Affiliation:
1Department of Oral Medicine, Faculty of Dentistry, Damascus University, Damascus 0100, Syrian Arab Republic
2The Higher Institute for Laser Research and Applications, Damascus University, Damascus 0100, Syrian Arab Republic
ORCID: https://orcid.org/0000-0002-2308-0547
Affiliation:
2The Higher Institute for Laser Research and Applications, Damascus University, Damascus 0100, Syrian Arab Republic
Affiliation:
2The Higher Institute for Laser Research and Applications, Damascus University, Damascus 0100, Syrian Arab Republic
Affiliation:
3Institute of Dentistry and Oral Science, Faculty of Medicine and Dentistry, Palacky University, Olomouc 77147, Czechia
4DCM Clinic, Hradec Kralove 50301, Czechia
ORCID: https://orcid.org/0000-0001-9194-6923
Affiliation:
5Master laser Dentistry, Universita Cattolica del Sacro Cuore di Roma, 00168 Rome, Italy
Affiliation:
6Department of Oral Medicine, Faculty of Dentistry, Al-Andalus University, Qadmous town 02400, Tartous Governorate, Syrian Arab Republic
Email: hsnkhal@gmail.com
ORCID: https://orcid.org/0000-0002-4100-7853
Explor Med. 2025;6:1001325 DOI: https://doi.org/10.37349/emed.2025.1001325
Received: April 04, 2025 Accepted: May 08, 2025 Published: May 26, 2025
Academic Editor: Gaetano Isola, University of Catania, Italy
Background: Lasers have demonstrated their potential as an effective alternative to the scalpel for gingivectomy procedures. Therefore, it is essential to evaluate their efficacy and safety. This article summarizes human studies comparing the effectiveness of laser-assisted gingivectomy with conventional surgical methods.
Methods: A comprehensive electronic search was conducted in Cochrane, PubMed/MEDLINE, ScienceDirect, and Google Scholar using the terms “Gingivectomy”, “Gingivoplasty”, “Crown lengthening”, “Gingival surgery”, and “LASER” to identify human studies that compared laser-assisted gingivectomy with traditional surgical methods up until December 2022. The inclusion criteria were as follows: English language, use of laser as the primary treatment tool, and study designs including randomized controlled trials, controlled clinical trials, clinical trials, and comparative studies.
Results: Twenty-two studies met the inclusion criteria and were analyzed. Diode lasers (810–940 nm) and Erbium, chromium-doped yttrium scandium gallium garnet laser (Er,Cr:YSGG) lasers caused less postoperative pain than conventional flap surgery, while the neodymium-doped yttrium aluminum garnet (Nd:YAG) laser resulted in higher initial pain. The diode 808 nm laser yielded mixed results—one study reported pain levels comparable to those of scalpels, while another noted reduced pain with laser use. However, one study indicated greater use of analgesics in laser-treated patients, suggesting increased discomfort. Lasers, particularly carbon dioxide (CO2) and diode 810 nm lasers, provided superior hemostasis compared to scalpels, with the Er,Cr:YSGG laser in flapless osteotomy minimizing bleeding. Additionally, no sutures were required in the laser-treated groups. The stability of the gingival margins after laser treatment was found to be similar to that of the scalpel.
Discussion: All lasers discussed in this article can be safely and effectively used for gingivectomy as an alternative to conventional surgical methods. Laser treatment demonstrated superior clinical outcomes in terms of pain, patient satisfaction, hemostasis, recovery period, and periodontal health.
Cosmetic dentistry is founded on the fundamental principles of facial and oral aesthetics, enabling clinicians to fulfill patients’ aesthetic expectations while achieving optimal clinical outcomes. These principles identify three essential components that determine smile beauty: teeth, gingiva, and lips [1]. The harmonious interplay of these elements creates an aesthetically pleasing smile that evokes positive emotional and psychological responses.
When this aesthetic harmony is lacking, gingival modifications to height, thickness, and contour may be required to achieve optimal results. A classic example is the gummy smile, characterized by exposure of ≥ 2 mm of gingival tissue above the fully visible maxillary anterior teeth. In such cases, gingivectomy—with or without accompanying osteotomy—represents the treatment of choice [2–4]. Gingivectomy is further indicated for multiple clinical scenarios, including: treatment of gingival enlargement; facilitating access to sound tooth structure margins; prosthetic indications such as crown lengthening; and various cosmetic enhancements including gingival reshaping, contour modification, and gingival zenith point adjustment [5, 6]. Additionally, gingivectomy may be indicated in orthodontics—for example, to improve oral hygiene, facilitate bracket placement, or expose superficially impacted teeth [7, 8].
