Affiliation:
1Volga District Medical Center, Federal Medical and Biological Agency, 603001 Nizhny Novgorod, Russia
2Department of Eye Diseases, Privolzhsky Research Medical University under the Ministry of Health of Russia, 603005 Nizhny Novgorod, Russia
Email: svetozarskij@rambler.ru
ORCID: https://orcid.org/0000-0002-7472-4883
Affiliation:
1Volga District Medical Center, Federal Medical and Biological Agency, 603001 Nizhny Novgorod, Russia
ORCID: https://orcid.org/0000-0002-9891-9730
Affiliation:
1Volga District Medical Center, Federal Medical and Biological Agency, 603001 Nizhny Novgorod, Russia
ORCID: https://orcid.org/0000-0001-5642-6420
Affiliation:
1Volga District Medical Center, Federal Medical and Biological Agency, 603001 Nizhny Novgorod, Russia
ORCID: https://orcid.org/0000-0001-7129-274X
Affiliation:
2Department of Eye Diseases, Privolzhsky Research Medical University under the Ministry of Health of Russia, 603005 Nizhny Novgorod, Russia
Explor Med. 2023;4:453–460 DOI: https://doi.org/10.37349/emed.2023.00155
Received: February 21, 2023 Accepted: May 24, 2023 Published: August 30, 2023
Academic Editor: Margaret M. DeAngelis, University at Buffalo, USA
Aim: The use of 5-fluorouracil in glaucoma surgery is associated with a high risk of corneal complications, as even minimal doses of the drug at the ocular surface inhibit corneal epithelial cell division and lead to corneal epitheliopathy and erosion. The aim of this study was to evaluate the clinical and functional results of the proposed method of postoperative adjuvant subconjunctival injection of 5-fluorouracil after non-penetrating deep sclerectomy (NPDS) in comparison with the control group.
Methods: Patients with primary open-angle glaucoma who underwent NPDS and received at least 1 subconjunctival injection of 5-fluorouracil in the postoperative period were included in a two-group retrospective comparative study. Patients who received a subconjunctival injection of 5-fluorouracil after surgery using the standard technique were included in Group 1; Group 2 included patients who received an injection using the proposed method. Best-corrected visual acuity (BCVA), intraocular pressure (IOP), rate of corneal complications, and number of office visits during the first 4 weeks after surgery were analysed.
Results: The compared groups did not differ in demographic characteristics, preoperative BCVA, and IOP parameters. Fluorescein-stained corneal epithelial defects were statistically significantly more frequent in Group 1 compared to Group 2, P < 0.001. Four weeks post NPDS IOP reduction was greater in Group 2, P = 0.042. Mean BCVA loss was 1.9 lines in Group 1 and 1.3 lines in Group 2, P < 0.001. The number of follow-up visits during the first month after surgery was lower in Group 2 than in Group 1, P = 0.002.
Conclusions: The proposed method was simple and effective in reducing the risk of corneal epithelial defects after subconjunctival injection of 5-fluorouracil, significantly improving clinical and functional outcomes of NPDS and reducing the need for outpatient visits.
Modifications in surgical technique, the use of various drainage, shunt, and valve devices as well as specific methods of pharmacological modification of the healing process are currently addressing the problem of prolonging the hypotensive effect of antiglaucoma surgery [1–4]. In particular, the use of antimetabolites in glaucoma surgery has found wide application both as intraoperative treatment and as postoperative injections, reliably reducing the risk of scleral scarring in the surgical zone [5–7]. The main drugs used for this purpose are mitomycin C and 5-fluorouracil (5-FU). 5-FU is a pyrimidine analogue that selectively inhibits DNA synthesis in the S and G2 phases of the cell cycle. It suppresses cell proliferation for approximately 2 weeks and blocks RNA synthesis and vascular endothelial growth factor antibodies [8]. Subconjunctival application of 5-FU in the postoperative period inhibits fibrocyte division and improves long-term prognosis by prolonging the hypotensive effect both in traditional filtering surgery, in various variants of drainage, and in valve glaucoma surgery [9–13]. According to data from randomised trials, the subconjunctival use of 5-FU reduced the risk of filtering surgery failure from 50% to 27% in the high-risk group during the first year of follow-up [14].
Meanwhile, the use of 5-FU is associated with a high risk of corneal complications, as even minimal doses of the drug on the ocular surface inhibit corneal epithelial cell division and lead to corneal epitheliopathy and erosion [14, 15]. Cases of conjunctival hyperplasia [16] and limbal stem cell deficiency after 5-FU use [17] have been described. Corneal complications result in reduced vision and quality of life for the patient and increase the number of visits to the doctor and the financial burden. The problem of harmful effects of cytostatics on the health of patients and medical staff is widely discussed, but prevention methods, which mainly focus on improving organisational measures, the use of supply and exhaust ventilation, and separate waste collection, cannot provide the necessary level of patient safety [18, 19].
