Affiliation:
1Centre for Medical Biotechnology, Amity Institute of Biotechnology, Amity University, Noida 201303, Uttar Pradesh, India
Email: bbondhopadhyay@amity.edu; bbanerjee218@gmail.com
ORCID: https://orcid.org/0000-0002-6679-7791
Affiliation:
2Molecular Diagnostics & Molecular Oncology, Molecular Biology Group, Division of Molecular Biology, ICMR-National Institute of Cancer Prevention and Research (NICPR), Noida 201301, Uttar Pradesh, India
ORCID: https://orcid.org/0000-0003-1456-9217
Affiliation:
2Molecular Diagnostics & Molecular Oncology, Molecular Biology Group, Division of Molecular Biology, ICMR-National Institute of Cancer Prevention and Research (NICPR), Noida 201301, Uttar Pradesh, India
Explor Med. 2023;4:1094–1108 DOI: https://doi.org/10.37349/emed.2023.00197
Received: May 29, 2023 Accepted: September 28, 2023 Published: December 29, 2023
Academic Editor: Andrea Nicolini, University of Pisa, Italy
The article belongs to the special issue Breast Cancer: Basic and Clinical Advances
Cancer cure with immunotherapy is an innovative step towards cancer treatment with better survivability, but it is mostly dependent on the response of the patient’s immune system to the immunotherapeutic approach. This descriptive review article emphasizes the conventional and advanced treatment modalities currently available for breast cancer management. This review also highlights the clinical management of breast cancer concerning immune response especially to unravel the prospects for manipulation of immune cells: such as lymphocytes, including T-cells, T-regulatory cells and natural killer cells, and others like macrophages, dendritic cells, and the panel of interleukins or interferons released by them which has made a significant impact on breast cancer research. In addition, an effort was made to emphasize the different clinical trials and their future implication for the reduction of breast cancer cases. Overall, an attempt has been made to shed light on the possibilities of immunotherapeutics in breast cancer care, as well as the role of immune response in the incidence, aggressiveness, and survival of breast cancer.
Breast cancer is the highest occurring cancer in women globally [1]. Recently, India has also shown a rapid increase in breast cancer cases [2]. However, if we look back at the past few decades, the 5-year survival rate has gradually increased from 63% to 90% significantly. Taking into consideration the stages of breast cancer, in the case of localized diseases the survival rate is 99% and for regional spreading, when the disease is spreading to neighboring lymph nodes the rate decreases to 84%. But, in the case of patients with highly metastatic and aggressive tumorous conditions, the 5-year survival rate drastically drops to 24% [3]. This suggests that early detection and diagnosis is essential to increase the survival rate. Moreover, better treatment options and personalized care can also help to reduce mortality. It is imperative to know that breast cancer has a hereditary link, especially in light of the BReast CAncer gene 1 (BRCA1)/BRCA2 mutation burden. Therefore, regular screening and genetic testing should be part of every woman’s health routine. Early detection is key to reducing the mortality rate of breast cancer. BRCA1 and BRCA2 are the most commonly responsible genes for hereditary breast cancer. There is an 80% risk of being diagnosed with breast cancer in women having an abnormal BRCA1 or BRCA2 gene (or both) during their lifetime. In young women, the occurrence of breast cancer is mostly related to abnormal BRCA1 or BRCA2 genes [4]. There is evidence that BRCA+ (BRCA mutated) breast cancer cases have a higher tumor mutational load than BRCA– (wild type) breast cancer cases. Tumor mutational load can either positively or negatively impact important immune cells or checkpoints that are necessary for a tumor microenvironment. This high mutational load can lead to an inflamed tumor microenvironment, which can then lead to an impaired immune response, allowing tumor cells to evade detection and destruction by the immune system [5].
Breast cancer has been considered a poorly immunogenic cancer as compared to cancers like non-small cell lung carcinoma and malignant melanoma, which are highly immunogenic. This is due to a lack of expression of antigens on the surface of breast cancer cells, which is necessary for recognition by the immune system. Consequently, more effective immunotherapy strategies need to be developed to increase the immunogenicity of breast cancer. Although earlier studies on tumor-infiltrating lymphocyte (TIL) and cancer genome sequences have suggested that estrogen receptor (ER) negative, human epidermal growth factor receptor 2 (HER2) positive, and triple-negative breast cancer (TNBC) subtypes are more immunogenic than ER-positive-HER2-negative subtypes [6]. Especially for TNBCs, TILs can serve as a biomarker for prognosis and good immunotherapy responses. As compared to luminal breast cancer, TNBC, and HER2-enriched breast cancer have a higher tumor mutation load and more T-cell penetration. The tumor mutation load may not reflect immunogenicity in luminal subtypes since it is inversely related to their prognosis [7]. Various factors other than BRCA1 and BRCA2 like genetic, epigenetic, and external stress make a person more susceptible to breast cancer. Among these different factors, an important role is played by the immune system in the occurrence and maintenance of cancer (Figure 1). The immune system can respond to changes in the tumor environment and can be activated by the presence of cancer antigens. Therefore, the immune system can be used to develop a personalized approach to cancer treatment. Over time, a great deal of information has been added on immunotherapy against breast cancer but still, the success rate is very low. This review deals with those important factors because of which such a wide differential immune response is observed in patients with the same disease status and treatment module, which is very intriguing to look upon in the future for higher success rates in immunotherapeutics and breast cancer.
