Affiliation:
1Department of Psychiatry, Epidemiology of Psychiatric Disorders and Mental Health Research Unit, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand
2Center of Excellence for Maximizing Children’s Developmental Potential, Department of Pediatric, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand
ORCID: https://orcid.org/0000-0002-5690-8216
Affiliation:
1Department of Psychiatry, Epidemiology of Psychiatric Disorders and Mental Health Research Unit, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand
ORCID: https://orcid.org/0000-0002-2012-871X
Affiliation:
3Epidemiolgy unit, Faculty of Medicine, Prince Songkla University, Songkla 90110, Thailand
ORCID: https://orcid.org/0000-0001-6753-8567
Affiliation:
1Department of Psychiatry, Epidemiology of Psychiatric Disorders and Mental Health Research Unit, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand
4Department of Psychiatry, King Chulalongkorn Memorial Hospital, Bangkok 10330, Thailand
Email: rasmon.k@chula.ac.th
ORCID: https://orcid.org/0000-0002-5311-1930
Explor Med. 2023;4:409–420 DOI: https://doi.org/10.37349/emed.2023.00151
Received: November 16, 2022 Accepted: February 01, 2023 Published: June 30, 2023
Academic Editor: Richard M. Sherva, Boston University School of Medicine, USA
The article belongs to the special issue The Biological Basis of Substance Use Disorders
Aim: There is a strong comorbidity between methamphetamine (MA) and alcohol use whereby MA use may contribute to increased alcohol consumption. This study aims to determine the associations between alcohol drinking and MA-associated behaviors among MA users in relation to mood disorders, suicidal ideation, and health-related quality of life (HR-QoL).
Methods: Substance use characteristics were obtained in 106 participants with MA use at a substance abuse treatment center by using the Severity of Dependence Scale (SDS) and the Thai version of the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA). Current alcohol drinking behaviors were estimated using the Substance Outcomes Profile (SOP), which was developed and translated from the Treatment Outcomes Profile, by computing the number of alcohol units x days per month of alcohol use. The Mini-International Neuropsychiatric Interview (M.I.N.I.) was used to estimate lifetime histories of mood disorders and substance abuse and current suicidal ideation.
Results: Current suicidal ideation in MA users is to a large extent predicted by the severity of current alcohol and MA use, female gender, and a lifetime history of mood disorders (major depression, dysthymia, and hypo-mania). Moreover, a lifetime history of mood disorders is positively associated with the severity of MA, but not with alcohol use. Partial least squares (PLS) path modeling shows that lowered HR-QoL in MA users is predicted by the SDS score and alcohol dosing (both inversely) and that lifetime diagnoses of mood disorders and MA use (both inversely) and alcohol dependence (positively) have significant effects on HR-QoL which are completely mediated via the SDS score.
Conclusions: In MA users, the severity of dependence, and MA and/or alcohol use exert adverse effects on current suicidal ideation and HR-QoL. Mechanistic explanations are given which may explain the inverse associations between the severity of MA and alcohol use in MA abusers.
Mood disorders may be caused by substance use disorders including methamphetamine (MA) and alcohol use disorders [1]. According to a record from a large treatment centre for substance abuse in Thailand, 40,419 patients received treatment for illicit substance use in 2018, while this number increased to 53,420 in 2022 [2]. In addition, 79.2% of Thais receiving treatment for illicit substance abuse used MA, and 46.39% of all subjects using addictive substances used MA, followed by alcohol (20.18%) [2]. According to information from the 2013 Thai National Mental Health survey, the lifetime and current prevalence of alcoholism are 18.0% and 5.3%, respectively [3].
MA is a derivative of amphetamine that stimulates and energises the user [4], whereas alcohol is a central nervous system depressant [5]. Alcohol use disorder is associated with other substance use disorders and psychiatric disorders, as well as a multitude of adverse health effects. For instance, excessive and long-term alcohol consumption can lead to obesity, hypertension, liver cirrhosis, and cancer if used excessively and continuously [6]. Furthermore, the concurrent use of alcohol and MA may exacerbate the health risks associated with each substance alone [7].
