention toward the present moment, accompanied by an attitude of curiosity and nonjudgment. It involves being aware of both internal and external experiences, including bodily sensations, thoughts, and emotions. Research using functional imaging has shown that mindfulness practices activate the prefrontal cortex, anterior cingulate cortex, and insula, areas involved in emotional regulation, interoception, and attentional control [58].
Guided imagery, meanwhile, involves the use of focused mental visualization, often accompanied by verbal cues or scripts, to promote relaxation, reduce pain perception, and foster positive psychological states. It may evoke multisensory experiences (visual, auditory, tactile) that influence autonomic nervous system responses—such as reducing heart rate, blood pressure, and cortisol levels—via the mind-body interface [59].
Both practices modulate the HPA axis and have been associated with lower levels of inflammatory cytokines, improved immune function, and reduced sympathetic arousal [60].
MBIs and guided imagery offer a range of clinical benefits for both cancer patients and their caregivers. For patients, these practices have been shown to reduce psychological distress significantly [61]. Meta-analyses have confirmed that MBIs are effective in decreasing symptoms of anxiety, depression, and fear of cancer recurrence in individuals at both early and advanced stages of disease. Among these, mindfulness-based stress reduction (MBSR) programs are the most extensively studied, with demonstrated efficacy in populations affected by breast, prostate, and colorectal cancers [62, 63].
In addition to emotional regulation, MBIs contribute to physical symptom management. Guided imagery, in particular, has been associated with reductions in perceived pain, chemotherapy-related fatigue, and anticipatory nausea. These benefits can be observed even with short, 10–15 min sessions conducted before or after treatment, highlighting the accessibility and practicality of these approaches in clinical settings [64].
Caregivers also experienced significant improvements through mindfulness training. Given the high levels of chronic stress, emotional exhaustion, and sleep disruption often reported among family and professional caregivers, MBIs can serve as practical tools to reduce burnout and compassion fatigue. Studies indicate that regular mindfulness practice enhances resilience and increases caregivers’ confidence in their ability to manage care responsibilities [5, 65, 66].
Furthermore, when patients and caregivers engage in mindfulness practices together, they often report enhanced communication, increased empathy, and a deeper emotional connection [67]. Dyadic mindfulness has shown promise in fostering relational coping strategies, particularly during the emotionally intense phases of cancer treatment and end-of-life care [68]. This collaborative engagement underscores the relational and supportive value of these non-pharmacological interventions.
One of the key advantages of mindfulness and guided imagery interventions is their high degree of scalability and adaptability across care settings. These techniques can be delivered through a variety of accessible formats, including mobile applications (such as Headspace or Insight Timer), pre-recorded audio sessions, live brief sessions led by therapists, chaplains, or psychologists, and structured group-based programs such as 8-week MBSR courses [69, 70]. This flexibility allows patients and caregivers to select formats that best suit their needs, preferences, and available resources.
Importantly, even short daily practices of just 5 to 15 min have demonstrated measurable benefits, making these interventions particularly well-suited for patients undergoing active treatment who may be fatigued or have limited time. Their brevity and ease of use enhance feasibility without compromising effectiveness [71, 72].
In home settings, mindfulness and guided imagery can be practiced individually or jointly by patients and caregivers. Using simple scripts or digital recordings tailored to stress reduction, grief processing, or emotional regulation, these practices can foster a sense of control, calm, and mutual support [73]. This at-home integration supports continuity of care and extends the therapeutic impact beyond clinical environments.
These interventions are generally safe and well-tolerated. However, care should be taken in individuals with a history of trauma or dissociative disorders, as guided imagery or extended silence may occasionally evoke distressing thoughts or emotions. In such cases, a trauma-informed approach to mindfulness is recommended, with emphasis on grounding and titration of emotional exposure.
Implementation in clinical practice benefits from collaboration with psychologists, palliative care teams, or certified mindfulness instructors who can tailor practices to meet the needs of both patients and caregivers. Table 3 presents a concise summary of mindfulness and guided imagery in oncology care.
Summary of mindfulness and guided imagery applications in cancer.
| Domain | Details |
|---|---|
| Primary benefits | • Reduced anxiety, depression, and emotional distress• Improved sleep and mood• Decreased caregiver burden and burnout• Enhanced coping and emotional resilience |
| Mechanisms of action | • Regulation of the HPA axis and cortisol levels• Activation of prefrontal and insular brain regions (emotion regulation)• Parasympathetic nervous system activation• Reduced inflammatory cytokine activity |
| Delivery formats | • Mobile apps (e.g., Headspace, Insight Timer)• Audio recordings (e.g., for guided imagery)• Group classes (MBSR)• Short daily practices (5–15 min) |
| Settings for use | • Hospitals and outpatient clinics• Home care and hospice settings• Dyadic patient-caregiver sessions |
| Practical advantages | • Low cost, highly scalable• Minimal training required for basic use• Adaptable to physical limitations and disease stage |
| Safety considerations | • Generally safe and well tolerated• Caution in trauma-exposed individuals; consider trauma-informed mindfulness approaches• Emotional responses should be monitored when used in early grief or advanced disease |
Mindfulness and guided imagery are particularly well-suited for integration into holistic care plans and patient empowerment strategies across various stages of cancer and settings. HPA: hypothalamic-pituitary-adrenal; MBSR: mindfulness-based stress reduction.
Music therapy and art therapy are expressive, non-pharmacological interventions that support the emotional, cognitive, and spiritual well-being of cancer patients and caregivers. Both modalities are adaptable to a wide range of clinical settings and patient needs, offering accessible tools to process emotions, reduce stress, and foster meaning-making throughout the cancer trajectory.