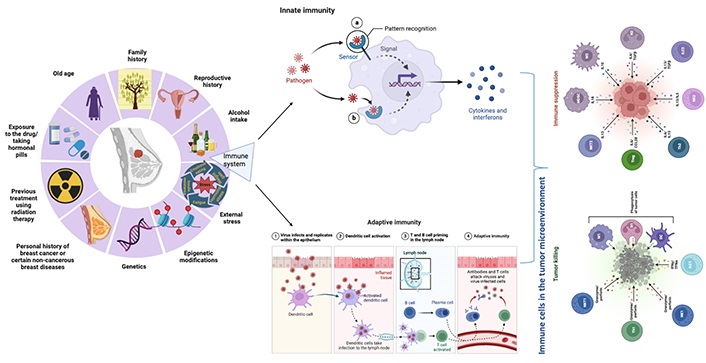
The factors contributing to breast cancer risk including genetics, epigenetics, external stress, and the immune system. Created with BioRender.com. NKT: natural killer T cell; Th: T helper cell; NK: natural killer cell; ILC: innate lymphoid cell; M1: type 1 macrophage; N1: type 1 neutrophil; DC: dendritic cell; Treg: T-regulatory cells; IL: interleukin; CCL: C-C motif chemokine ligand; MDSC: myeloid-derived suppressor cell; TGF: transforming growth factor; IFN: interferon; TFN: film nanocomposite; in innate immunity section dashed line arrow: internalization of products/signals in the nucleus of the cell; solid line arrow: pathogen recognition by pattern recognition receptors (PRRs)
Normal breast development and breast cancer are influenced by adaptive and innate immune systems. Although breast cancer is associated with fewer infiltrating immune cells, primarily T lymphocytes, than other types of cancer, infiltration is still more common [8]. Innate immune cells play a significant role in suppressing the immune response during breast cancer progression [9]. To design and develop immunotherapies for breast cancer, the innate and adaptive immune systems must be considered [10]. The immune system mainly includes lymphocytes (T- and B-cells), NKs, macrophages, dendritic cells, and a huge panel of substances called interleukins and interferons. In both early stages and metastatic conditions, T-cells and NKs play an important role in inhibiting cancer [11].
The main types of lymphocytes (T- and B-cells) have a surface expression on receptors, T-cell receptor (TCR) and B-cell receptor (BCR) respectively for the detection of antigens. These lymphocytes are activated to initiate immunity in response to the specific binding of their receptors to antigens such as tumors and viruses. Naive T-cells prime activate a normal cascade of specialized antigen-presenting cells (dendritic cells). This activation leads to the expansion and differentiation of T-cells into effector and memory T-cells. Effector T-cells are responsible for killing the infected cells, while memory T-cells are responsible for long-term protection against future infections. T-cells prepared for use in the tumor microenvironment are divided into antigen-specific T-cells and clonally expanded T-cells. In various cancers like breast, melanoma, ovarian, gastrointestinal, and other solid tumors, histology shows spontaneous T-cell infiltration that might act as a positive prognostic factor [12]. T-cells play an important role in cancer immunotherapy and can be used for targeted therapy. Vaccines and adoptive cell transfer can also be used to boost anti-tumor immunity. A significant decrease in CD4+ (helper T-cells) and CD8+ (cytotoxic T-cells) T lymphocytes in breast cancer patients, compared to healthy controls, was observed [13]. In patients with larger breast cancer tumors, the cytokine response was significantly lower. In the tumor microenvironment, T-cells expressing γδ-TCRs are also immunomodulatory. Even γδ-T-cells are found to have an anti-tumor effect [14] hence, Vγ9Vδ2 TCR-transduced T-cells synthesized by adoptive transfer strategies are used to directly recognize tumor cells [15]. The usage of bisphosphonate zoledronic acid increases γδ-T-cell function in cancer patients [16]. However, γδ-TCR-expressing T-cells isolated from breast cancer patients were not found to promote T-cell activation in vitro [17]. This suggests that the therapeutic effect of zoledronic acid is mediated by indirect mechanisms, such as activating other immune cells. Additionally, zoledronic acid may stimulate γδ-T-cells in vivo but not in vitro. Therefore, further investigation is needed to understand the full potential of zoledronic acid. TILs represent pre-existing immunity, and lymphocyte-predominant breast cancer (LPBC) which has more than 50–60% lymphocyte infiltration in the stroma. LPBCs may respond better to zoledronic acid than non-LPBCs. This suggests that zoledronic acid may act synergistically with pre-existing immunity to achieve better therapeutic effects. The incidence of LPBC is 20% for TNBC, 16% for the HER2 subtype, and 6% for the ER-positive luminal subtype [18]. TIL was reported by Aaltomaa et al. [18] to be an independent prognostic factor for high-grade highly proliferative breast cancer, and accumulating evidence suggests that high levels of TIL are associated with a better prognosis and a better response to chemotherapy in TNBC. Therefore, zoledronic acid may act as a potential adjuvant therapy to improve the efficacy of existing treatments [19]. Additionally, TIL levels may be a good predictor of response to zoledronic acid adjuvant therapy.