The supracrestal tissue attachment (STA) represents the primary determinant of gingival margin position following gingivectomy procedures [9]. Other factors should be taken into account, including the width of attached gingiva and the position of the cementoenamel junction [10], and the gingival margin’s relationship to both the tooth’s long axis and incisal edge, as well as its aesthetic alignment with the lip line and adjacent teeth [11]. Clinicians should always consider both the potential for gingival margin changes following crown lengthening procedures and the required stabilization period for these margins, which is generally estimated to be at least six months—particularly when planning anterior esthetic restorations [12–14].
The primary modalities for performing gingivectomy include surgical scalpels, electrosurgery, and lasers [15]. Scalpel gingivectomy offers several advantages: ease of execution, precise incisions with well-defined margins, rapid wound healing, and absence of lateral tissue damage. However, this technique typically induces significant intraoperative bleeding, necessitating periodontal dressing placement—a requirement that patients may find uncomfortable [16].
Extensive clinical evidence demonstrates that the use of lasers for soft tissue procedures offers a valuable alternative to conventional scalpel surgery. In orthodontic applications, laser-assisted gingivectomy serves multiple purposes: enhancing oral hygiene and bracket placement, improving gingival aesthetics, and facilitating exposure of superficially impacted teeth. This approach may additionally reduce postoperative discomfort and potentially shorten overall treatment duration [8, 17].
Laser technology offers superior surgical control, reduced postoperative pain/analgesic requirements, minimal inflammation, excellent hemostatic properties, and suture-free wound healing [8]. However, its primary limitations include higher costs and longer procedural times compared to conventional methods. Multiple laser systems are clinically available, including carbon dioxide (CO2), diode, neodymium-doped yttrium aluminum garnet (Nd:YAG), and erbium-doped yttrium aluminum garnet (Er:YAG) lasers [18]. These lasers differ in terms of their absorption within the tissue, which is related to the wavelength, power, laser spot size, exposure time, and pulse repetition rate (frequency). In addition to other tissue-specific parameters including, absorption and scattering coefficients, tissue thickness, heat capacity, and thermal conductivity [19].
In the past, non-contact ablative lasers (including CO2 and erbium systems) have been used for oral soft tissue surgery. In orthodontic practice, diode lasers are now preferentially employed for incision/excision procedures. Compared to scalpel techniques, diode lasers maintain a clean surgical field with superior hemostasis, while offering the added benefit of photobiomodulation—enhancing wound healing and significantly reducing postoperative patient discomfort [17].
In dental practice, what is the effect of using a laser for gingivectomy on treatment efficacy and safety, compared to conventional surgical methods, both during and after the procedure?
This article was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PRISMA, guidelines [20] and has been registered at the International Prospective Register of Systematic Reviews (PROSPERO) under number CRD42024501110.
This article summarizes a wide range of human studies on effectiveness of using lasers for gingivectomy compared to surgical methods.
The research question framed was ‘‘What is the effect of using a laser for gingivectomy on treatment efficacy and safety compared with surgical methods during and after treatment?’’.
The PICO question was based on the following:
Participants: patients undergoing gingivectomy.
Intervention: laser.
Comparator: other surgical methods for gingivectomy.
Primary outcomes: pain, discomfort, and patient satisfaction.
Secondary outcomes: bleeding, use of sutures, duration of procedure, healing and periodontal health, and stability of biologic structures.
Our electronic search strategy employed Boolean operators (AND/OR) to systematically combine key terms, including (“Gingivectomy” OR “Gingivoplasty” OR “Crown Lengthening” OR “Gingival Surgery”) AND (“LASER” OR “Laser Therapy” OR “Laser Surgery”), across Cochrane, PubMed/MEDLINE (with MeSH terms), ScienceDirect, and Google Scholar from database inception through December 2022. The search targeted title, abstract, and keyword fields, with additional variations such as (“Periodontal surgery” AND “Diode laser”) and (“Dental lasers” AND “Clinical outcomes”). Manual screening of reference lists and citation tracking ensured comprehensive coverage.
Eligible studies were selected based on the following criteria:
Publications in English language.
Utilization of laser as the primary gingivectomy treatment modality.
Study designs including randomized controlled trials (RCTs), controlled clinical trials (CCTs), clinical trials (CTs), and comparative studies.
No restrictions were applied regarding sample size, laser type, or publication date. Database searches were conducted without filters.
Studies were excluded if they:
Were non-comparative (case reports, case series, etc.).
Involved animal subjects.
Were theoretical reviews or opinion articles.
Promoted commercial products or devices.