Music therapy is a structured therapeutic approach that utilizes music, facilitated by trained professionals, to address emotional, physical, cognitive, and social needs in patients. It can take the form of receptive experiences, such as listening to music, or active participation, such as singing, songwriting, or playing instruments. The choice of method is typically guided by the patient’s preferences, clinical status, and functional capacity [74, 75].
Clinically, music therapy offers several well-documented benefits. Emotional regulation and mood enhancement are among its most prominent effects. Numerous studies have shown that music therapy can significantly reduce anxiety, depression, and emotional distress in cancer patients, particularly those undergoing chemotherapy, surgery, or palliative care [76]. These effects are linked to music’s activation of brain regions involved in emotional processing and reward, such as the amygdala and ventral striatum, which help promote a sense of calm and well-being.
In addition to its emotional impact, music therapy also plays a role in pain and symptom management. Both live and recorded music interventions have been shown to decrease the perception of pain and support relaxation during invasive procedures or end-of-life care. In pediatric oncology, music therapy plays a vital role in enhancing communication, alleviating anxiety, and facilitating coping strategies. Among geriatric populations, it may improve memory, provide orientation, and ease feelings of isolation [77].
From an implementation standpoint, music therapy is highly adaptable and accessible [78]. It can be delivered at the bedside, in group settings, or through headphones using personalized playlists. This approach requires minimal equipment and can be easily tailored to individual cultural and musical preferences. In palliative care, music therapy is sometimes combined with legacy projects or life review exercises, providing patients with opportunities for reflection, emotional closure, and meaning-making during the advanced stages of illness [79].
Art therapy is a form of expressive therapy that uses visual arts media—such as drawing, painting, and collage—to support emotional healing and psychological well-being [80]. Facilitated by trained art therapists, this modality emphasizes self-expression, symbolic communication, and emotional processing, rather than artistic skill. It allows patients to externalize internal experiences and explore complex emotions related to their illness in a non-verbal, creative format [81, 82].
The clinical benefits of art therapy are increasingly supported by empirical research. It offers patients a means of processing their illness experience, particularly emotions related to diagnosis, body image changes, and mortality. Engaging in art-making promotes reflection, emotional exploration, and psychosocial adaptation [83].
Studies have also demonstrated that art therapy can significantly reduce fatigue, alleviate anxiety, and improve mood, especially among patients undergoing chemotherapy or radiation [84, 85]. Moreover, art therapy is not limited to patients alone; caregivers also benefit from participating in these sessions. It provides them with a safe space to decompress, reflect on their caregiving role, and express their own emotional burdens, thus helping to reduce burnout and compassion fatigue [86].
From a practical perspective, art therapy is both accessible and adaptable. It requires only simple, inexpensive materials and can be conducted individually or in group settings [87]. With the rise of digital platforms, art therapy sessions can also be delivered remotely, expanding access to those in outpatient or home-based care. It is beneficial for populations with limited verbal communication abilities, such as children or individuals coping with trauma or grief [88].
The implementation of art therapy in oncology care is most effective when led by a certified therapist; however, patients may still derive benefits from self-directed creative activities, such as journaling or drawing [89]. Contraindications are rare but may arise in cases of cognitive impairment or sensory sensitivities that limit participation. Effective integration into routine care often requires coordination with psycho-oncology teams, rehabilitation services, or palliative care providers to ensure that the therapy is tailored to each patient’s functional capacity and emotional needs. Table 4 presents a summary of music and art therapy applications in cancer care.
Summary of music and art therapy applications in cancer care.
| Domain | Details |
|---|---|
| Primary benefits | • Reduction in anxiety, depression, and emotional distress• Improved mood and self-expression• Enhanced quality of life and spiritual well-being• Decreased caregiver burden and emotional fatigue |
| Mechanisms of action | • Modulation of the limbic system (music: amygdala, striatum)• Externalization of inner experiences (art)• Distraction and relaxation• Facilitation of emotional processing and coping |
| Delivery formats | • Music: live sessions, recorded playlists, songwriting, lyric analysis• Art: drawing, painting, collage, journaling• Individual or group settings, in person or virtual |
| Settings for use | • Hospitals (inpatient and outpatient)• Oncology clinics• Home care and hospice• Pediatric and geriatric units |
| Practical advantages | • Minimal equipment needed• Easy to personalize based on preferences• Accessible to patients with verbal or mobility limitations |
| Safety considerations | • Generally well tolerated• Caution with sound sensitivity or emotional overwhelm• Requires facilitator support in trauma-informed contexts |
Music and art therapy are most effective when integrated as part of a comprehensive care model that acknowledges the psychosocial and spiritual aspects of cancer.
Sleep disturbances are among the most common and debilitating symptoms experienced by cancer patients and their caregivers. Insomnia affects up to 75% of individuals undergoing cancer treatment and often persists into survivorship or end-of-life care [19]. Poor sleep quality is associated with fatigue, impaired cognitive function, reduced treatment adherence, and diminished overall quality of life. It can also exacerbate anxiety and depressive symptoms, creating a self-reinforcing cycle of psychological and physical burden. Importantly, caregivers are also at heightened risk for chronic sleep disruption due to stress, nighttime caregiving responsibilities, and anticipatory grief.
Sleep hygiene education and relaxation-based interventions offer evidence-based, low-cost strategies to mitigate sleep problems in oncology populations. These methods are accessible and adaptable to clinical and home settings, and can be taught as part of routine supportive care without the risks associated with pharmacologic sedatives.
