Affiliation:
Department of Chemistry, Ottawa-Carleton Chemistry Institute, Carleton University, Ottawa, ON K1S 5B6, Canada
Email: edward.lai@carleton.ca
ORCID: https://orcid.org/0000-0002-6046-1138
Affiliation:
Department of Chemistry, Ottawa-Carleton Chemistry Institute, Carleton University, Ottawa, ON K1S 5B6, Canada
ORCID: https://orcid.org/0000-0002-7062-0833
Explor Foods Foodomics. 2025;3:101099 DOI: https://doi.org/10.37349/eff.2025.101099
Received: January 07, 2025 Accepted: August 17, 2025 Published: September 17, 2025
Academic Editor: Guoliang Li, Shaanxi University of Science and Technology, China
The health benefits of milk have been acknowledged throughout human history, with scientific research over the past 50 years elucidating its nutritional composition and functional benefits. This article presents a contemporary analysis of modern casein chemistry, emphasizing the specialized engineering of dairy proteins for optimizing resource utilization. It explores the unique structure of casein micelles as supramolecular complexes, where ionic interactions promote electron sharing between phosphoserines and calcium phosphate nanoclusters. This review aims to synthesize recent literature on casein nanocomplexes and explore their potential in industrial applications such as drug delivery and sustainable food engineering. Casein-based bio-nanocomposites have emerged as a significant research interest in food science, offering considerable potential for a wide array of scientific applications, such as drug formulation and nutraceutical delivery. It is crucial for scientists to engage in ongoing research and development efforts to encourage sustainable progress, enhance commercial viability, improve manufacturing processes, and expand the engineering applications of casein micelles toward fostering an eco-friendly industry.
In food science, a primary objective is to link the physicochemical properties of material components to their functional performance [1]. Milk comprises approximately 87% water and 13% solids, containing essential constituents such as the protein casein (CN), calcium phosphate, and fats [2]. As a complex fluid, mammalian milk contains proteins, peptides, oligosaccharides, minerals, lipids, fatty acids, phytochemicals, and vitamins, all critical for nourishing newborns and conferring immunity [3]. Human milk is particularly rich in lactoferrin and serum albumin, containing between 20% and 60% of its protein as CN [4]. Comparatively, camel milk resembles human milk most closely in terms of whey protein composition. Cowʼs milk exhibits a higher total CN content, comprising a diverse range of CN proteins, notably including αs1-CN, αs2-CN, and either A1 or A2 β-CN [5]. Sheepʼs milk has a protein profile similar to that of cowʼs milk, but varies in the relative proportions of specific CN proteins. The variations in CN content and composition among these milk types hold significant implications for nutritional and allergenic considerations [6]. The reduced CN content in human milk is tailored to meet the specific nutritional requirements of infants [7]. Conversely, the higher CN levels in cowʼs and sheepʼs milk may pose challenges for individuals with CN sensitivities or allergies [8], particularly due to the presence of A1 β-CN in cowʼs milk [9]. Scientific research continues to investigate the health implications of these CN differences or explore alternative hypoallergenic milk sources [10]. There is a knowledge gap regarding the existence of critical periods during which human development is particularly vulnerable to nutritional influences [11]. Furthermore, there is a lack of understanding concerning the role of infant microbiome composition and its development in the potential epigenetic programming of long-term growth patterns or adiposity in later life [12].
Dairy proteins are versatile, used in various structural forms such as liquid, semi-solid, and solid. For example, the serum component of liquid milk is a colloidal dispersion of fat globules and CN micelles, which can transition into a soft gel, like yogurt, upon acid coagulation. Similarly, cheese is a semi-solid product derived from rennet-coagulated, CN-rich milk. The complex structural composition of these dairy products results from interactions between proteins, fats, and water, forming a substantial matrix. Food materials science investigates how the structures of components operate across different scales. A recent study highlights the application of material science techniques to explore the crucial link between processing, structure, and properties, addressing significant challenges within the dairy industry [13]. The creation of emulsions through non-covalent interactions among CN biomolecules has gained substantial interest from food scientists [14]. These distinctive properties suggest innovative ways to valorize milk proteins across industries, including cosmetics, functional foods, bioplastic packaging, pharmaceuticals, 3D printing, textiles, and wood [15–21], aligning with the United Nations Sustainable Development Goals [22, 23]. CN is prevalent in dietary supplements worldwide, such as α-CN-B12 and β-CN-B12 vitamin complexes [24]. The interactions of CN with polysaccharides and polyphenols play a pivotal role in regulating the functional properties and textural quality of dairy products [25]. Treating CN with polyphenols like quercetin enhances foam and emulsion formation, boosts antioxidant activity, and reduces stability. Quercetin incorporation decreases surface hydrophobicity through both covalent and non-covalent interactions with CN, laying the groundwork for studies on CN-polyphenol complexes and their food industry applications [26].