Lacked clearly defined measurement methodologies.
Failed to specify laser parameters.
All co-authors independently participated in the study selection process through a rigorous dual-phase screening protocol: initial evaluation of titles/abstracts followed by comprehensive full-text assessment. Each researcher autonomously applied the predefined inclusion criteria, with inter-reviewer discrepancies resolved through consensus discussion. Final inclusion determinations were principally based on full-text content analysis. A flowchart for the studies included in the research was created using the flowchart template of PRISMA generated by the website (https://www.prisma-statement.org/prisma-2020-flow-diagram) [21].
The data were extracted from the selected articles and arranged in tables; these data included the following: author names, sample size, age and gender of the patients, indications for gingivectomy, comparative treatment types, the laser used (along with its parameters), the follow-up period, and clinical outcomes (Tables 1 and 2).
Parameters used in included studies
| Laser type | Reference | Comparator | Type of laser | Mode | Fiber/Tip diameter | Power (Watt)/Frequency (Hz) | Pulse energy (mJ) | Pulse width |
|---|---|---|---|---|---|---|---|---|
| Other lasers | Altayeb et al. [26] (2022) | Open flap/flapless | Er,Cr:YSGG (2,780 nm) | Pulsed | 0.6 mm | 3/50 | 60 | 700 µm |
| Naidu and Gajendran [44] (2022) | Diode versa Er,Cr:YSGG | Diode (940 nm) | Gated pulsed | 400 µm | 1–1.5/Not-reported | Not-reported | Not-reported | |
| Er,Cr:YSGG (2,780 nm) | Pulsed | MZ5 | 3/18 | 382.1 | Not-reported | |||
| Tianmitrapap et al. [25] (2022) | Scalpel for soft tissue removal | Er,Cr:YSGG (2,780 nm) | Pulsed | 0.6 mm | 1.5/Not-reported | Not-reported | Not-reported | |
| Rotary instrumentation for hard tissue removal | Er,Cr:YSGG (2,780 nm) | Pulsed | 0.6 mm | 1.5/Not-reported | Not-reported | Not-reported | ||
| Thuaksuban and Nuntanaranont [42] (2003) | Scalpel | CO2 (10,600 nm) | Not-reported | Not-reported | Not-reported/Not-reported | Not-reported | Not-reported | |
| Jensen et al. [40] (2010) | Scalpel | Nd:YAG (1,064 nm) | Pulsed | Not-reported | 7/50 | Not-reported | 250 µs | |
| White et al. [31] (1991) | Scalpel | Nd:YAG (1,064 nm) | Pulsed (contact mode) | 320 µm | 1.25–3/15–20 | Not-reported | 150 µs | |
| Kazakova et al. [36] (2018) | 1-Scalpe2-Cercamic bur3-Electrocautery | Diode (810 nm) | CW (contact mode) | 300 µm | 1.5/N/A | N/A | N/A | |
| Er:YAG (2,940 nm) | Pulsed | Spot diameter 0.8 mm | 5.4/18 | 300 | 400 µs | |||
| CO2 (10,600 nm) | Pulsed (non-contact) | Spot diameter 2.5 mm | 9.69/200 | Not-reported | Not-reported | |||
| Taskan et al. [32] (2020) | 1-Electrosurgery2-Scalpel | Er:YAG (2,940 nm) | Pulsed | 1.3 mm | 2/10 | 200 | 1,000 µs | |
| Nd:YAG (1,064 nm) | Pulsed | 300 µm | 4/50 | Not-reported | 180 µs | |||
| Diode lasers | Lione et al. [41] (2020) | 1-Scalpel2-Nonsurgical periodontal treatment | Diode (810 nm) | CW (contact mode) | 300 µm | 1–1.5/N/A | N/A | N/A |
| Elmahal et al. [33] (2018) | Scalpel | Diode (808 nm) | Pulsed (contact mode) | 400 µm | 2/26 ms pulsed interval | Not-reported | Not-reported | |
| Koppolu et al. [34] (2017) | Scalpel | Diode (808 nm) | CW | 300 µm | 0.8–1/N/A | N/A | N/A | |
| Elif et al. [30] (2017) | Scalpel | Diode (940 nm) | CW | 400 µm | 0.9/N/A | N/A | N/A | |
| Farista et al. [28] (2016) | Scalpel | Diode (940 nm) | CW (contact mode) | 400 µm | 0.8–1.5/N/A | N/A | N/A | |
| Kumar et al. [39] (2015) | Electrocautery | Diode (980 nm) | Not reported | 200 µm | 5/Not-reported | Not-reported | Not-reported | |