To study the clinical and functional outcomes of the proposed method of postoperative adjuvant subconjunctival injection of 5-FU after non-penetrating deep sclerectomy (NPDS) in comparison with the control group.
A retrospective comparative study was conducted in 2 groups. Inclusion criteria: (1) patients with moderate and advanced stages of primary open-angle glaucoma; (2) who underwent NPDS, and (3) at least 1 subconjunctival injection of 5-FU in the postoperative period. Exclusion criteria were (1) a history of previous glaucoma surgery; (2) conjunctival and corneal diseases detected before surgery; (3) observation for less than 28 days after surgery. If a patient underwent surgery on 2 eyes, the eye operated on first was included in the study. Group 1 included patients who underwent standard postoperative subconjunctival 5-FU injection; Group 2 included patients who underwent subconjunctival 5-FU injection with prevention of corneal complications.
Clinical data from the medical records of patients who underwent glaucoma surgery with NPDS in the Ophthalmology Department of the Volga Regional Medical Centre of the Federal Medical and Biological Agency (FMBA) of Russia during the period from January 2013 to December 2021 were analysed. This time period was chosen based on the uniformity of surgical technique, postoperative management of patients, and principles of primary medical records in the department, including objective registration of all adverse events from January 2013.
The procedure was indicated for patients failing to achieve target intraocular pressure (IOP) despite adequate topical medical therapy. The technique of NPDS corresponded to the classical technique of Fedorov and Kozlov [20]. There was no intraoperative use of antimetabolites. Postoperatively, patients received instillations of antibiotics (tobramycin 0.3% for 14 days), steroids (dexamethasone 0.1% for 8 weeks on a tapering schedule), and non-steroidal anti-inflammatory drugs (indomethacin 0.3% for 5 weeks). All patients were examined on postoperative days 3, 7 (± 1 day), 28 (± 2 days), or more frequently as clinically indicated. At each visit, visual acuity, ocular tonometry, biomicroscopy, corneal, and conjunctival staining with fluorescein to detect external wound leakage and corneal epithelial defects were performed.
Patients received subconjunctival injections of 5-FU in a volume of 0.1 mL (5 mg) in the area of the filtering bleb starting 4–5 days after surgery, if there was no wound leakage. Depending on the degree of vascularization in the surgical area, 1 to 5 injections were administered. In the first group, where surgery was performed before 2016, 5-FU was injected into the inferior quadrant of the conjunctiva without any special precautions. In the second group, where surgery was performed from 2016 onwards, 5-FU was injected with specific safety measures in order to prevent corneal complications. After NPDS, the location of the scleral flap was identified, then a conjunctival puncture was performed at a distance of approximately 5 to 10 mm from the edge of the scleral flap. A 30G needle was inserted into the subconjunctival space to the location of the flap, and 0.1 mL of 5-FU was injected into the subconjunctival space (Figure 1A). The needle was then withdrawn, immediately followed by irrigation of the injection site with 10–20 mL saline or sterile water from a blunt-tipped syringe (Figure 1B). Subsequently, antibiotic solution was instilled into the conjunctival cavity and dexapanthenol 5% or carbomer 0.2% was applied (Figure 1C).
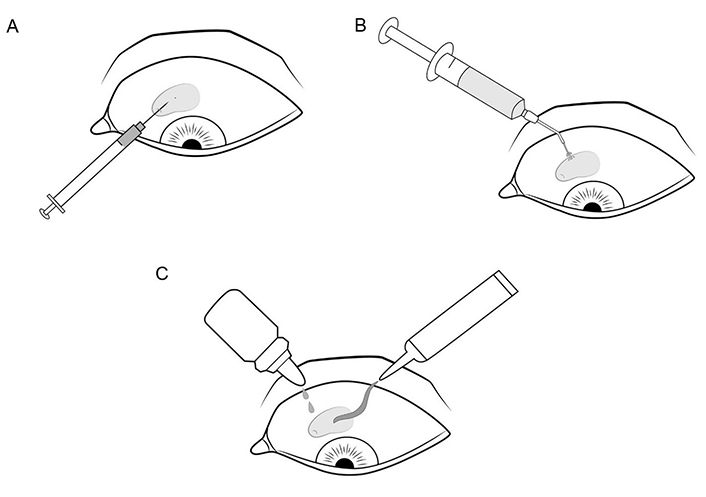
Modified method of subconjunctival administration of 5-FU. (A) Conjunctival puncture approximately 5–10 mm from the scleral flap edge, subconjunctival needle insertion to the flap edge, 0.1 mL drug injection; (B) irrigation of the injection site; (C) application of antibiotic solution and gel
The analysis included sex, age, number of preoperative topical glaucoma medications, number of 5-FU injections, best-corrected visual acuity (BCVA), and IOP measured by Maklakov tonometry before and 4 weeks after surgery. Wound leakage, choroidal detachment, and corneal complications, defined as fluorescein staining epithelial defects, were recorded for 28 days postoperatively. The number of doctor visits during the first 4 weeks after surgery was evaluated.