Significant knowledge gaps exist in the field of CN chemistry research concerning its structure, functions, and applications. Key challenges include structural complexity, functional properties, and nutritional applications. In terms of molecular dynamics, a comprehensive understanding of the tertiary and quaternary structures of CN micelles is lacking [27], especially regarding the precise spatial arrangements and interactions among the different types of CN: alpha, beta, and kappa. Concerning micelle assembly, the detailed processes and mechanisms under various conditions remain partially understood, leaving room for investigation in environments such as the human stomach [28] or industrial synthetic systems [29]. For functional properties, the bioactive roles and health impacts of individual CN components, such as peptides released during digestion [30], require further elucidation. The interactions between CN and digestive enzymes, along with the resulting implications for nutrient bioavailability [31], have not been fully explored. Regarding nutritional applications, deeper research is needed to modify CN structures to reduce allergenicity for sensitive populations [32]. Moreover, CN-based functional food ingredients could be further developed to enhance nutrition without compromising taste [33] or texture by exploring novel processing methods and formulations [34]. The application of CN in nanotechnology and its potential as a nanocarrier for drug delivery is promising [35], though still in its early stages. Research into genetic variations in CN content and composition among different breeds of dairy animals is needed, as well as understanding how environmental factors such as feed and management practices can enhance CN synthesis and quality [36]. Advancing the understanding of CN chemistry demands interdisciplinary approaches encompassing molecular biology, food science, nutrition, and materials science [37].
Combining protein complexation with water kefir-assisted fermentation significantly enhances the solubility of native lentil proteins from 58 to over 86% through the formation of lentil-CN complexes. This approach effectively modifies plant-based proteins [38]. When combined with polycaprolactone, CN forms bandage-like fibers of 1.4 µm diameter, suitable for wound dressing applications [39]. CN-based films become water-insoluble after heat treatment, optimizing degradation rate, mechanical properties, and water uptake, essential for wound management. These films exhibit proteolytic activity during degradation or release antioxidants, thus promoting healing [40]. A novel dual-network conductive hydrogel incorporates CN micelles and multi-walled carbon nanotubes into polyacrylamide (PAAm), creating bio-based sensors with enhanced adhesiveness, toughness, self-healing capabilities, and a near-infrared photothermal response [41]. Sodium caseinate acts as the active component in a bio-material-based resistive memory device, supporting memory retention for at least 20 days, ensuring cyclic endurance over 6 × 104 cycles, and maintaining a switching voltage of 1.06 V [42]. A robust, reusable fire warning composite utilizes CN for film formation and Ti3C2Tx as a conductive skeleton, with tannic acid functioning as both a crosslinker and a free radical scavenger. This composite exhibits mechanical flexibility and high thermal stability (T10% at 239.6°C), providing excellent fire safety with total heat release, smoke production, and CO production rates of only 1.35 mJ/m2, 0.19 m2, and 0.42 mg/s, respectively. Importantly, it provides reliable warnings under both direct flame exposure and contactless fire threats [43]. Additionally, CN-based adhesives prove advantageous in woodworking, bookbinding, and other applications requiring robust, durable, and water-resistant bonds [44]. Heated and acid-exposed CN molecules unfold and form chains, enabling solidification post-3D printing via baking [45]. CN protein improves the 3D printing performance of cassava starch (CS) gel by enhancing the starchʼs multi-scale structural and gel properties [46]. This review utilizes a thematic literature analysis within a formative assessment framework to explore the development of CN nanocomplexes for diverse applications in food nutrition and health-related pharmaceuticals.
Bovine milk is one of the most extensively studied sources of protein within dairy science [47, 48]. The mammary glands of lactating cows secrete substantial amounts of phosphoproteins. Free amino acids and inorganic phosphate from the bloodstream serve as precursors for CN biosynthesis [49]. This process occurs in two stages: initially, the polypeptide chain is synthesized; this is followed by phosphorylation. Phosphate groups are added to the developing CN by a Golgi-localized protein kinase using adenosine 5'-triphosphate as the phosphate donor, a process that requires divalent cations. The specific serine residues that become phosphorylated are determined by adjacent amino acids [50]. The four principal polypeptide families of CNs—αs1- (38%), αs2- (10%), β- (30–36%), and κ- (13%)—exhibit varying degrees of phosphorylation, typically containing 8, 10–13, 5, and 1 seryl phosphate residue per monomer, respectively [51, 52]. The electrophoretic heterogeneity observed in κ-CNs is due to the presence of glycosylated molecules alongside others lacking carbohydrate residues [53]. Desirable alleles of CN and whey protein genotypes in cows influence the content, quality, and technological properties of milk proteins [54]. The genetic distinctiveness of indigenous cattle has been elucidated by evaluating the A1/A2 alleles, the predominant β-CN gene variants. Typically, conventional milk contains about 75% A1 β-CN and 25% A2 β-CN [55]. There is speculation that high consumption of milk from cows with the A1 allele may increase the risk of various human diseases, whereas A2 milk is considered to be safer. The A1 variant has been associated with specific gastrointestinal disorders [56]. A specific qPCR assay can be employed to determine the genotype of β-CN (A1A1, A1A2, and A2A2) using DNA samples isolated from the raw milk of individual cows [57].