| Bhat et al. [38] (2015) | Scalpel | Diode (940 nm) | Pulsed (contact mode) | Not-reported | 2/1 ms on, 1 ms off | Not-reported | 100 µs | |
| Kumar et al. [50] (2015) | Scalpel | Diode (980 nm) | CW | Not-reported | 1.8/N/A | N/A | N/A | |
| Sobouti et al. [29] (2014) | Scalpel | Diode (940 nm) | CW | 400 µm | 0.9/N/A | N/A | N/A | |
| Ize-Iyamu et al. [37] (2013) | Scalpel | Diode (810 nm) | Not-reported | Not-reported | Not-reported/Not-reported | Not-reported | Not-reported | |
| To et al. [43] (2013) | Nonsurgical periodontal treatment | Diode (940 nm) | Pulsed (contact mode) | 300 µm | 1/0.2 ms pulse interval | Not-reported | 50 µs | |
| Mavrogiannis et al. [35] (2006) | Scalpel | Diode (810 nm) | Not-reported | Not-reported | Not-reported/Not-reported | Not-reported | Not-reported | |
| Alwan et al. [27] (2021) | Scalpel | Diode (940 nm) | Pulsed | Not-reported | 1/Not-reported | Not-reported | Not-reported | |
| Musaa et al. [45] (2017) | Scalpel | Diode (940 nm) | Pulsed | 400 µm | Avg = 1.5/Not-reported | 60 | 20 ms |
nm: nanometer; mJ: millijoules; Hz: hertz; CW: continious wave; N/A: not applicable; CO2: carbon dioxide; Nd:YAG: neodymium-doped yttrium aluminum garnet; Er:YAG: erbium-doped yttrium aluminum garnet; Er,Cr:YSGG: chromium-doped yttrium scandium gallium garnet laser
Summary of included studies
| Reference | Type of study | Number of patients (F) | Adverse events/Complication | Follow-up | Results |
|---|---|---|---|---|---|
| Altayeb et al. [26] (2022) | Clinical trial | 36 | Not-reported | 9 Months | E |
| Naidu and Gajendran [44] (2022) | RCT | 40 | Not-reported | 14 Days | E |
| Tianmitrapap et al. [25] (2022) | Clinical trial | 25 (21) | None | 3 Months | E |
| Thuaksuban and Nuntanaranont [42] (2003) | Comparative study | 14 | None | 7 Days | E |
| Jensen et al. [40] (2010) | RCT | 8 (4) | Root sensitivity in the first 4 weeks | 6 Months | E |
| White et al. [31] (1991) | RCT | 29 | None | 1 Month | E |
| Kazakova et al. [36] (2018) | Histological study | 18 | None | - | E |
| Taskan et al. [32] (2020) | RCT | 37 (17) | None | 15 Days | E |
| Lione et al. [41] (2020) | RCT | 56 (25) | None | 6 Months | E |
| Elmahal et al. [33] (2018) | Clinical trial | 11 | None | 3 Weeks | E |
| Koppolu et al. [34] (2017) | RCT | 14 (8) | None | 4 Weeks | E |
| Elif et al. [30] (2017) | RCT | 20 (12) | None | - | E |
| Farista et al. [28] (2016) | RCT | 22 | None | 10 Days | E |
| Kumar et al. [39] (2015) | RCT | 17 (7) | Charring | 3 Months | E |
| Bhat et al. [38] (2015) | RCT | 20 | None | 4 Weeks | E |
| Kumar et al. [50] (2015) | RCT | 70 | None | 3 Weeks | E |
| Sobouti et al. [29] (2014) | RCT | 30 (18) | None | - | E |
| Ize-Iyamu et al. [37] (2013) | RCT | 23 (17) | None | - | E |
| To et al. [43] (2013) | RCT | 30 (15) | None | 6 Months | E |
| Mavrogiannis et al. [35] (2006) | RCT | 23 (2) | None | 6 Months | E |
| Alwan et al. [27] (2021) | Comparative study | 18 | Not-reported | 2 Weeks | E |
| Musaa et al. [45] (2017) | Comparative study | 30 | None | 2 Weeks | E |
F: female; RCT: randomised clinical trial; E: effective
The Cochrane risk of bias 2 (RoB 2) tool was employed to evaluate methodological quality across five critical domains: (1) randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) outcome measurement, and (5) selective reporting. Studies were classified as having low risk, some concerns, or high risk of bias [22]. A detailed risk-of-bias assessment for each included study was tabulated and visualized using the ROBVIS tool (https://www.riskofbias.info/welcome/robvis-visualization-tool), presenting domain-specific judgments and overall bias classifications [23].
The Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool was systematically applied to evaluate potential biases across seven critical domains: (1) confounding variables, (2) participant selection, (3) intervention classification, (4) deviations from intended interventions, (5) missing data, (6) outcome measurement, and (7) selective reporting. Each domain received a standardized risk assessment categorized as: low, moderate, serious, critical risk of bias, or no available information [24].
Following quality assessment, all extracted laser parameters were systematically categorized according to the strength of recommendation taxonomy (SORT) criteria to establish evidence-based treatment recommendations.
A flow diagram of the selection process is shown in Figure 1. The search strategy yielded 1,896 results in the four databases. During the pre-screening phase, 26 duplicate records were removed. Following deduplication, 1,870 records underwent title and abstract screening, resulting in the exclusion of 1,769 irrelevant studies. Of the remaining 101 potentially eligible studies, 7 were unavailable for retrieval.
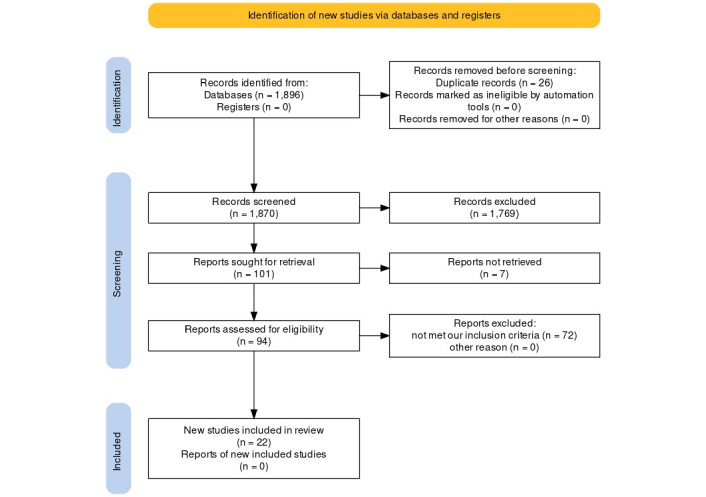
Flow chart of included study
Note. Adapted from “The PRISMA 2020 statement: an updated guideline for reporting systematic reviews” by Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. BMJ. 2021;372:n71 (https://doi.org/10.1136/bmj.n71). CC BY.
Ninety-four studies were screened by full-text reading, as a result, 22 articles met our inclusion criteria, in which gingivectomy was performed for several indications such as clinical crown lengthening (CCL), gingival enlargement, altered passive eruption, restorative and esthetic purposes.
Fifteen of the included studies were RCTs, and seven were non-randomized studies. The RCTs varied in design: eight followed a parallel-group design, four used a split-mouth design, two employed a stratified sampling design, and one utilized a split-mouth crossover design.
All included studies compared various laser types with conventional surgical methods (using either a traditional scalpel or an electrosurgical blade).
The most frequently utilized lasers were diode lasers, with the 808–810 nm wavelength employed in six studies and the 940 nm wavelength in seven studies. Nd:YAG and chromium-doped yttrium scandium gallium garnet laser (Er,Cr:YSGG) lasers were each reported in three studies, while Diode (980 nm), Er:YAG, and CO2 lasers were used in two studies each. The included studies encompassed a total of 597 patients, with ages ranging from 10 to 85 years and follow-up periods spanning 7 days to 9 months. Notably, only two studies evaluated CCL procedures involving osteotomy [25, 26]. Due to the apparent heterogeneity in the types of lasers used as well as in the irradiation parameters of the studies included in the review, it was not possible to perform a quantitative analysis.
Risk of bias in randomized studies was assessed using the RoB 2 tool developed by Cochrane. Among the 15 RCTs, the final overall judgment indicated:
4 studies had a low risk of bias.
7 studies had some concerns.
4 studies had a high risk of bias.
Specifically:
2 studies were rated as high risk in the randomization process.
2 studies were rated as high risk due to missing outcome data (Figure 2).
The risk of bias in non-randomized studies was assessed using the ROBINS-I tool, and the final overall judgment of the 7 included studies resulted in all studies being of low risk of bias except one study of moderate risk (Figure 3).
Comparative analyses revealed distinct pain profiles among laser modalities. Diode lasers (810–940 nm) [27–30] and Er,Cr:YSGG lasers (2,780 nm) [25] demonstrated superior postoperative pain control compared to conventional flap surgery. In contrast, Nd:YAG laser (1,064 nm) procedures were associated with significantly greater immediate postoperative pain (day 1) relative to scalpel techniques [31, 32].