This information shows the importance of examining patterns of alcohol and MA co-use on well-being and suicidal behaviors which are directly associated with alcohol use. Alcohol plays a role as a catalyst for suicidal ideation and behaviors through disinhibition, impulsiveness, and impaired judgment, and is often used as a way to cope with the distress associated with suicidal ideation [8]. Alcohol drinking patterns also predict an increased risk of suicide in those with psychiatric disorders including mood and anxiety disorders [8–14]. Mood and anxiety disorders, suicidal behaviors, and alcohol use are very strongly interrelated phenomena [15–18]. Moreover, MA use and alcohol use disorder are associated with poor health-related quality of life (HR-QoL). For example, long-term use of MA is associated with disabilities and illnesses, including cardiopulmonary disease, cognitive deficits, depression, anxiety, and suicide, which all cause a lowered HR-QoL [19–21]. A few examples among many others, co-occurrence of MA use and alcohol drinking may cause an increased risk of cardiovascular dysfunctions when compared to either drug taken alone [22]. Most clinical trials on MA dependence have excluded alcohol-dependent participants or did not present information on alcohol use [23].
Hence, the aim of this study is to examine the associations between alcohol drinking and MA use among MA users in relation to suicidal behaviors, mood disorders, and HR-QoL. The specific hypotheses are that the severity of alcohol and MA use in MA users are interrelated phenomena that adversely affect HR-QoL and increase suicidal ideation, and which are predicted by a lifetime history of mood disorders.
In this cross-sectional study, Thai-speaking MA users, aged 18 years or above, both males and females were included. They were recruited from Princess Mother National Institute on Drug Abuse Treatment (PMNIDAT), a substance abuse treatment center in Central Thailand where they were hospitalized between July 2017 and September 2017. The diagnosis of substance dependence is based on the Diagnostic Statistical Manual of Mental Disorders 4th text-revision (DSM-IV-TR). Exclusion criteria were lifetime diagnoses of DSM-IV-TR axis I diagnoses other than substance use disorders, including schizophrenia, autism, and psycho-organic disorders. Subjects with neurological diseases such as epilepsy, brain tumors, and multiple sclerosis, and major medical illness including autoimmune disorders were excluded.
Socio-demographic and substance use data were collected using sections A and B of the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA), a semi-structured questionnaire comprising items such as gender, age, level of education, marital status, income, types of treatment received, and length of recent treatments. The severity of MA dependence was measured using the Severity of Dependence Scale (SDS) [24] in a Thai-validated translation [25]. This scale consists of 5 questions which are rated from 0–3, yielding a total score of 0–15. Higher scores on the SDS indicate more severe MA dependence. The test-retest reliability of the SDS score is very good, namely 0.89 [26]. We also employed section F, “methamphetamine section”, of the SSADDA to assess MA use data, including onset, duration, amount, and frequency during the period of heaviest use [27]. The SSADDA has high inter-instrument validity (κ = 0.97) and inter-rater reliability (κ = 0.97). The Mini-International Neuropsychiatric Interview (M.I.N.I.) [28] was used to assess the lifetime diagnoses of affective and substance abuse disorders. We used a Thai-validated translation developed by Kittirattanapaiboon [29]. The inter-rater and test-retest reliabilities of the M.I.N.I.-lifetime are 0.88–1.00 and 0.76–0.93, respectively. M.I.N.I. was used to assess diagnoses of the 19 most common mental disorders, namely 17 axis I disorders, suicide, and axis II disorder.
The Substance Outcomes Profile (SOP) was developed from the Treatment Outcomes Profile [UK National Treatment Agency (NTA), 2007] and translated into Thai by Kalayasiri and modified by Jirakran and Kalayasiri [30]. This scale assesses substance use outcomes in 4 domains (substance use, injecting risk behavior, crime, and health and social functioning) comprising 38 questions with a 28-day recall. In the current study, we assessed alcohol drinking behaviors, namely a) days of alcohol use in the last 4 weeks before hospitalization for MA dependence treatment with a range from 0–28 (higher scores on the alcohol drinking behaviors questions indicate more days of use) and b) the average alcohol drinking units per day during that period. Consequently, we have computed a severity of alcohol use index as days of use in the last 4 weeks x average alcohol drinking units per day. The SOP also allows the participants to subjectively score physical and mental health functioning and quality of life (QoL) with a range from 0–20 (0 = the worst ever, 20 = the best ever).