Statistical analysis was performed using Minitab 14 (Minitab, Inc., USA). Continuous variables are presented as mean ± SD, where M is the arithmetic mean and SD is the standard deviation. The normality of distribution was assessed using quantile plots and the Shapiro-Wilk test. Student’s t-test for independent samples was used when the sample was normally distributed. The Mann-Whitney test was used to compare two independent samples that did not follow a normal distribution. Categorical binary variables were compared using the Pearson chi-square test. The accepted significance level was 5% (P < 0.05).
The study included 277 patients (277 eyes) who underwent NPDS surgery between January 2013 and December 2021. Group 1 included 101 patients (101 eyes) who underwent subconjunctival injection of 5-FU using the standard technique. Group 2 consisted of 176 patients (176 eyes), where subconjunctival injection of 5-FU was performed with corneal complication precautions (Table 1). The modified technique has been used in all patients injected with 5-FU since 2016, which explains the unequal distribution of patients between groups. The groups did not differ in demographic characteristics and had similar values for visual acuity parameters, IOP, and the number of topical glaucoma medications before surgery (Table 1).
Baseline characteristics of patients in the study groups
| Parameter | Group 1 | Group 2 | P |
|---|---|---|---|
| Number (patients/eyes) | 101/101 | 176/176 | N/A |
| Age, years (mean ± SD) | 68.32 ± 9.49 | 70.44 ± 8.71 | 0.167 |
| Male/female | 53/48 | 91/85 | 0.663 |
| BCVA (mean ± SD) | 0.441 ± 0.392 | 0.510 ± 0.372 | 0.154 |
| IOP (mean ± SD, mm Hg) | 24.99 ± 6.47 | 25.32 ± 4.88 | 0.659 |
| The number of glaucoma medications (mean ± SD) | 3.139 ± 0.617 | 3.233 ± 0.593 | 0.215 |
P: statistical significance level; N/A: not applicable
In the postoperative period, the incidence of wound leakage and choroidal detachment was not statistically significantly different between the study groups (Table 2). Group 1 received one to five injections (2.97 ± 1.20), and Group 2 received one to five injections (3.16 ± 1.13), P = 0.187. Fluorescein-stained corneal epithelial defects were statistically significantly more frequent in Group 1 (38.6%) compared to Group 2 (7.4%), P < 0.001. The mean corneal defect healing time was 8 days in Group 1 and 6 days in Group 2, P = 0.047.
Clinical characteristics of the study groups after surgery
| Parameter | Group 1 | Group 2 | P |
|---|---|---|---|
| Number of 5-FU injections | 2.97 ± 1.20 | 3.16 ± 1.13 | 0.187 |
| BCVA, 4 weeks after NPDS (mean ± SD) | 0.252 ± 0.241 | 0.380 ± 0.318 | < 0.001* |
| IOP, 4 weeks after NPDS (mean ± SD, mm Hg) | 18.62 ± 5.32 | 17.34 ± 4.42 | 0.042* |
| Number of patients with wound leakage, n (%) | 5 (5.0) | 11 (6.3) | 0.652 |
| Choroidal detachment, n (%) | 4 (4.0) | 12 (6.8) | 0.313 |
| Number of patients with corneal defects, n (%) | 39 (38.6) | 13 (7.4) | < 0.001* |
| The duration of corneal defect healing, days | 7.6 ± 3.1 | 5.6 ± 2.1 | 0.047* |
| Number of outpatient visits during the first month | 5.0 ± 1.4 | 4.4 ± 1.4 | 0.003* |
* P < 0.05, differences between groups are statistically significant
Four weeks after surgery, IOP was statistically significantly lower in Group 2, where corneal complication prophylaxis was used, than that in Group 1, P = 0.042. The IOP reduction was 25.5% in Group 1 and 31.5% in Group 2. At 4 weeks after surgery, there was a statistically significant difference in BCVA between groups, with Group 2 showing better values, P < 0.001. The mean BCVA loss was 1.9 lines in Group 1 and 1.3 lines in Group 2. The number of visits to the doctor in the first month after surgery was 0.6 visits less in Group 2 than that in Group 1, P = 0.003.