There are various reports that Tregs may be encouraged by tumors and can try to prevent the immune response against tumor antigens in cancer. Tregs are more abundant in peripheral blood in human breast adenocarcinomas, which indicates that they are essential for tumor microenvironment development [20]. A recent meta-analysis reported that high infiltration of Treg was associated with a poor prognosis in breast cancer [21]. However, the clinical impact of Treg infiltration varied across the subtypes of breast cancer. The association with a favourable prognosis was observed in ER-negative and HER2-positive breast cancer but not in ER-positive luminal breast cancer [22]. There might be a chance that in tumor Tregs, there is a mechanism for switching off the normal immune response against tumors. Any natural substance or endogenous molecule, that can inhibit this property of Tregs, then it could synergize with physiologic immunity to kill tumor cells. These types of approaches might help increase the immune response against breast cancer.
Previously, breast cancer received minimal attention for immunotherapeutic techniques since it was thought not to be an immunogenic tumor type. The low immunogenic profile of breast cancer was explained by its low mutational load when compared to other kinds of cancer with significant mutational burden, such as non-small cell lung cancer and melanoma, where immune-based treatments were largely successful [23]. However, there have been instances of clinical inefficacy following programmed cell death protein 1 (PD1) inhibition in individuals with a significant number of non-synonymous mutations [24], or even long-term therapeutic effectiveness in individuals with modest mutational load in response to anti-PD-1 medication [24], with no objective responses in immunotherapy patients whose tumors were barred from T-cell infiltration due to differential activation of the tumor cell-intrinsic signaling pathway, which has recently reframed this hypothesis [25]. Furthermore, recent publications have provided persuasive evidence that TILs and tumor infiltrating immune cells (TIICs) selectively infiltrate tumors of breast cancer patients with certain molecular subtypes, and there are multiple articles detailing the context of the immune complex in breast tumor tissue [26]. This immunological setting suggests an endogenous intratumoral anticancer response rather than random recruitment of immune cells from the circulation, suggesting that such a response may emerge ectopically in the absence of secondary lymphoid organs. Another sort of ectopic antitumor immunity is thought to be generated within tertiary lymphoid structures (TLSs), which are seen within or near tumors and represent a type of lymphoid neogenesis [27]. TLS acts as an in-situ activation site for immune lymphocytes, allowing them to provide antitumor properties and, as a result, contribute to the development of protective immunity against tumors; however, they may be associated with a poor prognosis if they harbor Tregs or express inflammation-related genes [19]. As a result, their predictive value varies depending on the circumstances. Consequently, combining the terms “immunoscore” [28], TLS can provide the most robust and comprehensive cancer prediction, which is characterized by the kind, location, and density of intra-tumoral immune cells [25].