We used analyses of variance (ANOVA) to check differences in scale variables between groups. We used analysis of contingency tables (χ2 test) to check associations between two or more categories. Multivariate regression analyses were employed to assess the most important predictors of the SDS score and alcohol use. Binary logistic regression analysis was used to assess the best predictors of subjects with high versus low alcohol use. Nagelkerke values were used as effect size estimates. Statistical analyses were performed using Statistical Package for the Social Science (SPSS) Windows version 22 and Statistica 8. Tests were 2-tailed, and a P value < 0.05 indicated a statistically significant effect.
SmartPLS analysis was employed for structural equation modeling using partial least squares (PLS) pathway modeling with latent variables coupled with a structural equation modeling algorithm. The final outcome variable was a latent construct reflecting “HR-QoL”, whereby three SOP items (i.e. mental health, physical health, and QoL) were entered as indicator variables. All other variables shown in Figure 1 were direct or indirect explanatory (input) variables. Suicidal ideation, alcohol use, and total SDS score were entered as secondary outcome variables. PLS path modeling was only performed when the model and constructs complied with quality criteria, including adequate model fit as estimated using standardized root mean square residuals (SRMR) values (< 0.08), good reliability and discriminant validity [Cronbach’s alpha values > 0.7, composite reliability > 0.7, and adequate convergent validity, namely average variance extracted (AVE) > 0.5], and factor loadings > 0.7 with P values < 0.001, good cross-validated redundancies and communalities. Path coefficients with exact P values, total effects, total indirect and specific indirect effects were computed.
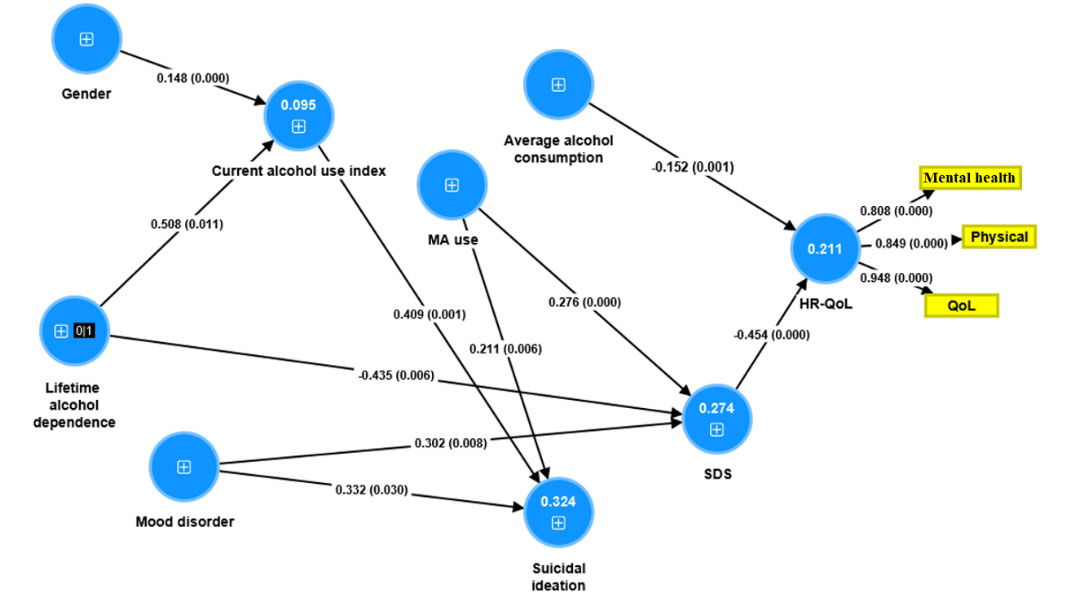
Associations between substance use variables, mood disorder, suicidal ideation, and QoL by using PLS way analysis. Shown are the path coefficients (with P values) of the inner model, and loadings (with P value) of the outer model. Figures in the blue circles denote explained variance. No figures in the blue circles means there are no predictors. The sign in the blue circles denote single indicator. MA use: days of MA use per month during heaviest use; mood disorder: major depression, dysthymia, mania, and hypomania; current alcohol use index: alcohol units x days per month of alcohol use; average alcohol consumption: average alcohol drinks per day; physical: physical subdomain
The socio-demographic and clinical data in participants with high versus low alcohol days x alcohol units were shown in Table 1. We used the median-split method (cut-off value of 10.4) to divide the patients into two groups. There were no significant differences in age, marital status, income, SDS score, age at onset of MA use, and days per month of MA use in the past month between both groups. There were significantly more males in subjects with higher as compared with lower alcohol use index. Days per month of MA use at the heaviest MA use in the lifetime was significantly lower in those with high versus low alcohol use index. Only the alcohol lifetime diagnosis of alcohol dependence was associated with the current level of alcohol use, whereas there were no significant differences in other psychiatric diagnosis or substance use between both groups.