CN is typically considered an intrinsically unstructured protein, lacking well-defined secondary structures [58]. Variations in amino acid sequences at specific sites in β-CN can lead to gene mutations that affect the physicochemical properties of dairy products and the sites of digestive enzyme hydrolysis. Bovine A1 and A2 β-CN mutations are distinguished by the presence of histidine versus proline at position 67 in the peptide chain [59, 60] (as illustrated in Figure 1), causing significant conformational changes in secondary structure [61]. The β-CN genotype also affects cheese texture; after 180 days of ripening, cheese made from A1A1 homozygous milk is the softest, while A2A2 cheese is the most fracturable [62]. Sensory analyses have shown that β-CN genotypes significantly influence the overall acceptability of stirred yoghurt [63]. Milk with the A1A1 genotype is preferred for cheese production, while A2A2 milk suits formulations requiring stability, such as yoghurts [64]. During the proteolysis of A1 β-CN, a peptide known as β-casomorphin-7 (BCM-7) is released. This bioactive peptide binds to and activates the μ-opioid receptor in various tissues [65]. The suggested link between A1 β-CN/BCM-7 and adverse health effects remains an area for further research [66]. Notably, yogurt and cheese contain the highest levels of BCM-7 per serving [67]. Despite being less ordered, CNs possess considerable secondary/tertiary structure and are more flexible than globular whey proteins.
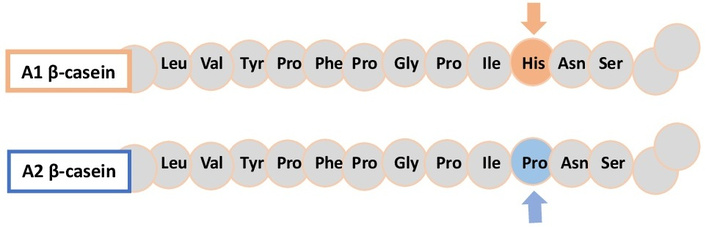
Partial structures showing the difference between A1 β-casein (histidine at position 67) and A2 β-casein (proline at position 67).
α-Lactalbumin (α-LA; 14.178 kDa) and β-lactoglobulin (β-LG; 18.300 kDa) are the principal structural proteins associated with major milk allergens [68]. CN also ranks as a significant milk allergen, raising global health concerns, with αs1-CN being the predominant allergen in cow milk. Allergies occur when the immune system mistakenly identifies CN proteins as harmful, leading to severe IgE-mediated reactions. Processing methods have shown promise in reducing allergenicity by altering protein structures and breaking down epitopes that trigger immune responses. Enzymatic hydrolysis is frequently used to reduce the allergenicity of milk proteins [69], while heat treatment provides another modification approach. Peptidome analysis has demonstrated that papain effectively disrupts the key epitope (Y154-T174) by selectively cleaving critical amino acid residues (Y154 and Y165), significantly reducing the antigenicity of αs1-CN and decreasing IgE and IgG binding [70]. As an alternative method used for the same purpose, hypoallergenic responses can be achieved by structurally modifying these proteins using polyphenols, such as those derived from orange peel [71]. For individuals who are lactose or CN-intolerant, alternative milk options include cantaloupe seed milk, coconut milk, dry bean milk, potato flour milk, tiger nut milk, sesame milk, sorghum milk, and white corn milk [72]. CN, a byproduct of skim milk production, is precipitated by acidification to produce acid CN or treated with rennet to yield rennet CN [73]. Its flexible linear structure makes CN an excellent stabilizer for colloidal dispersions [74]. The water-holding capacity, thermal stability, and microstructure of CN micelle gels, which are induced by rennet, improve with the addition of 0.5% agar due to hydrogen bonding within the CN micelle/agar mixture, thus enhancing the protein digestion rate during gastric digestion [75]. Recent findings suggest that polypropylene nanoparticles reduce the rate of cowʼs milk protein digestion in an infant model of gastric digestion [76].