Sleep hygiene education is a foundational, evidence-based strategy that involves teaching both patients and caregivers behavioral and environmental practices aimed at promoting more restorative sleep [90, 91]. Key components of this approach include maintaining a consistent sleep-wake schedule, even on days without treatment, and creating a sleep environment that is dark, quiet, and cool. Patients are advised to avoid stimulants such as caffeine and nicotine, as well as large meals close to bedtime, as these can interfere with sleep onset and quality. Limiting screen use and exposure to blue light before bed is also recommended, as these can disrupt circadian rhythms. Additionally, incorporating light physical activity during the day, when appropriate, may help improve sleep at night [92]. While sleep hygiene alone may not fully resolve complex cases of insomnia, it remains an essential first-line intervention. It allows patients to regain a sense of structure and control, particularly during periods of active treatment or hospitalization, when routines are often disrupted.
PMR is a guided technique that involves systematically tensing and releasing muscle groups. It promotes parasympathetic activation, reduces somatic tension, and has been shown in RCTs to improve sleep onset latency and total sleep time in patients with cancer-related insomnia [93]. PMR can be delivered via audio recordings, bedside guidance, or mobile apps.
Deep, diaphragmatic breathing and autogenic phrases (e.g., “my body is calm and heavy”) have been used effectively to reduce physiological arousal. These techniques decrease heart rate and cortisol levels, making them ideal for pre-sleep routines. Studies indicate that guided breathing practices, especially when practiced regularly, can reduce nighttime awakenings and help manage anticipatory anxiety before treatments [94].
CBT-I is widely recognized as the gold-standard, non-pharmacological treatment for chronic insomnia and has strong evidence supporting its effectiveness in individuals with cancer [95]. This structured intervention focuses on identifying and modifying maladaptive beliefs and behaviors related to sleep. Common examples include negative thoughts such as “I’ll never sleep without medication” or unhelpful habits like clock-watching and taking long daytime naps. CBT-I typically incorporates several core strategies, including stimulus control, which involves using the bed only for sleep and intimacy; sleep restriction therapy, which limits the time spent in bed to consolidate sleep; and cognitive restructuring, which challenges and reframes distorted thoughts about sleep [96]. In recent years, digital CBT-I programs, such as Sleepio and CBT-I Coach, have shown promising results and offer a practical solution for patients with limited mobility, those living in rural areas, or those lacking access to trained therapists. These digital tools increase the accessibility and scalability of CBT-I, making it a valuable option within oncology care settings [97].
When addressing sleep disturbances in oncology, it is important to distinguish between CRF and true insomnia. Fatigue is characterized by a persistent sense of physical and mental exhaustion that is not necessarily linked to sleep patterns, whereas insomnia refers explicitly to difficulty initiating or maintaining sleep [98]. Although these conditions can co-occur, they require different management strategies, and tailored interventions should be selected accordingly. Another key consideration is the potential interaction between sleep-promoting medications and cancer treatments such as chemotherapy, corticosteroids, or opioids [99]. In many cases, these drug interactions may limit the feasibility of pharmacologic sleep aids, making non-pharmacological interventions particularly valuable and safer alternatives.
Additionally, sleep education and relaxation techniques are not only beneficial for patients but can also be taught to caregivers. Enhancing caregivers’ sleep quality contributes to greater resilience and allows them to maintain their caregiving role more effectively, reducing the risk of burnout [100, 101]. Table 5 presents a summary of sleep hygiene and relaxation techniques in cancer care.
Summary of sleep hygiene and relaxation strategies in cancer care.
| Domain | Details |
|---|---|
| Primary benefits | • Improved sleep onset, duration, and quality• Reduced nighttime awakenings and arousals• Decreased fatigue and anxiety• Enhanced cognitive functioning and emotional regulation |
| Mechanisms of action | • Parasympathetic activation (e.g., PMR, breathwork)• Reduction of cortisol and sympathetic arousal• Cognitive restructuring of maladaptive beliefs (CBT-I)• Behavioral sleep pattern correction |
| Delivery formats | • Verbal instruction (nursing, psycho-oncology)• Audio-guided PMR or breathing sessions• Digital CBT-I platforms (e.g., Sleepio, CBT-I Coach)• Printed or digital sleep hygiene handouts |
| Settings for use | • Hospital wards, oncology clinics• Home-based or palliative care environments• Suitable for both patients and caregivers |
| Practical advantages | • Low-cost and non-invasive• Easily integrated into routine care• Beneficial for a wide range of ages and cancer types |
| Safety considerations | • Generally very safe• CBT-I may require supervision for patients with psychiatric comorbidities• Caution with sleep restriction in severely fatigued or advanced disease states |
When delivered consistently and supported by healthcare teams, sleep-focused interventions significantly enhance patients’ physical and psychological resilience across the cancer care continuum. CBT-I: cognitive behavioral therapy for insomnia; PMR: progressive muscle relaxation.
While non-pharmacological interventions are generally considered safe and well-tolerated, it is essential to recognize that each modality carries specific risks, limitations, and contraindications that must be considered during clinical implementation [102]. A careful, individualized assessment is necessary to avoid unintended harm, particularly in oncology populations who may be medically fragile, immunocompromised, or emotionally vulnerable.
Aromatherapy is widely used for symptom relief; however, the topical application of essential oils may cause skin irritation or allergic reactions, particularly in patients with compromised skin integrity due to radiation or chemotherapy [103, 104]. Essential oils with high phenolic content (e.g., clove, cinnamon) should be avoided in frail or pediatric patients [105]. Photosensitizing essential oils, such as Citrus aurantium, should not be applied before sun exposure [106]. Inhalation is typically safer; however, individual scent aversions and potential interactions with respiratory conditions (e.g., asthma) should be considered.