Socio-demographic and clinical data in participants that were divided into two groups with high versus low alcohol days x alcohol units index (≥ 10.4 versus < 10.4)
| Variables | Alcohol index | F/χ2df | P value | |
|---|---|---|---|---|
| Low (< 10.4, n = 71) | High (≥ 10.4, n = 35) | |||
| Age (years)a | 29.8 (6.9) | 29.2 (6.6) | 0.1511/104 | 0.699 |
| Gender (female/male) | 16/55 | 2/33 | 4.711 | 0.030* |
| Marital status (single/married/divorced) | 35/26/10 | 20/11/4 | 0.592 | 0.74 |
| Income (baht)a | 16,338 (21,487) | 10,500 (5,579) | 2.491/104 | 0.118 |
| Average alcohol drinks per daya | 1.3 (4.5) | 17.9 (9.9) | 143.211/104 | < 0.001*** |
| Alcohol day x unitsa | 1.2 (2.5) | 128.7 (122.4) | 77.701/104 | < 0.001*** |
| SDS total scorea | 5.3 (3.0) | 4.6 (3.1) | 1.171/104 | 0.282 |
| Age at onset of MA (years)a | 18.8 (4.7) | 17.9 (3.5) | 0.891/104 | 0.347 |
| Days of MA use in last montha | 17.5 (10.2) | 14.8 (10.1) | 1.591/104 | 0.210 |
| Days of MA use per month, heaviest usea | 24.1 (8.3) | 19.4 (11.0) | 5.971/104 | 0.016* |
| Dysthymia (no/yes) | 62/9 | 30/5 | 0.051 | 1.000 |
| Anxiety disorder (no/yes) | 66/5 | 28/7 | 3.921 | 0.058 |
| MA-induced psychotic (no/yes) | 41/30 | 21/14 | 0.051 | 0.825 |
| Antisocial personality (no/yes) | 62/9 | 28/7 | 0.981 | 0.322 |
| Opioid use (no/yes) | 69/2 | 33/2 | 0.541 | 0.597 |
| Cannabis use (no/yes) | 69/4 | 29/6 | 3.631 | 0.078 |
| Nicotine use (no/yes) | 21/50 | 13/22 | 0.621 | 0.433 |
| Mitragyna speciosa use (no/yes) | 65/6 | 31/4 | 0.241 | 0.727 |
| Alcohol dependence (no/yes) | 49/22 | 12/23 | 11.571 | 0.001** |
a Data are shown as mean [standard deviation (SD)]. F: results of ANOVA; χ2: results of analyses of contingence tables; df: degree of freedom. * P < 0.05; ** P < 0.01; *** P < 0.001
The results of multiple regression analysis with the total SDS score as the dependent variable are shown in Table 2. We found that 21.4% of the variance in SDS score was explained by the regression on lifetime diagnosis of mood disorders [major depression, dysthymia, and (hypo) mania] and lifetime alcohol dependence (inversely). Regarding regression analysis with the severity of the current alcohol use index as the dependent variable, 19.5% of the variance in the severity of alcohol use was explained by the regression on lifetime diagnosis of alcohol dependence, lifetime cannabis use (both positively), and days per month of MA use (inversely, Table 2).