Individual tumors are generally classified into three immune landscapes: immunologically active, immunologically quiet, and immunologically excluded [29] while a single tumor might have a range of these environments. The immune system is assumed to have two roles in cancer development [30]. To begin with, when an appropriate immune response is produced, the immune system can remove neoplastic cells that arise from early tumor-initiating processes (immunoediting). In contrast, the immune system can activate signaling of wound repair pathways, which can aid in the creation of an environment favorable to carcinogenesis. The interaction between cancer cells and their microenvironment, also known as the immunological landscape, is critical in tumor formation and progression. Aside from growing tumor cells, the tumor microenvironment includes extracellular matrix (ECM) cells, stromal cells (e.g., fibroblasts, mesenchymal cells, endothelial cells, pericytes, adipocytes), and innate and adaptive immune system cells. Tumor microenvironment includes lymphoid lineage cells such as T-cells, NKs, and B-cells, as well as myeloid lineage cells such as dendritic cells, neutrophils, and macrophages. Tumor microenvironment activity is also represented by several immunomodulatory substances such as cytokines, chemokines, and growth factors, which are examples of important signals released by tumor, stromal, and immune cells which are responsible for cell-to-cell communication [31]. While it is widely recognized that the adaptive immune system can play a substantial role in the elimination of existing tumors, the involvement of the adaptive immune system in the clearance of cancer cells during early starting events has remained a mystery. Despite the fact, that immunosuppression has been related to an increased risk of cancer in humans [32], it has remained difficult to quantify early immunoediting events and to ascribe the clearance of precancerous lesions in immunocompetent persons for clearance of tumor-derived neoantigens, as opposed to alternative processes such as the removal of cells containing cancer-inducing viruses. A recent study employed immunohistochemistry to closely examine the topographical presentation of immune infiltration in 965 histological tissue slides from 177 people with varied cancer histology [33]. Based on the number of cells per mm2, tissue samples were categorized into three geographic categories: outer invasive margin (0–500 μm outside the tumor invasion front), inner invasive margin (0–500 μm within the tumor invasion front), and tumor core (> 500 μm within the invasion front). A preliminary investigation found a significant link between tumor core infiltration and internal invasive margin. As a result, these two categories were combined to define immune-active or “hot” cancers. Tumors with a high immune cell density in the invasive edge but a low density in the nucleus were labeled as “excluded” immune tumors. The term “cold tumors” refers to tumors that have a low density in all compartments. Cutoff values were used for many immune cell types, including CD3+ (363 cells/mm2), CD8+ (295 cells/mm2), forkhead box protein p3-positive (FoxP3+, 62 cells/mm2), PD1+ (6 cells/mm2), CD68+ (310 cells/mm2), and CD163+ (559 cells/mm2). Immune infiltration patterns in distinct tumor types were remarkably different [34].
The immune system of women with breast cancer and undergoing breast cancer surgery is usually compromised. In addition, tumor cells contain antigens that are related to the blood such as Lewis antigens like glycoproteins or glycolipids [35]. Disobedience of cancer cells might be caused by altered structural formation of carbohydrates with lipids or proteins [36]. Several studies have reported that Lewis antigens associated with blood grouping act as tumor-associated molecules [37]. A study found that increased expression of Lewisy/b antigens is related to reduced survival in lymph node-negative breast carcinomas [37]. Tumor-associated carbohydrate antigens (TACAs) are associated with metastasis and cancer progression depending on their expression by tumor cells; increased levels of TACAs increase the risk of cancer and vice versa [36]. TACAs have been identified by the National Institutes of Health as key indicators of cancer prognosis [38]. TACAs alone are inadequately immunogenic in many cases, failing to elicit a T-cell-reliant immune response, which has been identified as crucial for cancer treatment [39]. TACAs were first conjugated with T-cell activating protein carriers such as keyhole limpet hemocyanin (KLH), tetanus toxoid (TT), bovine serum albumin (BSA), and diphtheria toxin (CRM197) [40]. The responses of the monovalent vaccinations were first encouraging, but subsequent research revealed that those protein carriers themselves serve as self-immunogenic and reduce antigen-specific immunogenicity [41]. TACAs were then linked with polysaccharides [zwitterionic polysaccharide A1 (PS A1)] [42], Toll-like receptor 2 (TLR2) ligand, Pam3CysSerLys4 [43], and T-cell peptide epitopes [44], to create partially to totally synthetic, self-adjuvating, multi-component cancer vaccines, among other things. Some of the vaccines have progressed to various stages of clinical trials; for example, a hexavalent vaccine construct incorporating ganglioside GM2, globohexaosylceramide (Globo H), Lewisy, clustered Thomsen nouveau (Tn), clustered Thomsen-Friedenreich (TF), and glycosylated mucin 1 (MUC1) antigens has been used to treat phase II prostate cancer patients [45]. It has been suggested that the conformational change of covalent glycan chains may trigger cancer malignancy in glycoproteins and glycolipids [44]. In breast cancer, the cells of the mammary gland displayed huge antigenic differences in recognizing glycoproteins associated with glycan chain modifications [45]. A high proportion of circulating immune complexes (CICs) are deranged in the sera of cancer patients [46]. A patient’s CIC levels may differ regardless of whether their cancer is the same as another’s; the CIC, MUC1, has been linked to breast cancer [47]. Breast cancer CICs are thought to play a dual role, either defensive [47] or aggressive [48] depending on the microenvironmental circumstances. This segment of the review emphasizes that two patients of breast cancer having the same treatment module can react differently because of the differential tumor immune response exerted by the same treatment module. Nevertheless, breast cancer immunotherapeutic is not able to achieve success.