Massage therapy is contraindicated in patients with severe thrombocytopenia, neutropenia, deep vein thrombosis, active infections, or unstable bone metastases [107]. Oncology-trained therapists should modify techniques to avoid tumor sites, surgical wounds, lymphedema risk areas, and indwelling medical devices. Even a light touch must be carefully adapted to the patient’s tolerance and clinical condition [108].
Mindfulness and guided imagery are highly beneficial for many patients but may pose psychological risks for those with unresolved trauma, dissociative symptoms, or severe emotional instability [109]. Mindfulness practices that involve extended silence or body scanning can inadvertently trigger flashbacks or anxiety. In such cases, trauma-informed adaptations (e.g., anchoring to external stimuli or limiting exposure) are strongly recommended, and a trained facilitator with mental health expertise should ideally introduce practices [110].
Music and art therapy are generally low-risk, but care must be taken in individuals with sound sensitivities, cognitive impairments, or difficulty with sensory processing [111]. Emotionally evocative activities, such as legacy projects or autobiographical art, may stir distress and should be guided by trained professionals who can offer appropriate containment and support [112].
Sleep hygiene and relaxation techniques, including PMR and breathwork, are safe for most patients. However, interventions such as sleep restriction (as part of CBT-I) may not be suitable for individuals with severe fatigue, advanced disease, or cognitive impairment [113]. Additionally, some patients may find the focused attention on bodily sensations distressing, particularly if they are experiencing pain or anxiety.
In all cases, informed consent, patient education, and close monitoring are essential. Interventions should be adjusted to the individual’s medical status, preferences, and psychosocial context, ideally in collaboration with interdisciplinary teams [114]. Establishing safety protocols, screening tools, and clear referral pathways can enhance the responsible integration of these therapies into supportive oncology care.
While non-pharmacological therapies are often categorised as supportive or adjunctive, accumulating evidence suggests that they exert meaningful effects through specific molecular, neuroendocrine, and cellular pathways. Understanding these mechanisms reinforces their clinical relevance and supports their integration into evidence-based cancer care.
Essential oils contain volatile organic compounds that, when inhaled, interact with olfactory receptors in the nasal epithelium. Unlike other sensory inputs, olfactory signals bypass the thalamus and project directly to the limbic system, specifically to the amygdala, hippocampus, and hypothalamus—regions involved in emotion regulation, memory, and autonomic control [31].
Lavandula angustifolia (Lavender): Rich in linalool and linalyl acetate (Figure 1), lavender acts via gamma-aminobutyric acid (GABA)ergic modulation, producing anxiolytic and sedative effects. Animal models show reduced spontaneous motor activity and decreased plasma corticosterone levels following inhalation [115].
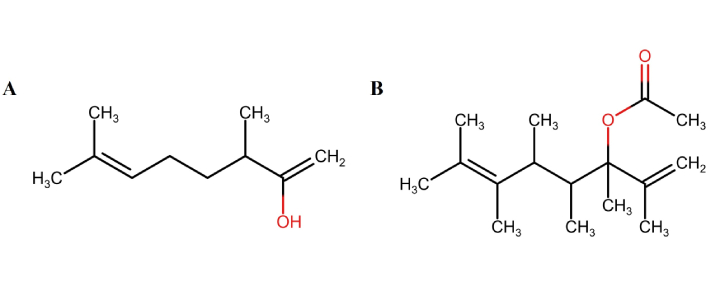
Chemical structures of major constituents of lavender essential oil. Linalool (A) and linalyl acetate (B) are two major constituents of lavender essential oil. Marvin JS was used for drawing, displaying, and characterizing chemical structures (RCSB PDB: Chemical Sketch). Marvin JS 17.21.0, ChemAxon (https://chemaxon.com) [116].
Citrus aurantium (Bitter orange): Contains limonene (Figure 2), which exhibits serotonergic and dopaminergic activity, associated with mood stabilization. Studies have shown a downregulation of HPA axis activity and a reduction in pro-inflammatory cytokines [e.g., interleukin-1β (IL-1β), IL-6] [117].
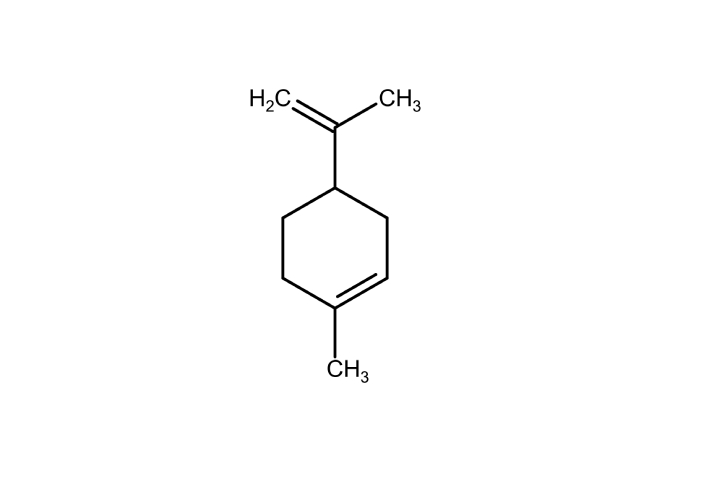
Limonene: principal constituent of citrus essential oils. Marvin JS was used for drawing, displaying, and characterizing chemical structures (RCSB PDB: Chemical Sketch). Marvin JS 17.21.0, ChemAxon (https://chemaxon.com) [116].