Results of multiple regression analysis with total SDS and current severity of alcohol use index score as dependent variables
| Predictor variables | t | P value | R2 | Fdf | P value |
|---|---|---|---|---|---|
| Model for SDS | 21.4% | 14.272/103 | < 0.001*** | ||
| Lifetime alcohol dependence | –2.88 | 0.005** | - | - | - |
| Lifetime mood disorders | +4.51 | < 0.001*** | - | - | - |
| Model for severity of current alcohol use | 19.5% | 8.233/102 | < 0.001*** | ||
| Lifetime alcohol dependence | +2.20 | 0.030 | - | - | - |
| Day per month of MA use | –3.17 | 0.002** | - | - | - |
| Lifetime cannabis use | +2.68 | 0.009** | - | - | - |
R2: the coefficient of determination; t: test statistic from a two-sided t-test; -: not applicable. ** P < 0.01; *** P < 0.001
In order to determine the best predictors of the subgroup of patients with high severity of current alcohol use, we performed a binary regression analysis with the group of patients with high use as the dependent variable, and using demographic data, days per month of MA use, SDS total score, and lifetime psychiatric diagnoses as explanatory variables (Table 3). We found that male sex, anxiety disorders, and a lifetime diagnosis of alcohol dependence (all positively) and days per month of MA use (inversely) were significantly associated with higher alcohol use in the last month.
Results of binary logistic regression analysis with high alcohol use index as the dependent variable
| Explanatory variables | Walddf | P value | ORs | 95% CI |
|---|---|---|---|---|
| Male | 3.981 | 0.046* | 7.28 | 1.04–51.12 |
| Lifetime anxiety disorders | 5.751 | 0.016* | 6.65 | 1.91–31.25 |
| Lifetime alcohol dependence | 8.301 | 0.009** | 3.91 | 1.55–9.91 |
| Day per month of MA use | 4.731 | 0.030* | 0.95 | 0.91–0.995 |
ORs: odds ratio; CI: confidence interval. * P < 0.05; ** P < 0.01
The results of a binary logistic regression analysis with suicidal ideation as the dependent variable (and no suicidal ideation as the reference group) and severity of alcohol use index and MA use (SDS score), age, sex, a lifetime diagnosis of mood disorders, anxiety disorders, and other drugs of use as explanatory variables are shown in Table 4. Suicidal ideation was best predicted by the severity of alcohol use index, a lifetime history of mood disorders, days per month of MA use, and female sex (χ2 = 27.18, df = 1, P < 0.001, Nagelkerke = 0.546); the accuracy was 96.2%, with a sensitivity of 75.0% and a specificity of 98.0%.
Results of binary logistic regression analysis with suicidal ideation as dependent variable
| Explanatory variables | Walddf | P value | ORs | 95% CI |
|---|---|---|---|---|
| Severity of alcohol use index | 6.481 | 0.011* | 1.04 | 1.01–1.07 |
| Lifetime mood disorders | 4.481 | 0.034* | 14.54 | 1.22–173.75 |
| Day per month of MA use | 5.401 | 0.020* | 1.38 | 1.22–1.81 |
| Female | 3.891 | 0.049* | 0.049 | 0.003–0.967 |
* P < 0.05
In order to decipher the complex associations between HR-QoL, the severity of alcohol use, SDS score, and different input variables, we have carried out SmartPLS pathway analysis with multiple independent variables (Figure 1). HR-QoL was the final dependent variable and SDS score, suicidal ideation, and severity of current alcohol use were employed as direct explanatory variables of HR-QoL. All variables (except HR-QoL) were entered as single indicator variables, while the three HR-QoL (physical and mental health and overall functioning) measurements were entered as indicator variables reflecting the latent construct “HR-QoL”, whereby the causal arrows go from the HR-QoL latent vector to the three measured variables in a reflective model. Only the significant paths were shown in Figure 1. All quality model data were excellent including model fit SRMR = 0.031, AVE = 0.757, Cronbach’s alpha = 0.837, and composite reliability = 0.903. We found that 21.1% of the variance in HR-QoL was explained by the effects of SDS and average alcohol consumption (inverse association). The regression on a lifetime diagnosis of alcohol use (inversely) and lifetime history of mood disorders and MA use (both positively) explained 27.4% of the variance in the total SDS score. There were significant total indirect effects of a lifetime history of mood disorders (t = –2.39, P = 0.017), MA use (t = –2.91, P = 0.004), and lifetime alcohol dependence (t = 2.62, P = 0.008) on HR-QoL, which were mediated by SDS. There were significant indirect effects of a lifetime history of alcohol dependence (t = 1.99, P = 0.047) and gender (t = 2.44, P = 0.015) on suicidal ideation.