The evidence for 808 nm diode lasers appeared equivocal, with one study showing comparable pain levels to scalpel [33], and another demonstrating analgesic benefits [34]. The consumption of analgesics, patient satisfaction, and discomfort levels generally followed the same trend as pain, with laser groups tending to show better outcomes in terms of patient satisfaction and reduced need for analgesics compared to traditional surgery. However, in Mavrogiannis’s study [35], it was reported that patients in the laser group required more analgesics, suggesting higher pain levels than those in the scalpel group.
The included studies consistently demonstrated lasers’ superior hemostatic performance compared to conventional scalpel techniques across all surgical phases. Among the evaluated laser systems, CO2 and 810 nm diode lasers exhibited particularly effective coagulation capabilities, producing thicker coagulation layers than Er:YAG lasers [36]. Notably, flapless Er,Cr:YSGG laser osteotomy procedures achieved exceptional hemostasis, outperforming both traditional flap surgery and Er,Cr:YSGG laser treatments with flap elevation [25, 26].
All types of lasers showed a clear superiority as they did not need any surgical sutures, unlike the surgery with the traditional flap [25, 26, 29, 37].
Local anesthesia was used before the initiation of laser therapy in two studies [27, 36]. However, other studies reported different percentages of patients’ need for anesthesia ranging from 30% to 90% of patients [29–31, 34, 37–40].
It seems that there was no significant difference in the time required to complete the surgical work depending on the method of intervention [31, 39], Nevertheless, the operation time was shorter in the laser group in two studies [29, 33], and longer in one study [41].
Tissues cut with Nd:YAG and CO2 lasers exhibited a recovery period similar to that of the electric scalpel, though generally longer than with Er:YAG lasers[32]. In contrast, the CO2 laser, 980-nm diode, 808-nm diode, and traditional scalpel showed comparable recovery times [33, 39, 42].
Er:YAG and CO2 lasers demonstrated superior cutting margin alignment with minimal tissue rupture compared to ceramic burs, electric scalpels, and 810-nm diode lasers [36].
For flapless/open-flap procedures, the Er,Cr:YSGG laser provided a minimally invasive alternative, yielding better outcomes in bleeding control, probing depth (PD), gingival inflammation, and papilla index versus traditional scalpel flaps [25, 26].
Regarding periodontal parameters [bleeding on probing (BOP), clinical attachment level (CAL), and PD], most studies found no significant difference between lasers and scalpels, with results comparable to non-surgical periodontal therapy for adenoid management [32, 41, 43]. However, the 940-nm diode laser showed statistically significant improvement in periodontal parameters over both Er,Cr:YSGG lasers and scalpels during the first two weeks post-treatment for gingival enlargements [44, 45] and CCL [27].
The 940 nm diode laser demonstrated comparable gingival margin stability to conventional scalpel techniques at one-month follow-up. However, while scalpel-treated sites showed progressive changes (including recession and rebound), laser-treated margins maintained complete stability throughout the observation period. Similarly, Er,Cr:YSGG laser techniques (both flapless and open-flap approaches) achieved equivalent stability outcomes to scalpel procedures at 3-month [25] and 9 months [26] postoperative evaluations. The most significant margin alterations consistently occurred during the initial three-month healing phase [41].
These parameters were arranged according to the SORT [46] depending on the design of the studies and the consistency of their results (Table 3).
Recommended laser parameters for gingivectomy
| Level of evidence | Laser | Parameters | |||
|---|---|---|---|---|---|
| Level 1 evidence | Diode 810 | CW | P = 0.8–1.5 Watt | ||
| Diode 940 | CW | P = 0.8–1 Watt | |||
| Pulsed | D = 0.25 | Pavg. = 1 Watt | |||
| D = 0.5 | Pavg. = 1.5–2 Watt | ||||
| Nd:YAG | F = 50 Hz | Pulse width = 180 μs | Pavg. = 4 Watt | ||
| Pulse width = 250 ms | Pavg. = 7 Watt | ||||
| F = 15 Hz or 20 Hz | Pulse width = 150 μs | Pavg. = 1.25–3 Watt | |||
| Er:YAG | F = 10 Hz | Pulse width = 1,000 μs | Pavg. = 2 Watt | ||
| Level 2 evidence | Diode 980 | CW | P = 1.8 Watt | ||
| CO2 | F = 200 Hz | Pavg. = 9.69 Watt | |||
| Er:YAG | F = 18 Hz | Pulse width = 410 μs | Pavg. = 5.4 Watt | ||
CW: continuous wave; D: duty cycle; F: frequency; Hz: hertz; P: power; Pavg.: average power; μs: microsecond; ms: millisecond; CO2: carbon dioxide; Nd:YAG: neodymium-doped yttrium aluminum garnet; Er:YAG: erbium-doped yttrium aluminum garnet
Numerous studies have shown that the use of lasers for soft tissue procedures provides an alternative adjunct to traditional scalpel surgery. Laser-assisted gingivectomy is utilized in orthodontics to enhance oral hygiene and bracket placement, improve gingival aesthetics, and expose superficially impacted teeth, potentially reducing post-operative pain and decreasing the duration of orthodontic treatment [8, 17].