Mentha piperita (Peppermint): Menthol (Figure 3) modulates TRPM8 channels, contributing to the analgesic and cooling sensations associated with this plant. It also influences cholinergic transmission, which may reduce chemotherapy-related nausea.
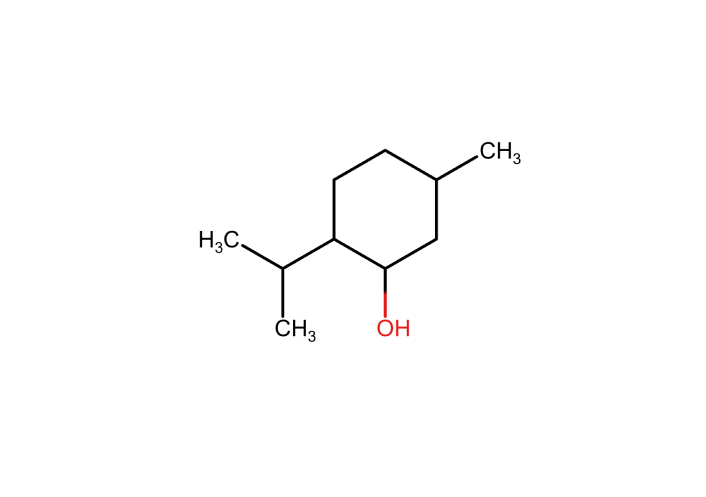
Menthol: a major constituent of peppermint essential oil. Marvin JS was used for drawing, displaying, and characterizing chemical structures (RCSB PDB: Chemical Sketch). Marvin JS 17.21.0, ChemAxon (https://chemaxon.com) [116].
These biochemical interactions are complemented by autonomic effects, including lowered heart rate, blood pressure, and respiratory rate, which are often measurable within minutes of exposure.
Massage stimulates cutaneous and deep pressure receptors, activating Aβ afferent fibers that inhibit nociceptive C and Aδ fibers at the spinal cord level, consistent with the gate control theory of pain [49]. Beyond pain inhibition, the stimulation of mechanoreceptors during massage fosters parasympathetic nervous system dominance. This shift is physiologically evident through decreased levels of stress-related hormones such as cortisol and norepinephrine, alongside increased secretion of oxytocin and serotonin, neurochemicals associated with relaxation, bonding, and mood regulation [118, 119]. Additionally, massage contributes to a reduction in pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and IL-6. Studies involving cancer patients have demonstrated that therapeutic massage can modulate natural killer cell activity, reduce sympathetic nervous system arousal, and improve sleep quality [120]. Together, these effects support improved homeostatic balance and bolster psychological resilience during the cancer journey [51].
MBIs have been shown to produce measurable changes in both brain function and gene regulation. Neuroimaging studies reveal that consistent mindfulness practice leads to increased activation of the prefrontal cortex, which is associated with executive control and decision-making. Simultaneously, there is a reduction in amygdala reactivity, which helps to mitigate emotional overarousal. Enhanced connectivity between the anterior cingulate cortex and the insula has also been observed, improving interoceptive awareness and the ability to monitor internal bodily states [121]. On a molecular level, mindfulness is associated with the downregulation of nuclear factor kappa B (NF-κB) and other pro-inflammatory transcription factors, thereby contributing to decreased systemic inflammation. Additionally, mindfulness practices are associated with the upregulation of telomerase activity, which plays a role in cellular longevity, and the reduced expression of genes involved in the stress response and glucocorticoid receptor resistance. Guided imagery, a technique often used in conjunction with mindfulness, further supports these benefits by reducing hyperactivation in the limbic system and promoting the restoration of the parasympathetic nervous system. This dual impact on neural and physiological systems enhances the body’s capacity for self-regulation and stress resilience [122].
Music therapy activates the mesolimbic dopaminergic system, particularly the nucleus accumbens and ventral tegmental area, which are central to the brain’s reward circuitry [123]. This engagement fosters sensations of pleasure, emotional reward, and personal meaning. At the neurochemical level, music therapy has been associated with increased dopamine release, enhancing motivation and positive affect. It also modulates endogenous opioid pathways, contributing to pain relief, and leads to reduced cortisol levels, indicating a reduction in physiological stress responses [124].
In parallel, art therapy primarily engages the brain’s visual-spatial processing centers, including the occipital and parietal cortices, as well as the default mode network, which is involved in self-referential thought and introspection. Through these mechanisms, art therapy facilitates emotional expression, supports the construction of personal narratives, and encourages meaning-making during the cancer experience. Neuroimaging studies have demonstrated that expressive arts activate cognitive reappraisal pathways, which are associated with reduced anxiety and increased psychological flexibility [125].
Relaxation techniques such as PMR and diaphragmatic breathing shift autonomic balance toward parasympathetic dominance. This shift can be measured through various physiological markers, including lower HRV, which reflects an increase in vagal tone [126]. Additionally, these techniques lead to reduced levels of salivary cortisol and alpha-amylase—biomarkers of stress—and an increase in melatonin secretion, particularly when combined with consistent sleep hygiene practices [127]. These changes support improved sleep quality and overall well-being. CBT-I affects neural connectivity in regions associated with arousal (e.g., the insula and thalamus). It reduces catastrophic thinking about sleep, improving sleep architecture and latency over time [128]. Table 6 presents a summary that encapsulates the core mechanisms of action for each non-pharmacological intervention.