The results of our study show that in individuals who used MA, there is an inverse association between the severity of current MA and alcohol use and between a lifetime history of alcohol dependence and the severity of MA use. These inverse associations may perhaps be explained by the opposite properties of both drugs, whereby alcohol, a sedative depressant, may counteract the stimulant effects of MA [31]. Moreover, alcohol may inhibit the metabolism of MA as indicated by lowered p-hydroxylated metabolites in the urine of MA users [32]. Thus, it is possible that persons who used MA attenuate their alcohol use in an operant fashion to reduce the adverse effects of their MA use. It is also plausible that patients with alcohol dependence attenuate their MA use to decrease possible interactions between both drugs. Thus, persons who used MA have an increased risk when drinking alcohol to reach intoxication levels [33] as shown in a study that alcohol dependence was a predictor of MA-induced paranoia [34]. The co-use of MA with alcohol increases MA levels and the metabolite amphetamine [35]. In addition, both MA and alcohol stimulate mesocorticolimbic dopaminergic metabolism causing possible synergistic effects [36, 37]. These and other interactions may have undesirable clinical effects including deficits in learning and spatial memory and increased heart rate [35]. It is also plausible that the severity of MA use depends more on internal factors such as substance preference and other personal characteristics rather than on external factors, including preexisting use of alcohol [38].
Here we underscore that these inverse associations observed in our study between the severity of MA and alcohol use do not contradict the well-known comorbidity between both disorders. A recent review indicates that, in persons with MA dependence, the prevalence of alcohol use disorder is 75% [35]. Moreover, some studies report that persons with MA use disorder may have a higher consumption of alcohol or alcohol dependence than those without MA use [32], whereby co-use may mask clinical signs of alcohol intoxication (e.g., sedation) with the risk of developing alcohol toxicity [34]. According to Kirkpatrick et al. [22], MA and alcohol use can augment the pleasurable effects of each other and additionally reduce the negative behavioral effects of each other, indicating that there is a predictable pattern in co-using alcohol and MA. This relationship seems more intense in individuals with low MA dependence severity and individuals with heavier alcohol problems [31]. Although alcohol use is related to a greater risk of MA use on the same day, lagged analyses show no relationships between the previous day’s alcohol use and the risk of MA on the following day after controlling for the previous day’s MA use, or vice versa [39].
Another finding of our study is that the severity of alcohol and MA use, a lifetime history of mood disorders, and female sex predict to a large extent suicidal ideation. This may be explained by findings that alcohol use predisposes towards suicidal behaviors following depressogenic effects of alcohol or greater negative life events following alcohol use [40, 41]. The same authors also proposed that a decrease in problem-solving skills and aggravation of impulsive personality traits, related to serotonin neurotransmission, may be involved [41]. In adolescents, there is also a strong relationship between alcohol-related disorders and suicide attempts as shown in the National Comorbidity Survey [42]. Moreover, psychological autopsy studies report a strong relationship between alcohol use and suicide [42]. Results from meta-analysis studies indicate that drug use and alcohol dependence are largely associated with suicide and that individuals who show heavy alcohol consumption display a five-fold increased tendency towards suicide [43]. There are also reports that injected MA use is accompanied by increased suicidal behaviors [44]. However, Kennedy et al. [45] found that alcohol use is directly associated with a higher risk of suicidal behaviors independent of the use of illicit drugs, while in our study both severity of alcohol and MA use were associated with increased suicidal ideation, although the impact of alcohol use was more important.