This systematic review provides a comprehensive synthesis of current evidence on the efficacy and safety of laser-assisted gingival resection. The analysis encompassed 22 clinical studies, including 15 RCTs and 7 non-randomized clinical studies, offering a substantial evidence base for clinical evaluation.
The methodological assessment revealed significant heterogeneity in study quality. Among RCTs evaluated using the Cochrane RoB 2 tool (n = 15), approximately 27% (4/15) demonstrated low risk of bias, 47% (7/15) raised some concerns, while the remaining 27% (4/15) were classified as high risk. The critical appraisal identified specific methodological limitations: 13% (2/15) of studies exhibited deficiencies in randomization procedures, and an equivalent proportion (2/15) were compromised by incomplete outcome data. These findings reflect well-documented challenges in surgical trial methodology, where practical constraints often impede proper allocation concealment and follow-up adherence.
In contrast, non-randomized studies assessed via ROBINS-I demonstrated comparatively stronger methodological quality, with 86% (6/7) rated as low risk and only 14% (1/7) showing moderate risk of bias.
Pain is often the most significant concern for patients during and after treatment, as it results from the activation of the nervous system and induces a sensation of discomfort. The concept of discomfort expresses the psychological and emotional aspects of the patient; however, it is not definitively associated with pain [47].
The inherent challenges in achieving patient blinding in surgical studies necessitate meticulous environmental control by researchers to ensure objective pain assessment and minimize confounding variables. Current evidence from numerical rating scales (NRS) demonstrates generally reduced pain perception in laser-treated groups [25–29], except for Nd:YAG laser procedures which showed increased discomfort [30, 31]. Laser techniques consistently demonstrated superior patient satisfaction with reduced analgesic requirements compared to conventional surgery [25–31, 33, 34]. However, multiple confounding factors—including variations in anesthetic protocols (depth, type, and administration), the potential for pain transference in split-mouth designs, and the inherently subjective nature of pain perception—collectively preclude definitive conclusions regarding laser superiority in pain control, despite these observed favorable trends.
Studies have unanimously agreed on the superiority of almost all types of lasers in bleeding control compared to the scalpel during and after surgery. Intraoperative hemostasis provides a clear and dry surgical field that makes it easier to see, in addition to a greater ability to access the targeted tissue [48]. The persistent hemostatic effect of laser treatment was evident postoperatively, typically eliminating the need for additional bleeding control measures. However, histological analyses revealed significant variations among laser systems: Er:YAG and Er,Cr:YSGG lasers demonstrated minimal to absent hemostasis at tissue margins, in contrast to the pronounced hemostatic effects observed with CO2 and 810 nm diode lasers. This disparity was similarly reflected in coagulation layer thickness—while erbium-family lasers produced thin marginal coagulation zones, CO2 and diode lasers generated substantially thicker coagulated layers [36, 49]. The reduced thickness of both coagulation and thermal effect layers results from water-cooled systems that limit temperature elevation within tissues, consequently diminishing hemostatic efficacy [49]. Paradoxically, two additional studies reported minimal bleeding with flapless Er,Cr:YSGG osteotomy and gingival excision compared to both open-flap Er,Cr:YSGG laser techniques and traditional flap methods [25, 26].
Regarding the need for the use of surgical sutures, laser sites showed a clear superiority as they did not need any surgical sutures unlike the scalpel and the traditional flap [25, 26, 29, 37].
The surgical technique and the difference in the targeted chromophores may affect the short-term outcomes, and procedure-related morbidity and contribute to the advantages and limitations of each laser type [26]. In addition to other complications, such as causing allergic reactions, which lasted for four weeks [40].