CN can form branched chains comprising two to five proteins, interlocked by calcium phosphate nanoclusters. As a lyophilic colloid of phosphoproteins, CN typically exists as salts that are easily dispersed in water and resistant to precipitation. With an isoelectric pH between 4.6 and 4.8, CNʼs solubility in water is approximately 0.01% [77]. Below pH 4.6, CN forms moderately soluble salts such as CN chloride, whereas above pH 4.6, it forms salts with bases. Extrusion-porosified milk protein concentrate powders demonstrate high dispersibility (96%), with rehydrated dispersions containing sub-micron particles after two hours [78]. In milk powder, CN is the predominant component, leading to dispersion behaviors similar to CN suspensions. For effective CN volume fractions under 0.54, viscosity depends on concentration akin to hard-sphere suspensions, with no divergence at the glass transition due to CNʼs elastic deformation. For volume fractions between 0.55 and 0.61, viscosity shows minimal concentration dependence but increases sharply, diverging at an effective volume fraction of 0.69. Rheology and diffusing wave spectroscopy indicate that suspensions jam at volume fractions above 0.69 [79]. CN-coated paper outperforms zein-coated paper in waxy pick tests [80]. The chemical composition and primary/secondary structures of CN offer intriguing research opportunities [81]. Molecular weights of CN range from 19 to 25 kDa, with each milk protein containing numerous genetic variants that can significantly alter milk functionality. A single amino acid substitution can modify the properties of intrinsically unstructured proteins such as β-CN, which adsorb onto hydrophobic surfaces forming multilayers, in contrast to β-LGʼs compact monolayer formation [82]. Methods for preparing and purifying isoelectric CN significantly affect its electrophoretic properties. Peptization with sodium hydroxide removes γ-CN and some β-CN, and the pH of the wash water markedly influences the relative composition of α-, β-, and γ-CN [83].
The components of CN (αs1, αs2, β, and κ) are effectively separated through various methods, including precipitation from a 50% alcohol solution, and alterations in temperature, pH, and ionic strength, as well as isoelectric precipitation from water and solubility in aqueous urea [84]. Quantitative analysis of αs-, β-, and κ-CN is conducted via column chromatography using alkylated samples on diethylaminoethyl cellulose in the presence of urea. This chromatographic method is well-suited for CN mixtures with widely varying carbohydrate compositions per κ-CN molecule and is unaffected by γ-CN and temperature-sensitive variants [85]. Whole CN fractions such as β-CN, κ-CN, αs1-CN, and αs2-CN can also be separated using cation-exchange fast protein liquid chromatography (FPLC) [86]. Utilizing a urea-acetate buffer at pH 5, along with a NaCl gradient ranging from 0.00 to 0.26 M, facilitates the separation, with γ-CNs eluting first, followed by β-CNs, κ-CN, αs1-CN, and αs2-CN. These compositional analyses align closely with values obtained through anion-exchange FPLC at pH 7. The λ-CN fraction is primarily composed of αs1-CN fragments that are produced in vitro through bovine plasmin incubation [87]. Milkʼs κ-CN protein is crucial for regulating micelle functionality, size, and stabilization [88]. Pure κ-CN is isolated using Sephadex G-150 gel filtration in a tris-citrate buffer at pH 8.6 with 6 M urea, where it emerges at the void volume [89]. κ-CN constitutes approximately 13% of milk proteins and is encoded by a gene located on chromosome 6, consisting of 162 amino acids. It acts as a stabilizing factor in cheese production, influencing curd formation rates [90]. Mutational diversity within κ-CN generates nine different forms, with A, B, and E being predominant. Six κ-CN genotypes (AA, AB, AE, BB, BE, and EE) result from parental transmission, affecting κ-CN forms in milk and, consequently, cheese quality. Molecular docking and simulations confirm that hydrogen bonds and hydrophobic interactions are the primary forces in β-CNʼs binding with aldehyde aroma compounds [91].
PAAm gel electrophoresis (PAGE) and high-resolution mass spectrometry are utilized for sequence analysis, identifying four distinct variants of cow CN and examining genetic polymorphisms [92]. Significant differences are observed in the amino acid content and peptide composition of CN fractions following simulated gastrointestinal digestion. Notably, the β + κ-CN group demonstrates superior calcium absorption activity in cellular and murine models, while the κ-CN group also exhibits enhanced bone mineral activity. This provides a theoretical foundation for developing infant formulas aimed at boosting calcium absorption and bone health [93]. Proteoforms of α-CN, cytochrome C, and myoglobin are effectively separated using capillary isoelectric focusing with a precise pH gradient, enabling accurate mass spectrometry detection [94]. Comparative genomic analyses of CN loci across various mammals uncover a highly conserved 147 bp region, identified in silico as a potential regulatory element and validated as a unidirectional repressor element through functional studies in cell lines [95].