In the present study, we found a significant association between mood disorders (lifetime diagnosis) and severity of MA use, but not alcohol use in persons who used MA. These data extend previous findings showing that individuals who use MA injections have significantly more symptoms of depression [46]. Another study found that individuals who abstained from MA have significant reductions in depressive symptoms during treatment [47]. In this respect, it is interesting to note that negative emotionality predicts later substance use disorders in longitudinal studies, which is explained by the effects of negative reinforcement [48]. The current study found that the severity of alcohol use, but not MA use, is greater in males than in females. It is known that men show a greater prevalence of co-occurrence of alcohol and other drug use disorders as compared with women [49]. Male drinkers are more likely to drink high volumes than women, while women are more likely to abstain from alcohol during their lifetime [50].
Finally, the current study found that in persons who used MA, the severity of MA and average alcohol consumption had a strong effect on HR-QoL. These findings extend those of previous papers showing that MA-dependent individuals who completed treatment and continued care have a better HR-QoL [51]. HR-QoL improvement following a residential treatment is shown to correlate with low HR-QoL scores at admission, whereas improvement in physical domain of HR-QoL is related to less alcohol intake and better somatic status [52]. In this respect, Neale [53] reported that drug users have poor physical health expectations and therefore, perceive their health status better than the actual HR-QoL. One study showed that increasing regularity of alcohol use is associated with a better HR-QoL in both male and female subjects [54]. MA use may affect mental and physical HR-QoL through a variety of mechanisms including the induction of side effects such as weight loss, insomnia, mood swings, increased heart rate, delusions, tweaking, cognitive deficits in episodic memory and executive functions, confusion, cardiovascular disorders, inflammation, neuroinflammation, oxidative stress, and increased gut permeability [55–60].
The current study’s findings should be discussed in light of its limitations. First, the study would have been more interesting if we had also assayed biomarkers of MA use and suicidal behaviors, including the cytokine network. Second, it is interesting that females in this group seem to be more prone to suicidal ideation when drinking, however, there are only two female subjects in the high alcohol use disorder group. Third, our finding that high MA use may predict less alcohol use may initially appear puzzling. Nonetheless, we have an overall population of heavy drinkers, and the results presented pertain to heavy alcohol consumption in general. The knowledge gained by amphetamine and cocaine users who consume alcohol more frequently may be applicable to a lower intensity.
In conclusion, the results show that, among MA users, the severity of alcohol use is significantly associated with lowered MA use and male sex, while predicting suicidal ideation but not HR-QoL. The intensity of MA use is the single best predictor of a lowered HR-QoL in MA users and is additionally associated with more suicidal ideation.
df: degree of freedom
HR-QoL: health-related quality of life
M.I.N.I.: Mini-International Neuropsychiatric Interview
MA: methamphetamine
PLS: partial least squares
QoL: quality of life
SDS: Severity of Dependence Scale
SOP: Substance Outcomes Profile
SSADDA: Semi-Structured Assessment for Drug Dependence and Alcoholism
We thank participants and staff from Princess Mother National Institute on Drug Abuse Treatment (PMNIDAT) for facilitating data collection.
KJ: Methodology, Data curation, Project administration, Writing—original draft. MM: Conceptualization, Formal analysis, Writing—review & editing. NB: Investigation. RK: Methodology, Supervision, Validation, Writing—review & editing.
The authors declare that they have no conflicts of interest.
The study was approved by the Faculty of Medicine, Chulalongkorn University Institutional Review Board (Med Chula IRB), number 155/60 and the Ethical Review Committee for Research in Human Subjects, and the Research Committee, PMNIDAT, and complies with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
Not applicable.
RK was supported for research career by the Centre for Addiction Studies (CADS), Department of Psychiatry, Faculty of Medicine, Chulalongkorn University, and the
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 4719
Download: 56
Times Cited: 0
Jiayi W. Cox ... Lindsay A. Farrer
Elisha M. Wachman ... Huiping Zhang
Richard Sherva ... Lindsay A. Farrer
Rui Fu ... Michael Chaiton
Rasmon Kalayasiri ... Witaya Sungkarat
Abuelgasim Elrasheed A. Alhassan ... Simon Elliott