A person diagnosed with breast cancer and further surgical treatments undergoes tremendous stress, which might affect their cellular immune response like NK toxicity, and T-cell responsiveness which are important players in cancer prognosis [49]. Obesity depresses the response of T-cells and the movement of macrophages. This could result in a weakened immune system, making it more difficult for the body to fight cancer. Additionally, obesity can also affect the effectiveness of cancer treatments, making them less effective. Sometimes, obesity causes a chronic inflammatory response that relates to both the immune system and adipose tissue. It is known that obesity damage’s immune function and alters leukocyte counts as well as cell-mediated immune responses [50]. Obesity affects mainly women in the phase of life when they gain weight and become obese. Therefore, obesity can have a significant impact on cancer treatment outcomes and morbidity. Early diagnosis and treatment of obesity are essential to improve cancer treatment outcomes. Epidemiologists reported that weight gain from 18 years to 50 years of age has been systematically linked with the threat of breast cancer after menopause. Similarly, premenopausal women are found to be threatened with breast cancer due to overweight and obesity. The increased levels of estrogen in obese women augment the chances of occurrence of breast cancer in postmenopausal women. During the postmenopausal stage, the ovaries stop producing hormones and fatty tissues become the most important source of estrogen. Estrogen levels are higher in obese women which causes the rapid growth of estrogen-sensitive breast tumors. The connection between obesity and the risk of breast cancer can also vary according to race and ethnicity [51]. The risk of developing breast cancer also increases with age, and the risk of developing the disease is higher in women who have a family history of breast cancer. Regular check-ups and mammograms are important for early detection and treatment. In addition to these, intake of heavy metals can also be a plausible factor for immune modulation aiding in the development of breast cancer. For example, mercury from dental amalgam can cause a reduction in the number of T-cells and decrease the function of the immune system [52]. Lead and cadmium can also have a similar effect, leading to an increased risk of cancer. Arsenic is another heavy metal that has been linked to an increased risk of breast cancer, likely because of its ability to damage DNA. Even the release of cadmium and lead from industrial pollution and cigarette smoke decelerates the production of antibodies from T- and B-cells. In this way, the activity of macrophages decreases with the increase in susceptibility to infection [53]. Furthermore, heavy metals can also interfere with hormones, leading to an increased risk of certain cancers, as well as reproductive and developmental disorders.
According to Daniel Stover, MD, Medical Oncologist and Computational Biologist at the Ohio State University Comprehensive Cancer Center, a breast cancer patient’s immune system plays a significant part in breast cancer management and therapy. He made a note of it. “However, we have a poor understanding of what patient factors may influence immune cells and breast cancer.” Stover presented findings from his research (abstract PD9-11: association of body mass index and inflammatory dietary pattern with breast cancer pathologic and genomic immunophenotype in the Nurses’ Health Study) at the recent 2021 San Antonio Breast Cancer Symposium in December [54]. The Nurses’ Health Study and the Nurses’ Health Study II are two of the biggest prospective studies of women’s risk factors for major chronic illnesses. Since the inception of the Nurses’ Health Study in 1976, many investigators have contributed to the continuing study effort. The study discovered that individuals with the biggest rise in body mass index (BMI) from the age of 18 to the time of breast cancer diagnosis had greater RNA evidence of these immune cell types—CD4+ and CD163+ immune cells [54]. The immune system is involved in both the prevention and advancement of breast cancer. This suggests that maintaining a healthy weight can help reduce the risk of breast cancer. Additionally, maintaining a healthy immune system can help reduce the risk of breast cancer. Cytotoxic T lymphocytes (CTLs) and NKs boost antitumor immunity by targeting breast cancer cells. Tregs, macrophages, myeloid-derived suppressor cells, and T helper cells promote the growth of breast cancer in a variety of ways, including blocking the activity of cytotoxic T-cells, secreting proinflammatory cytokines, and encouraging metastasis. B-cells may be antitumorigenic by producing tumor-neutralizing antibodies and pro-tumorigenic by inhibiting antitumor immunity. Inflammation can cause substantial harm to breast tissue and hasten the evolution of breast cancer by increasing proinflammatory cytokines including interleukins and tumor necrosis factors. Immunotherapy and immunological engineering are still developing topics, with new findings being discovered regularly. These sectors are attempting to manipulate the immune microenvironment or immune cells to either attack the cancer cells directly or release chemotherapeutic medications to eliminate the breast tumor. There has also been research in the field of immune engineering to improve the diagnosis of breast cancer and provide a more accurate prognosis. Many of these studies have a long way to go before they become viable therapeutic options, but these studies show promise in terms of being able to design the immune system to attack the breast tumor and increase antitumor immunity [55].