Fluorescence quenching and isothermal titration calorimetry have demonstrated that chlortetracycline can spontaneously bind to bovine CN via hydrogen bonds and hydrophobic interactions. Molecular docking has identified the amino acid residues forming the binding pocket, while saturation transfer difference NMR revealed that the binding involves the -N(CH3)2 group. Thermal treatment indicated a reduced first-order rate constant in the presence of CN; the removal of CN decreased the half-life of chlortetracycline by at least 48% [96]. The interactions between α-LA, β-LG, and β-CN with nano-resveratrol were examined in binary and ternary systems using transmission electron microscopy (TEM), field emission scanning electron microscopy (SEM), conductometry, isothermal titration calorimetry, and molecular dynamics simulations [97]. Ethylene glycol dimethacrylate-modified hydroxyapatite microbeads efficiently isolate α-LA from whole milk without the need for fat or CN removal. The fabrication of gold-capped nanoparticle substrates involved a core of surface-modified silica nanoparticles on a glass slide situated between gold layers, with an absorbance peak at approximately 520 nm [98]. In the presence of bovine β-CN, the diffusion rates of ferulic acid and epicatechin decreased from 2.76 × 10–11 and 1.33 × 10–11 to 8.51 × 10–12 and 1.00 × 10–12 m2/s, respectively. The magnitude of the diffusion rate decrease appears to be governed by the strength of the protein-bioactive compound interaction [99].
The phosphoserine residues in CN structures induce electrostatic repulsion between the charged hydrophilic N-terminal regions. This repulsion, coupled with attraction among hydrophobic domains, is fundamental to the micellization process. The resulting CN micelle, which functions as a colloid in milk, has a critical concentration of approximately 0.85 μg/mL [100, 101]. Recognized as a dynamic supramolecule, the CN micelleʼs structure is capable of adapting to environmental changes [102]. CN micelles contain both organic phosphates, specifically those covalently attached to phosphoserine side chains, and inorganic calcium phosphate nanoclusters, which are released upon acidification. Interaction between β-LG and κ-CN within native micelles occurs through sulfhydryl-disulfide exchange, forming intermolecular disulfide bonds that stabilize the micelles in cow milk. The processes of calcium chelation and acidification result in colloidal particles that are structurally distinct from naturally secreted CN micelles [103]. Notably, mouse mammary epithelial cells cultured on floating collagen gels exhibit 3- to 10-fold higher production of CN mRNA compared to cells cultured on plastic or affixed gels [104].
Cheese functions as an emulsion gel where milk fat globules form the dispersed phase and a CN gel network provides the continuous phase. This embedding of fat within the network allows manipulation of the viscoelastic and physicochemical properties of CN emulsion gels, enabling the creation of customized cheese products [105]. The presence of Ca2+ ions is crucial for forming a rigid interfacial network in CN blends; without Ca2+, the network becomes less stiff and more viscous, akin to a two-dimensional polymer solution [106]. CN-gellan gum emulsions enhance probiotic viability following storage, pasteurization, and digestion, with the highest stability observed in emulsions containing a CN-0.8% gellan gum complex [107]. A novel microcapsule has been developed using CN and gum Arabic as wall materials to improve the survival of probiotic bacteria during gastrointestinal digestion, storage, and lyophilization [108]. In the processing of skim milk using a polyvinylidene fluoride membrane, CN is the primary protein fouling agent, increasing hydraulic resistance and inhibiting serum protein passage through the membrane [109]. β-CN, an amphiphilic protein, self-assembles into well-defined core-shell micelles. TEM images reveal an interlocked lattice model where CN-calcium phosphate aggregates and CN polymer chains collaborate to maintain micelle integrity. Comparisons between commercial caseinates and CN micelles as raw materials for isolating food-grade β-CN show yields of 9–11% from the micelles and 2–3% from the caseinates [110]. The yield of β-CN via selective precipitation is likely due to the solubility of the raw CN material [111]. Micellar CN concentrate (MCC) is gaining importance as a supplement for acidified milk-derived products, offering extended functional possibilities through pre-chelation with trisodium citrate (0–15 mM) and high-pressure microfluidization [112]. Recent techniques achieved an average β-CN enrichment of approximately 90% (w/w) total protein from MCC using cold microfiltration combined with chymosin treatment [113]. The critical micelle concentration (CMC) of β-CN decreases with reduced pH (from 8.0 to 6.0) and increased temperature (from 25 to 50°C), while the critical micelle temperature also decreases with lower pH. Fluorescence spectroscopy indicates maximum surface hydrophobicity at pH 6.0. Circular dichroism spectroscopy shows an increase in α-helix content and a decrease in unordered structures with decreasing pH and increasing temperature [114]. Shifting pH from 6.7 to 5.7, and then to 2.3, significantly affects the release of CNs from micelles [115].