A summary of the core mechanisms of action for each non-pharmacological intervention.
| Intervention | Primary pathways involved | Key molecular/neuroendocrine effects |
|---|---|---|
| Aromatherapy | Olfactory-limbic axis (amygdala, hippocampus, hypothalamus) | ↑ GABAergic activity (linalool)↓ Cortisol and IL-6↑ Parasympathetic tone |
| Massage therapy | Somatosensory and neuroimmune modulationGate control of pain (spinal inhibition) | ↓ Cortisol, TNF-α↑ Oxytocin, serotonin↓ Sympathetic activation |
| Mindfulness & guided imagery | Prefrontal cortex, anterior cingulate cortex, amygdalaGene expression regulation | ↓ NF-κB pathway↑ Telomerase activity↓ Inflammatory gene expression |
| Music & art therapy | Mesolimbic reward system (VTA, nucleus accumbens)Default mode network (self-referential processing) | ↑ Dopamine↓ Cortisol↑ Endogenous opioid release |
| Sleep & relaxation | Autonomic nervous system balance (vagal activation)Sleep-related neurocognitive circuits (insula, thalamus, prefrontal cortex) | ↓ Cortisol and α-amylase↑ Melatonin↑ Sleep architecture and circadian alignment |
↑: Increased activity/secretion; ↓: decreased activity/secretion. GABA: gamma-aminobutyric acid; IL-6: interleukin-6; NF-κB: nuclear factor kappa B; TNF-α: tumor necrosis factor-alpha; VTA: ventral tegmental area.
The successful implementation of non-pharmacological interventions in cancer care depends on providing tailored, accessible, and evidence-informed guidance to all individuals involved in the care process. This section outlines specific recommendations for three primary groups: patients, caregivers, and healthcare professionals, with an emphasis on empowerment, collaboration, and safety.
Patients undergoing cancer treatment often experience multiple co-occurring symptoms such as pain, insomnia, anxiety, and fatigue. Engaging in supportive practices can improve symptom control and quality of life without the risks of polypharmacy.
To begin, patients are encouraged to adopt brief and manageable interventions, such as five minutes of guided breathing exercises or inhaling calming essential oils like lavender before bedtime [129]. Monitoring symptom changes through simple logs or mobile apps can help identify which strategies offer the most benefit. Creating a calming environment at home, using tools such as aromatherapy diffusers, soft background music, or soothing visual imagery, can further promote emotional and physical relaxation. Patients should also consider discussing with their healthcare team the possibility of integrating relaxation or massage therapies into their care, particularly during periods of intensive treatment such as chemotherapy or radiation [130]. When using essential oils, it is crucial to choose only clinically recommended varieties, such as Lavandula angustifolia or Citrus aurantium, and to ensure they are of high quality, pure, therapeutic grade, and appropriately diluted for safe use.
Caregivers are frequently at risk for psychological distress, sleep disruption, and physical exhaustion [4, 131]. Supporting caregivers’ well-being directly benefits patients and fosters sustainable care relationships. To promote resilience, caregivers are encouraged to incorporate daily self-care routines that may include mindfulness practices, creative art activities, or listening to calming music [132]. Engaging in these interventions alongside patients, such as participating in relaxation exercises together, can strengthen emotional bonds and create shared moments of relief. Learning simple therapeutic touch techniques, such as gentle hand massage or slow stroking, can provide comfort to both caregiver and patient while enhancing physical connection and reducing stress [55]. Guided imagery sessions, especially during waiting periods or hospital visits, offer a practical way to regulate stress responses and restore emotional balance. Additionally, caregivers may benefit from joining support groups, either in-person or online, that provide not only emotional validation but also structured coping tools that go beyond traditional verbal counseling.
Healthcare professionals play a crucial role in identifying suitable interventions, ensuring patient safety, and promoting consistent use. Though these practices are often termed “complementary”, they are increasingly recognized as part of whole-person, integrative oncology care.
To support their effective use, clinicians should routinely screen patients for symptoms such as anxiety, insomnia, and fatigue using validated assessment tools. Recommendations should be tailored to the patient’s needs, preferences, and care setting. For example, guided imagery may be beneficial for managing procedural anxiety, while CBT-I is suitable for addressing persistent sleep disturbances. When possible, professionals should provide or refer patients to structured programs such as MBSR, music therapy, or massage services available in oncology centers [90, 132].
Ensuring that these interventions are delivered safely requires that therapists are appropriately trained and credentialed. Licensed massage therapists, certified aromatherapists, and mindfulness instructors with experience in clinical settings should be involved in patient care. In addition, healthcare providers have a responsibility to educate interdisciplinary teams on the mechanisms, evidence base, and benefits of these approaches, fostering a collaborative and unified model of integrative care [133].
Importantly, equity in access must be prioritized. Non-pharmacological interventions should be available to all individuals, regardless of socioeconomic status, literacy level, or geographic location. The development of culturally adapted materials and the use of community-based resources—such as audio guides in native languages—can help extend the reach and impact of these supportive practices, ensuring inclusivity in cancer care delivery [134, 135].
Although a growing body of evidence supports the use of non-pharmacological interventions in cancer care, their translation into routine clinical practice remains inconsistent. Implementation efforts must address real-world barriers, including workforce limitations, institutional readiness, and economic feasibility. Furthermore, to ensure equitable access and long-term sustainability, these strategies must be embedded in health systems through targeted training, scalable delivery formats, and supportive policy frameworks. This section outlines key considerations for clinical integration, including professional training needs, equity in access, and cost-benefit implications, and concludes with recommendations for future research and policy development.