Chronic lack of sleep or insomnia can lead to a deficiency of the immune system. Therefore, taking deep sleep a day is necessary for the proper functioning of the immune system and even our body [56]. A lack of sleep can also lead to an increased risk of developing certain diseases. Furthermore, it can worsen existing medical conditions, such as diabetes and high blood pressure. The second is to exercise; judicious exercise can help in the functioning of the immune system. Extreme and extended intense exercise can briefly decrease immune function. The third is to reduce stress because any type of emotional, physical, or psychological stress can affect the immune system and release hormones related to stress, such as cortisol, which causes immune ailments. The fourth one is to use relaxation techniques such as breathing exercises, meditation, yoga, or other stress management techniques. The fifth is to eat foods rich in nutrients, especially fruits and vegetables. The sixth is to avoid the use of carcinogenic chemicals that can alter hormones and damage DNA. The seventh is to avoid excessive intake of sugar. Three ounces of sugar in a single session significantly inhibits lymphocytes from eliminating exogenous pathogens. The eighth is the consumption of healthy probiotic bacteria in yogurt or probiotic beverages. The good intestinal bacteria help improve immune function. The ninth is taking a large amount of tea since tea is a rich source of antioxidants and helps stimulate the immune system. The element is available in black, green, oolong, and pekoe teas which increases the ability of immune cells to attack exogenous pathogens. A person should try to avoid caffeine at the end of the day to get rid of interference with sleep. Therapies such as bodywork, energetic work, acupuncture, and massage reduce cortisol levels for better rest [57].
Previous studies have classified breast cancer into four subtypes: luminal A [ER+/progesterone receptor-positive (PR+)/HER2–, grade 1 or grade 2], luminal B (ER+/PR+/HER2+, or ER+/PR+/HER2–, grade 3), HER2 overexpression (ER–/PR–/HER2+), and TNBC (ER–/PR–/HER2–). Luminal A subtype has a good prognosis and is sensitive to endocrine therapy, so general treatment may be endocrine therapy alone. The luminal B subtype is associated with a high rate of tumor proliferation, of which HER2 negative luminal B subtype is usually treated with endocrine therapy + chemotherapy, while the HER2 positive luminal B subtype is usually treated with chemotherapy + anti-HER2 treatment + endocrine therapy. HER2 overexpression subtype features a worse prognosis and rapid progression. The main recommended treatment is chemotherapy + anti-HER2 treatment. The negative expression of ER, PR, and HER2 in TNBC has unique biological properties. This subtype has the worst prognosis and in the wild type, platinum-derived drugs are recommended; instead those who are BRCA1/BRCA2 carriers or programmed cell death ligand 1 (PDL1) + poly(ADP-ribose) polymerase (PARP) or PDL1 inhibitors respectively combined with chemotherapy are commonly administered. TNBC is very aggressive and more likely to recur, so it is important to monitor the patient closely and adjust treatment if necessary. Immunotherapy may be a promising option for TNBC patients, and more research is needed to determine the best treatments. Various treatment modules are available for breast cancer cure based on their classification into different stages and/or subtypes concerning their clinical response (Figure 2). In recent years, the internal mechanisms of the host immune system to eradicate cancer cells have achieved impressive success, and advances in immunotherapy have developed potential new therapeutic strategies mainly for HER2+ and TNBC subtypes. Immunotherapeutic strategies include the acceptance of cancer vaccines, oncolytic viruses, ex-vivo activated T-cells and NKs, and the administration of antibodies or recyclable proteins that stimulate cells or inhibit so-called resistance. Immunotherapy is a powerful tool that has the potential to revolutionize cancer treatment. Research is still ongoing to find more effective immunotherapeutic strategies and improve patient outcomes. These strategies are effective in treating different types of cancer. Furthermore, they are generally well tolerated and have fewer side effects than traditional chemotherapy. Recent successes in CTL-associated antigen 4 (CTLA-4) and PD1 blocking have led to improvements in this treatment method (Table 1). These strategies have shown promising results in reducing tumor growth and prolonging survival in cancer patients. Furthermore, immunotherapy can be combined with other treatments such as chemotherapy and radiation therapy to further improve patient outcomes.