β-CN, an intrinsically disordered protein, has been analyzed in both its calcium-bound and unbound states using neutron and light scattering techniques. Upon calcium binding, β-CN partially folds and stiffens [116]. Both phosphates and CNs have the capacity to bind divalent metal ions [117]. Bovine CN micelles consist of calcium-insensitive κ-CN, along with calcium-sensitive αs1-, αs2-, and β-CNs, which are phosphorylated at specific serine residues. Phosphorylation is crucial for micelle formation [118]. Except for regions surrounding the phosphorylation centers, CNs predominantly exhibit an all-β-strand conformation. The micelle reassembly capacity of a solution containing all four CN types is directly proportional to the degree of phosphorylation. As phosphorylation decreases, the gelation pH increases; fully dephosphorylated CNs fail to gel and precipitate at their isoelectric point (pH 5.5) [119]. However, condensation into β-sheets is inhibited by conserved primary structural features, allowing proteins to maintain an open and rheomorphic conformation [120]. Photon correlation spectroscopy, utilizing multi-angle measurements of the diffusion coefficient, has been used to study the size distribution of CN micelles [121]. The impacts of calcium-sequestering salts on their properties and the resulting physicochemical, textural, functional, and sensory attributes of processed cheese have been comprehensively reviewed [122]. Zinc ions also bind to αs1-, β-, and κ-CNs, forming specific metallocomplexes. The zinc-CN binding process is highly dependent on CN size, structure, amino acid sequence, and hydrophobicity. Zn2+ ions predominantly bind to the polar amino acid residues at the deprotonated carboxylic groups of glutamic and aspartic acids and the imidazole ring of histidine. Notably, in κ-CN-zinc interactions, the sulfur in cysteine may play a significant role. This easy surface modulation of colloidal CNs by Zn2+ offers new opportunities for the food industry [123].
Native CN micelles tend to settle under gravity during extended storage, leading to the formation of a sediment layer over several months, a phenomenon particularly pronounced in concentrated milk [124]. The removal of a subcritical amount of Ca2+ from CN micelles using EDTA results in the release of soluble CN and a gradual reduction in the sedimentation velocity of micelles, without altering their hydrodynamic radii. These findings imply that the removal of Ca2+ initially dissociates weakly bound CNs from the micelle while maintaining the micellar framework responsible for determining size [125]. Cryo-electron tomography has quantified changes in their major constituents, including CN proteins, colloidal calcium phosphate nanoclusters, and serum-filled voids/channels, in response to pH variations. The average micelle diameter was measured at 199 ± 29 nm at pH 6.0 and 233 ± 51 nm at pH 6.7 [126]. Colloidal calcium phosphate is crucial for CN micelle formation and integrity, with a molecular weight of approximately 7,450 g/mol [127]. Micellar and serum CNs do not form an equilibrium system governed by the solubilities of different CNs. Upon cooling, αs-, β-, and κ-CNs dissolve in the serum, with β-CN accounting for about 55% of the total increase in serum CN. Serum CN content decreases with calcium addition and increases with phosphate addition, acidification of pH from 6.7 to 5.3, and the removal of colloidal calcium phosphate [128]. Bovine cream demonstrates excellent whipping properties, forming stiff peaks in milk with low glycosylation levels. Cream separated from skim milk with larger CN micelles shows superior whipping properties, requiring shorter whipping times, achieving greater firmness, and having better overrun [129].
The interactive behavior of bovine CN micelles during processing is significantly influenced by the micellar surface structure. Although it is established that the surface is coated with κ-CN, detailed information regarding the surface topology is limited. The κ-CN molecules likely exhibit an uneven distribution on the micellar surface, with gaps potentially allowing other molecules to pass through [130]. In unheated milk at physiological pH, most κ-CNs are positioned on the CN micelle surface, leading to steric repulsion during their collisions [131]. CN micelles are polydisperse, with sizes ranging from approximately 50 to 600 nm in diameter. In vivo, they form through interactions of SH-κ-CN monomers with αs- and β-CNs, followed by S-S-κ-CN polymer formation via oxidation post-milking [132]. Rather than absorbing light, CN colloidal particles scatter it. Their internal structure has been investigated using small-angle neutron scattering (SANS), small-angle X-ray scattering (SAXS), and static light scattering (SLS), indicating a homogeneous mass distribution within a micelle as evidenced by the radius of gyration [133]. A localized surface plasmon resonance (LSPR) immunosensor was developed for detecting CN [134]. Treatment with reducing agents releases κ-CN, as identified by PAGE [135]. CN micelle dissociation was achieved by selective reduction at a stable colloidal calcium phosphate concentration [136]. Studies using TEM, ultraviolet-visible (UV-vis) spectroscopy, Fourier transform infrared (FTIR) spectroscopy, and fluorescence spectroscopy have shown that gold nanoparticles (GNPs) bind to the CN micelle surface, forming GNP-CN micelle conjugates. These conjugates exhibit stability against variations in salt concentration and pH. GNPs are presumed to bind to the micelle surface through complexation with carboxylate or amine groups rather than through electrostatic interactions [137]. Fluorescence spectroscopy has examined phycocyanin-CN micelle interactions, revealing complete suppression of CN fluorescence by phycocyanins at specific wavelengths, which underscores their antioxidant and immunomodulatory properties [138].