Practical and structural barriers often constrain the successful integration of non-pharmacological therapies in oncology. A central challenge is the lack of adequately trained personnel equipped to deliver these interventions safely in cancer settings [136]. Oncology nurses, psycho-oncologists, massage therapists, and integrative health providers may lack access to standardized training in modalities such as aromatherapy, mindfulness, or sleep education [137]. While some institutions offer in-house workshops or continuing education, there is no universal credentialing pathway for many of these interventions, leading to variability in quality and safety. Additionally, interdisciplinary teams may be unaware of referral processes or hesitant to recommend non-pharmacological approaches due to uncertainty about the strength of evidence or institutional guidelines [138].
The absence of structured protocols and documentation systems further complicates the clinical integration process. Supportive therapies are rarely tracked in electronic health records (EHRs), making it difficult to measure outcomes, communicate across teams, or justify resource allocation [139]. Embedding symptom screening tools and patient-reported outcome measures (PROMs) into routine oncology workflows can help identify candidates for non-pharmacological support and track the efficacy of interventions over time [140]. Finally, real-world implementation requires institutional buy-in and administrative support. This includes allocating time for staff training, budgeting for therapy supplies (e.g., essential oils, music devices), and recognizing these interventions as integral—not optional—components of holistic cancer care.
Equitable access to non-pharmacological interventions remains a significant challenge, particularly for patients in rural, low-income, or underserved communities. Many of these supportive therapies—such as massage, art therapy, and mindfulness training—are more readily available in academic or urban cancer centers [141]. At the same time, patients in community settings may have limited or no access. Additionally, cultural, linguistic, and literacy barriers can hinder patient engagement, especially when educational materials or therapeutic instructions are not adapted to diverse populations [142]. Addressing these disparities requires intentional efforts to develop inclusive models of care that account for differences in language, culture, socioeconomic status, and healthcare infrastructure.
Digital tools have emerged as promising avenues to overcome geographic and resource-based barriers. Mobile applications and web-based platforms now offer guided mindfulness, progressive relaxation, sleep hygiene programs, and even digital CBT-I, often at low or no cost [69]. These tools can extend the reach of care into the home environment, providing continuous support between clinical visits. For example, digital programs such as Sleepio, Headspace, and Insight Timer have demonstrated usability and clinical impact in oncology populations, particularly when supplemented with brief clinician guidance [143–145]. However, digital inclusion must be prioritized: interventions must be user-friendly, available in multiple languages, and compatible with varying levels of digital literacy. Partnerships with community organizations and patient advocacy groups can help promote uptake and ensure that technology-enhanced interventions are not limited to tech-savvy or high-resource users.
Equity-focused implementation also benefits from training lay caregivers and community health workers in basic supportive techniques, such as hand massage or guided breathing, thereby extending care beyond formal clinical teams [146]. Integrating these approaches into community clinics, hospices, and primary care settings provides a pathway toward more inclusive and scalable models of integrative oncology care.
While non-pharmacological interventions are often perceived as ancillary, emerging evidence suggests that they may offer significant economic benefits by improving symptom control, reducing reliance on medications, and enhancing the well-being of both patients and caregivers. Many of these strategies—such as aromatherapy, sleep hygiene education, guided imagery, and caregiver-led massage—are low-cost, low-risk, and relatively easy to implement, particularly when adapted for home use or delivered via digital platforms. By reducing symptom burden (e.g., anxiety, nausea, insomnia), they may help decrease the need for sedatives, antiemetics, or anxiolytics, which in turn could lower polypharmacy-related adverse events and healthcare utilization [147].
Preliminary studies indicate that integrative oncology programs incorporating non-pharmacological therapies can reduce hospital admissions, emergency department visits, and length of stay, particularly when used proactively during active treatment or in survivorship care plans [148, 149]. Furthermore, interventions that improve sleep and emotional resilience may enhance treatment adherence and reduce caregiver burnout, contributing to indirect savings for health systems and communities.
Despite these potential benefits, rigorous cost-effectiveness analyses remain limited. Most studies focus on clinical outcomes such as pain or mood, with few capturing long-term economic data or system-wide impact. Health economic evaluations should be prioritized in future research, particularly to assess whether investments in staff training, digital tools, or therapy infrastructure yield measurable returns in quality-adjusted life years (QALYs), caregiver productivity, or healthcare cost avoidance [150, 151]. Demonstrating financial value is especially important for informing reimbursement models, policymaking, and institutional resource allocation, factors that are critical for the sustainable integration of these approaches into standard cancer care.
To ensure that non-pharmacological interventions are sustainably integrated into cancer care, future research must focus on expanding the evidence base, standardizing delivery protocols, and informing supportive policy frameworks. Although existing studies support the clinical effectiveness of interventions such as mindfulness, massage, and aromatherapy, further high-quality RCTs are needed to clarify optimal dosing, delivery formats, and long-term effects across diverse cancer populations. Standardizing protocols, such as session duration for guided imagery or recommended dilution for essential oils, would enhance consistency, reproducibility, and safety across settings.
In parallel, research must prioritize inclusion. Populations such as pediatric, geriatric, rural, and ethnically diverse patients are often underrepresented in many trials, which limits the generalizability of the findings. Culturally tailored interventions, multilingual educational materials, and participatory research approaches are essential for ensuring that future models of care reflect the needs and realities of all patients and caregivers [152].
At the policy level, professional societies and regulatory bodies should collaborate to develop certification standards and best-practice guidelines for non-pharmacological therapies in oncology. These should outline minimum competencies for practitioners, safety criteria, and evidence thresholds for clinical recommendations. Additionally, health insurers and public health systems should consider revising reimbursement frameworks to support interventions that demonstrably improve quality of life, reduce medication use, and lower healthcare costs.