This study demonstrates the high efficacy of an innovative, simple, and accessible method to reduce the incidence of corneal complications and improve the clinical and functional outcomes of NPDS with adjuvant subconjunctival injection of 5-FU.
To solve the problem of 5-FU side effects, it has been suggested to reduce the dose of the drug or to change the way it is administered [21]. In particular, various modifications of the method presented in the work of Traverso et al. [22] have been proposed, which is performed as follows [15, 21]. A 30G needle was inserted under the conjunctiva up to the sleeve to inject the drug as far away from the puncture site as possible; when the needle was removed, light pressure was applied to the injection site using a surgical sponge or cotton applicator. However, we believe that pressure on the puncture site with the edge of the surgical sponge or cotton applicator increases the degree and area of trauma to the conjunctival epithelium and creates an additional risk of drug reflux, while complete absorption of the drug is not possible. As a result, the incidence of corneal defects after subconjunctival injection of a standard dose of 5-FU reached 47% according to Reiter et al. [15], which is significantly different from the results obtained in this study. The reduction of the erosion rate to 13% achieved by Traverso et al. [22] cannot be considered comparable, as in this study, the injection of 5-FU was performed in the inferior quadrant of the conjunctiva, opposite to the surgical site. This not only reduces the risk of complications, but also significantly increases the availability of the drug in the area of the filtering bleb [22].
An important finding of the present study is the statistically significant improvement in clinical and functional outcomes of glaucoma surgery. The frequent development of corneal erosions in Group 1 patients necessitated changes in the pharmacological therapeutic regimen, in particular, the temporary withdrawal of steroid anti-inflammatory drugs and the use of soft contact lenses and corneal protectors. In addition, in some cases, this led to the refusal of ongoing 5-FU injections. As a result, anti-inflammatory and anti-proliferative therapy used to prevent scarring around the filtering bleb was reduced for the period of corneal complication treatment, which compromised the hypotensive effect of surgery. Decreased frequency of complications causing visual disturbance and pain increased quality of life and patient satisfaction with medical care. The period of post-operative rehabilitation and temporary disability was reduced by minimising the loss of visual acuity. The results obtained in reducing the need for outpatient visits and the associated medico-economic effect can be considered as underestimated. Between 2016 and 2021, the average length of stay in the hospital during NPDS gradually decreased from 4 bed days to 1 bed day, which required an additional appointment for a patient on 3 days after surgery. Nevertheless, the use of preventative interventions not only did not raise the average number of visits but resulted in a statistically significant reduction of 0.6 visits.
Besides the obvious clinical significance and reduced quality of life associated with corneal complications, the effect of 5-FU on epithelial morphology should be considered. In more than 60% of patients, squamous metaplasia, nuclear atypia and apoptosis of conjunctival epithelial cells occur when 5-FU is used subconjunctivally without specific preventive measures [23]. Activation of caspase-8 and caspase-9, decreased mitochondrial membrane potential, increased p21 gene activity and Bcl-2-dependent signalling pathways have been implicated in the development of cellular abnormalities [24].
We believe that the problem of corneal complications is one of the main causes of the current contradiction between the demonstrated prolonging effect of 5-FU, the unceasing interest of researchers in the possibilities of its use as monotherapy and in various combinations, on the one hand [25–27], and the decrease in its use in real clinical practice from 39% in 2002 to 0.8% in 2016 [28], on the other hand. The application of the proposed method may significantly improve the safety profile of postoperative use of antimetabolites in glaucoma surgery, leading to wider use of 5-FU by practicing ophthalmologists and improving the long-term visual prognosis of patients with glaucoma.
In conclusion, the proposed modification of the method of 5-FU subconjunctival injection after NPDS reduces the risk of corneal epithelial defects from 38% to 13%. Prevention of corneal complications significantly improves clinical and functional outcomes of glaucoma surgery, reduces postoperative visual acuity loss from 1.9 to 1.3 lines, and provides a 6% more pronounced hypotensive effect of NPDS at 4 weeks follow-up. In addition, the introduction of preventive measures resulted in a statistically significant reduction in the number of visits to the doctor in the first month after surgery.
5-FU: 5-fluorouracil
BCVA: best-corrected visual acuity
IOP: intraocular pressure
NPDS: non-penetrating deep sclerectomy
SNS: Conceptualization, Methodology, Investigation, Data curation, Formal Analysis, Writing—original draft, Writing—review & editing. ANA: Conceptualization, Investigation, Writing—review & editing. AVS and SVS: Investigation, Writing—review & editing. ANS: Visualization, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
This study is approved by the Institutional Ethics Committee of Volga District Medical Center under the Federal Medical and Biological Agency No. 7 of 31 May 2022 and complies with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The primary data will not be shared with third parties due to the institution’s policy regarding patient data.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 5651
Download: 43
Times Cited: 0