Although the diode 808–810 and diode 940 lasers in general have approximate wavelengths to that of the Nd:YAG laser, they do not seem to cause the same complications according to the included studies, and for their high hemostasis capacity, however, their relatively deep tissue penetration, and they should be used at the lowest effective power setting to minimize side effects. This also applies to other lasers, as it seems unjustified to use the Nd:YAG laser with a power of 7 Watts [40]. When the same laser can cut with a power of 1.25–3 Watts as mentioned in White’s study [31]. Therefore, the practitioner must start with the lowest effective power for cutting and gradually increase it if it was proven to be ineffective, according to the thickness of the cut tissue and its statement (healthy/inflammatory/fibrous...). Using inappropriate parameters will also lead to undesirable side effects as increasing the exposure time, which will increase the risk of laser penetration into the tissue and thermal collateral damage.
The Er:YAG laser demonstrates exceptional precision in soft tissue excision, combining high water affinity with integrated cooling to achieve minimal penetration depth. This results in clean surgical margins with negligible thermal damage, surpassing conventional methods in tissue preservation [36]. Its unique dual capability for both soft tissue procedures and osteotomy presents a promising area requiring further high-quality clinical investigation Similarly, the Er,Cr:YSGG laser exhibits comparable clinical performance, with parameter-adjusted efficacy for both soft and hard tissue applications. This versatility stems from its ability to exploit the differential absorption characteristics of various tissue types [25].
The included studies demonstrate equivalent gingival margin stability between Er,Cr:YSGG laser techniques (flapless/open-flap) and conventional scalpel procedures at both 3-month [25] and 9 months [26] postoperative evaluations. Since the most substantial margin alterations occur during the initial three-month healing phase [41], clinicians must observe a mandatory ≥ 3-month healing period to ensure complete tissue reorganization before permanent restoration placement [26]. Furthermore, periodontal phenotype constitutes a critical determinant of healing outcomes, with thick biotypes exhibiting superior gingival margin stability and more predictable rebound characteristics compared to thin phenotypes [26].
In addition, achieving stable and predictable crown lengthening outcomes requires greater bone reduction with a more coronal flap positioning [48]. However, the characteristically limited hemostatic capacity of erbium-family lasers remains a critical consideration when selecting appropriate laser systems for these procedures.
The CO2 laser demonstrates dual advantages of precise tissue incision with minimal marginal rupture and superior hemostatic/coagulation capacity [36]. However, its lack of integrated cooling systems elevates thermal injury risks, potentially explaining the comparable recovery periods observed across diode 808/980 nm, Nd:YAG, and conventional scalpel techniques [33, 39, 42]. Current evidence remains limited by insufficient high-quality studies establishing reproducible laser parameters.
Given both the procedural versatility of multiple laser systems and the notable research gaps—particularly for CO2, Er:YAG, and Er,Cr:YSGG modalities—no single laser platform currently emerges as definitively superior for gingival resection procedures.
This systematic review demonstrates that all evaluated laser systems can serve as safe and effective alternatives to scalpel gingivectomy, though with varying therapeutic advantages. The Nd:YAG laser proves least favorable due to its propensity for thermal collateral damage from excessive tissue penetration. In contrast, Er:YAG, Er,Cr:YSGG, and diode 808–810–940 nm lasers emerge as preferable options when used within recommended parameters.
Beyond three established benefits—superior hemostasis during/after surgery, eliminated suture requirements, and reduced need for infiltration anesthesia—current evidence fails to demonstrate additional laser superiority over conventional scalpel techniques. However, laser intervention becomes the definitive treatment choice for patients with coagulopathies or contraindications to local anesthesia.
This systematic review is subject to several important limitations. The exclusive inclusion of English-language publications may have introduced potential language bias, while the inability to perform direct comparisons between laser types—each exhibiting distinct tissue interactions—was compounded by the absence of standardized irradiation protocols. Furthermore, the limited number of available studies precludes definitive conclusions regarding the relative superiority of specific laser systems.
To establish evidence-based clinical guidelines, future research should prioritize multicenter RCTs evaluating laser-assisted gingivectomy (with and without osteotomy) using CO2, Er:YAG, and Er,Cr:YSGG lasers. Such studies must incorporate adequate sample sizes and rigorously standardized protocols to ensure reproducible and clinically meaningful outcomes.
CCL: clinical crown lengthening
CO2: carbon dioxide
Er,Cr:YSGG: chromium-doped yttrium scandium gallium garnet laser
Er:YAG: erbium-doped yttrium aluminum garnet
Nd:YAG: neodymium-doped yttrium aluminum garnet
RCTs: randomized controlled trials
RoB 2: Cochrane risk of bias 2
ROBINS-I: Risk Of Bias In Non-randomized Studies of Interventions
OH, SA, RG, RM, WA, and MK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review & editing.
The authors declare that they have no competing interests.
Not applicable.
Not applicable.
Not applicable.
The dataset used during the study is available from the corresponding author upon request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 4752
Download: 65
Times Cited: 0