Ultimately, institutional policies must acknowledge the legitimacy and value of supportive care by integrating non-pharmacological interventions into care pathways, EHRs, and interdisciplinary team workflows. Clear referral mechanisms, informed consent procedures, and documentation practices can further support their routine use and alignment with ethical and professional standards. In combination, these research and policy efforts will be essential to moving non-pharmacological strategies from the margins to the mainstream of comprehensive cancer care. These research and policy priorities align with the current evidence landscape summarized in Table 7, where specific modalities, such as art and music therapy, are still classified as ‘promising but inconclusive’ due to variability in outcome measures and limited standardization of these approaches. Clarifying these gaps through coordinated research and guideline development will be essential to strengthening their clinical recommendation status.
Summary of non-pharmacological interventions in oncology care.
| Intervention | Recommendation status | Notes |
|---|---|---|
| Aromatherapy (lavender, citrus) | Recommended | Effective for anxiety, nausea, and sleep; good safety profile |
| Massage therapy | Recommended | Strong evidence for pain and emotional relief; needs trained staff |
| Mindfulness & guided imagery | Recommended | Effective for stress, mood, and sleep; widely adaptable |
| Music therapy | Promising, but inconclusive | Evidence supports mood benefits; more RCTs needed for symptom control |
| Art therapy | Promising, but inconclusive | Helpful for expression and coping; outcomes vary |
| Sleep hygiene education | Recommended | Effective for insomnia and fatigue; easily teachable |
| Essential oils (topical use) | Use with caution | Potential for skin irritation; dilution and quality control needed |
| Energy therapies (e.g., Reiki) | Not currently supported | Insufficient clinical evidence; further studies needed |
RCTs: randomized controlled trials.
To support clinical decision-making and reflect the variability in the current literature, Table 8 summarizes the strength of evidence for each intervention based on the Oxford Centre for Evidence-Based Medicine Levels of Evidence (2022) [153]. This framework categorizes the quality of evidence from Level 1 (highest) to Level 5 (expert opinion or case series), providing a structured approach to evaluating the robustness of available data. Table 8 also highlights common clinical indications and key research gaps.
Summary of non-pharmacological interventions by strength of evidence, outcomes, and research gaps (Oxford Levels of Evidence 2022).
| Intervention | Strength of evidence (Oxford Level) | Primary outcomes supported | Key research gaps |
|---|---|---|---|
| Aromatherapy | Level 2–3 | Anxiety, sleep, mild nausea | Standardization of dosages; long-term effects |
| Massage therapy | Level 1–2 | Pain, anxiety, and sleep quality | Cost-effectiveness; access in outpatient/rural settings |
| Mindfulness & guided imagery | Level 1–2 | Emotional distress, sleep, and caregiver burden | Pediatric/Geriatric data; implementation studies |
| Music therapy | Level 2–3 | Mood, anxiety, and coping | Larger RCTs; outcome heterogeneity |
| Art therapy | Level 3–4 | Emotional processing, caregiver support | Standardized outcome metrics; comparative trials |
| Sleep hygiene education | Level 1–2 | Insomnia, fatigue, anxiety | Personalization by cancer stage: digital intervention trials |
| Topical essential oils | Level 4 | Pain, skin care | Safety trials in oncology; dermatologic outcomes |
| Digital CBT-I | Level 1–2 | Insomnia, fatigue | Long-term adherence, low-literacy adaptation |
| Energy therapies (e.g., Reiki) | Level 4–5 | Subjective well-being | High-quality trials; mechanistic research |
RCT: randomized controlled trial; CBT-I: cognitive behavioral therapy for insomnia. Level 1: Systematic reviews of RCTs; Level 2: individual RCTs or observational studies with dramatic effects; Level 3: non-randomized cohort or case-control studies; Level 4: case series or poor-quality cohort/case-control; Level 5: mechanism-based reasoning or expert opinion.
Cancer care extends far beyond tumor management—it encompasses the relief of suffering, the preservation of dignity, and the support of both patients and caregivers through every phase of the illness. Non-pharmacological interventions such as aromatherapy, massage, mindfulness, music and art therapy, and sleep hygiene strategies offer accessible, evidence-based tools that can significantly alleviate symptom burden, enhance emotional well-being, and improve overall quality of life.
This review highlights the physiological mechanisms underlying these therapies, reinforcing their relevance within the broader scope of molecular and integrative medicine. Furthermore, it provides practical guidance tailored to the unique needs of patients, caregivers, and healthcare professionals, emphasizing simplicity, safety, and scalability.
As healthcare systems increasingly adopt holistic, person-centred models of care, the integration of these supportive interventions must be guided by rigorous research, inclusive implementation, and policy frameworks that prioritise equity and accessibility. By doing so, the oncology community can move closer to delivering comprehensive, compassionate, and scientifically grounded cancer care.
CBT-I: cognitive behavioral therapy for insomnia
CRF: cancer-related fatigue
EHRs: electronic health records
GABA: gamma-aminobutyric acid
HPA: hypothalamic-pituitary-adrenal
HRV: heart rate variability
IL-1β: interleukin-1β
MBIs: mindfulness-based interventions
MBSR: mindfulness-based stress reduction
NF-κB: nuclear factor kappa B
PMR: progressive muscle relaxation
PROMs: patient-reported outcome measures
RCTs: randomized controlled trials
TNF-α: tumor necrosis factor-alpha
SDG: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Validation, Supervision. The author has read and approved the submitted version.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