Intact CN imparts beneficial properties to food products, such as dairy items, sauces, and dressings, as well as to biomaterials like biodegradable films. However, the properties of proteins such as CN can be modified through hydrolysis—a process that enhances digestibility, reduces allergenicity, and improves functional characteristics. Additionally, hydrolysis can generate peptides with health-promoting properties and alter molecular structures for more effective coatings [139]. Enzymatic hydrolysis of CN in aqueous dispersion (pH 8.0) with trypsin at 40°C plateaus after 45 min. Peptides with molecular weights exceeding 50 kDa decrease significantly, while those in the 30–50 kDa range and below 20 kDa increase notably. The α-helix and β-turn content in hydrolysates increases relative to the original CN [140]. In the ripening of Italian cheese, β-CN hydrolysis results in fragments, predominantly yielding three γ-CNs containing the COOH-terminal portion of β-CN [141]. Enzymatic proteolysis of bovine milk CN was performed using three gastrointestinal proteases, revealing degrees of hydrolysis at one hour of 37% for pepsin, 97% for trypsin, and 47% for chymotrypsin, as verified by high-performance liquid chromatography [142]. Proteolysis of β-CN by trypsin was also detected using a multi-harmonic quartz crystal microbalance biosensor [143]. CN enzymatic hydrolysis at varying intervals showed antigenicity loss approximated by three-phase first-order kinetics, with significant loss in the first 10% of hydrolysis time. Hydrophobicity and emulsifying activity decreased, while net charge increased with the progression of hydrolysis [144]. The use of protein hydrolysates in food systems is often restricted by bitterness and hygroscopicity, but spray drying CN hydrolysate with gelatin and soy protein isolate successfully reduced bitterness [145].
Complex coacervation with soybean protein isolate/pectin as a wall material effectively microencapsulates and mitigates the bitter taste of CN hydrolysate, as confirmed by a trained sensory panel [146]. Nano- and micro-particles formed from carboxymethylcellulose and CN via complex coacervation are promising for controlled substance release in aqueous media, relevant to the food, cosmetics, pharmaceuticals, and wastewater treatment industries [147]. A delivery system using micellar CN with varying degrees of freezing achieved controlled anthocyanin release in the intestine [148]. A comprehensive review of spray drying-assisted fabrication of milk-based passive nanostructures—such as nano-emulsions, nanotubes, and nanoparticles—explored factors influencing their manufacturing. Spray drying is extensively used to convert liquid matrices into dry powders, primarily for food ingredients, nutrient delivery, and materials science [149]. CN hydrolysate can act as a dispersant to enhance quercetinʼs water dispersibility, improving its oral bioavailability [150]. The effects of the Maillard reaction on the glycation, structural, and functional characteristics of CN acid hydrolysate products were evaluated with reducing sugars (methylglyoxal, glucose, galactose, lactose, and maltose) under incubation conditions of 6 h at 80°C and 8 h at 100°C. This reaction improved the thermal, antioxidant, and emulsifying properties of all products [151].
CNs constitute 80% of the total protein content in milk and can be efficiently recovered through isoelectric precipitation or enzymatic coagulation [152]. Acid induces interactions between protein particles in milk, specifically between CN micelles and serum protein/κ-CN complexes, leading to coagulation. Heated serum proteins gel at higher pH levels compared to unheated ones [153]. The coagulation process upon the addition of proteolytic enzymes to milk is well-documented [154]. Chymosin, a protease enzyme, curdles CN in milk, and rennet traditionally separates milk into curds and whey by converting caseinogen into insoluble CN, which is essential in cheese production. Pasteurized skim milk was acidified using glucono-δ-lactone at temperatures ranging from 10 to 40°C to study gelation. The gelation process comprises three phases: (a) temperature-dependent dissociation of proteins from CN supramolecules occurs as the milk pH decreases, releasing proteins that form loosely entangled aggregates, (b) protein reassociation with these aggregates occurs between pH values of 5.3 and 4.9, and (c) rapid aggregation of colloidal CN supramolecules into a gel network occurs at pH 4.8 [155].
For rennet-induced CN gels at pH 5.3, structural rearrangements proceed faster than at pH 6.7, involving the enlargement of compact building blocks, partial fractal structure disappearance, and formation of breakable, straightened strands—changes due to particle fusion [156]. At pH 6.7, the addition of citrate or EDTA removes over 33% of the original colloidal calcium phosphate and releases 20% of CN from micelles, thus inhibiting gelation. Reintroduction of CaCl2 reverses this inhibition [157]. In comparison, goatʼs milk has an average composition of 3.6% fat, 3.0% protein, 2.5% CN, and 4.4% lactose [158]. Goatʼs milk with low αs1-CN coagulates faster, while high αs1-CN milk produces firmer curds [159]. Goat A2 β-CN exhibits physicochemical properties similar to bovine whole CN, providing good digestibility and hypoallergenic benefits, making it suitable for infants, the elderly, and individuals with metabolic disorders [160].