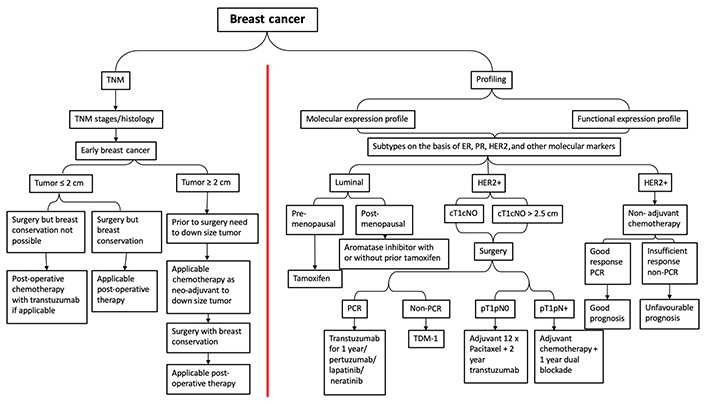
The various treatment strategies in breast cancer according to TNM and subtype. TNM: tumor-node-metastasis; PCR: polymerase chain reaction; TDM: therapeutic drug monitoring; cT1cN0: clinical stage is tumor size 1 with no lymph node involvement; pT1pN0: pathological stage is tumor size 1 with no lymph node involvement; pT1pN+: pathological stage is tumor size 1 with lymph node involvement; adjuvant 12 x paclitaxel + 2-year trastuzumab: 12 weeks or 12 cycles of paclitaxel followed by 2 year trastuzumab treatment (systematic adjuvant therapy)
Immunotherapeutic agents and their immune response in different subtypes of breast cancer
| Sl. No. | Immunotherapy | Immunotherapy agent | Breast cancer subtype | Immune response | References |
|---|---|---|---|---|---|
| 1 | CTLA-4 blockade in breast cancer | Tremelimumab | ER+/HER2– breast cancer | Significant increase in the ratio of ICOS+/FoxP3+ and CD4+ T-cells was observed | [58] |
| 2 | CTLA-4 blockade in breast cancer | Ipilimumab | Early breast cancer prior to mastectomy; any HR, HER2, and nodal status were permitted | Immunotherapy with cryoablation induced circulating T helper type 1 cytokines, ICOS+ and Ki67+ CD4+ and CD8+ T-cells, and an increased CD8+ T-cell/FoxP3+ Treg ratio within the tumor | [59] |
| 3 | PD1/PDL1 blockade in breast cancer | Avelumab | TNBC | Blocking PDL1, T-cells function better and the immune response is stimulated to find and kill cancer cells | [60] |
| 4 | PD1/PDL1 blockade in breast cancer | Atezolizumab | TNBC | Blocking PDL-1, T-cells function better and the immune response is stimulated to find and kill cancer cells | [61] |
| 5 | PD1/PDL1 blockade in breast cancer | Pembrolizumab | Metastatic PDL1+ TNBC | Human monoclonal antibodies that inhibit the interaction between PD1 and PDL1. This prevents the downregulation of T-cells and tumor cell evasion of normal immune surveillance | [62] |
| 6 | HER2-directed immunotherapy | Herceptin (trastuzumab) | Early and late-stage HER2-overexpressing breast cancer | Trastuzumab-dependent NK activation leads to cytokine secretion contributing to the recruitment and functional polarization of myeloid and T-cells. Exert a vaccine-like effect activating the adaptive as well as the innate immune system | [63] |
| 7 | HER2-directed immunotherapy | Pertuzumab | Early and late-stage HER-2-overexpressing breast cancer | Directed against the extracellular dimerization domain of HER2 (a different epitope than trastuzumab). Its binding inhibits dimerization of HER2 with other receptors of the HER family; increases the density of FcγR binding sites on HER2+ cells, possibly enhancing NK-mediated ADCC responses | [64] |
| 8 | HER2-directed immunotherapy | Lapatinib | HER2+ breast cancer | Reversible inhibitor of both HER2 and EGFR intracellular tyrosine kinase domains; promotes tumor infiltration by CD4+, CD8+, IFN-γ-producing T-cells through a Stat1 dependent pathway | [65] |
| 9 | HER2-directed immunotherapy | T-DM1 | HER2+ breast cancer | Antibody-drug conjugate formed by trastuzumab linked to the cytotoxic agent DM1. After binding HER2, T-DM1 is internalized, degraded in the endosome, releasing DM1. In addition, T-DM1 blocks HER2 signaling pathway and mediates ADCC | [66] |
| 10 | HER2-directed immunotherapy | Neratinib | HER2+ breast cancer | It is a pan-HER tyrosine kinase inhibitor. It bonds covalently to a conserved cysteine residue, leading to irreversible inhibition of all four HER receptors, block of downstream pathways, and in vitro inhibition of proliferation in tumor cells with trastuzumab resistance | [67] |
Sl. No.: serial number; ICOS: inducible costimulatory; HR: hormone receptor; ADCC: antibody-dependent cellular cytotoxicity; EGFR: epidermal growth factor receptor; DM1: emtansine; T-DM1: ado-trastuzumab DM1
In the past 40 years, breast cancer treatment has improved tremendously thanks to the lessons learned through clinical trials. Clinical trials investigate the benefits and safety standards of new therapies, as well as also check the new combination therapies. They also investigate additional points of care, including screening, diagnosis, and risk reduction. Improving response rates to immunotherapies remains a great challenge for breast cancer treatment including other methods. Considerably in breast cancer patients, restricted T-cell infiltration is important in the progression of novel approaches that aim to allow sufficient lymphocyte infiltration as well as generate de novo T-cell responses that overlap the tumor microenvironment with immunosuppressants, making this therapy successful [68]. Among the different approaches currently being considered, and despite their limited efficacy when administered as oncolytic viruses [69] and/or monotherapy [70], with combination therapy [71] these act as an exclusive platform for personalized and customized treatment of patients with advanced breast cancer. Recent evidence on the role of tumor-associated macrophages in breast tumor progression and drug resistance has paved the way for the development of new macrophage-targeted breast treatment strategies, such as tumor repolarization and inhibition of macrophage recruitment. Macrophages are associated with an antitumor phenotype, and enhancement of macrophage-mediated phagocytosis or tumor cell death, which are currently being evaluated in clinical trials [72]. Despite the promising results of preclinical studies, these therapies have shown limited clinical efficacy, so it is necessary to develop new strategies to improve the efficacy of these treatments. Another main drawback of immunotherapies, especially within a combined regimen, is the appearance of side effects related to the immune system that affect different organs such as the skin (rash, pruritus) or the gastrointestinal tract (diarrhea, colitis). Although the severity of these immune-related adverse events is generally mild, life-threatening complications can also occur [73], resulting in many cases in a reduction in the optimal dose of treatment or in the discontinuation of medication. In some cases, complications can lead to long-term health problems, including organ damage. It is therefore important to monitor patients carefully and to be vigilant for signs of adverse events. Early diagnosis and treatment can help minimize any long-term effects. Therefore, more research is still needed to develop biomarker panels for patient selection and prediction of immunotherapy response. Predictive biomarkers can also be used to identify individuals who are likely to benefit from immunotherapy, as well as those at high risk of serious adverse events. Additionally, biomarkers can be used to optimize treatment protocols for immunotherapy.
In conclusion, it can be inferred from the description of the immune system and breast cancer that the proper activity of the immune response is important in breast cancer. The balance between the different roles of immune cells in a particular tumor microenvironment either helps with tumor progression or regression. It can be said that immune status is an important part to be considered regarding the prevention of breast cancer relapse. Common therapies like chemotherapy and radiotherapy cause profound immunological impacts on breast cancer patients. So, immunological research is required to improve the activity of immune cells.
Improving immune status following breast cancer treatment might enhance the disease-free survival rate of breast cancer patients. However, so far, the success rate of breast cancer immunotherapy is poor, and vast disparities in immune response in the same kind of patient occur. Nonetheless, with persistent research and testing, maybe shortly patients suffering from breast cancer will have access to therapy modules for all subtypes of the disease.
BRCA: BReast CAncer gene
CICs: circulating immune complexes
CTLA-4: cytotoxic T lymphocyte-associated antigen 4
ER: estrogen receptor
HER2: human epidermal growth factor receptor 2
LPBC: lymphocyte-predominant breast cancer
NK: natural killer cell
PD1: programmed cell death protein 1
PDL1: programmed cell death ligand 1
PR: progesterone receptor
TACAs: tumor-associated carbohydrate antigens
TCR: T-cell receptor
TIL: tumor-infiltrating lymphocyte
TLS: tertiary lymphoid structures
TNBC: triple-negative breast cancer
Treg: T-regulatory cell
The authors acknowledge Amity University Utter Pradesh, Noida for providing the platform to continue with the research related to breast cancer immunobiology and the generation of ideas answering the present status of immune response in breast cancer. The authors acknowledge the ICMR-Indian Council of Medical Research for the opportunity to work in ICMR laboratories.
BB: Conceptualization, Investigation, Supervision, Writing—original draft, Writing—review & editing. SH: Writing—review & editing. VK: Writing—review & editing.
The authors declare no conflict of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Ali Abdul Hussein S. AL-Janabi ... Abdul Razzak Kalaf Hassan
Danila Coradini, Federico Ambrogi
Nadia Islam, Suneela Vegunta
Spoorthi Marada ... Yi Lu
Danila Coradini
Remo Poto ... Gilda Varricchi
Kaoutar Anouar Tadlaoui ... Moulay Mustapha Ennaji