Different dietary fibers (pectin, cellulose, lignin) affect CN digestibility under gastric and intestinal conditions by promoting flocculation, hydrogen bonding, and reducing proteolytic enzyme activity, thereby influencing amino acid homeostasis [161]. Insoluble dietary fibers (e.g., lemon peel) decrease fermentation time by forming CN-lemon peel fiber-CN clusters with a higher gelation rate. These fibers promote cross-linking through a bridging effect, creating a densely interconnected network that enhances link density and reduces pore fraction [162].
Edible films and coatings for food packaging incorporate proteins (soy, whey, wheat gluten, gelatin), polysaccharides (chitosan, starch, cellulose), and lipids [163]. CN-based edible films, emulsions, hydrogels, and micro/nanocapsules are pivotal in delivering bioactive compounds [164]. Utilized as a structural protein, CN is integral to edible coatings and protein-based hydrogels. Reducing hydrophobic and ionic interactions among CN proteins provides novel functionality. Similar to gelatin, pure CN films generally exhibit brittle mechanical properties; however, they can be rendered flexible through glycerol plasticization. Unlike gelatin–glycerol films, water is essential for CN films to exhibit the rheological properties characteristic of drawable materials [165]. Edible CN is processed from milk residue by dissolving it with sodium hydroxide (pH 9.6), followed by filtration to remove impurities, centrifugation/degreasing, high-temperature sterilization, citric acid addition (adjusting to pH 4.4), washing, prilling, and drying [166]. The final product composition includes moisture (10.24%), fat (1.23%), ash (1.87%), protein (96.16%), sodium (236 ± 56 mg/kg), and potassium (56 ± 72 mg/kg) [167]. Shear processing combined with homogenization is employed to create highly stable total nutrient emulsions based on CN and maltodextrin conjugates, serving as natural edible biopolymers for specialized food and pharmaceutical formulations [168]. Sodium caseinate and chitosan-based edible films infused with black pepper essential oil are being developed for sustainable active food packaging applications [169].
Proteolysis by Lactiplantibacillus plantarum enhances CN gel formation during fermentation, significantly improving gel hardness and water-holding capacity. This results in a denser and more uniform gel structure with increased hydrophobicity and disulfide bond content [170]. Compared to control CN/carrageenan gels, the addition of low concentrations of locust bean gum (0.1–0.3%) significantly augments the water-holding capacity, strength, and thermal stability of CN/carrageenan/locust bean gels. The unfolding of carrageenan macromolecules into chains optimizes the gel network structure [171]. A highly stretchable hydrogel was developed by integrating CN into PAAm. In the CN-PAAm hybrid gel, CN micelles and PAAm chains synergistically enhance mechanical properties, making it highly stretchable under uniaxial tension [172]. Rheological studies revealed that the gel-sol transition temperature, determined using a multiple waveform method, was significantly lower at 11.1°C (P < 0.05) compared to the conventional method range of 21–27°C [173]. High-pressure processing (100–500 MPa) is a promising technological approach in the dairy sector to enhance the functional characteristics of MCCs. This process improves the solubility of calcium and phosphorus, reduces particle size, decreases surface hydrophobicity, and alters α-helix/β-sheet content [174]. A hydrogel comprising 10% CN and 1% konjac glucomannan was formed using 0.4 wt% transglutaminase as a cross-linker, resulting in improved stability and effective docetaxel drug release behavior [175]. Several hydrogels were synthesized from 10 wt% CN using 5 wt% glutaraldehyde as a crosslinker at pH 7.5 [176]. These CN-based adhesives outperformed gelatin adhesives in terms of properties and versatile applications [177]. The cytotoxicity of CN-based protein biomaterials was evaluated through biochemical tests, showing no cytotoxic effects on cells from these naturally derived materials [178]. Albumin, CNs, and gelatin facilitate the formation of hybrid nanocomposite materials by catalyzing sol-gel processes and templating silica formed in situ. These proteins provide a unique opportunity to regulate silica morphology and properties by altering their secondary and tertiary structures through changes in pH and temperature [179].
Recent developments in processing and application focus on reducing costs while meeting market demands for flexibility and quality in ingredient usage, enabling holistic product design [180]. A CN phosphopeptide-amorphous calcium phosphate paste significantly repairs enamel microstructure by increasing hydroxyapatite crystal size and Ca/P molar ratio, proving effective in treating early childhood caries [181]. On enamel surfaces, CN phosphopeptide-amorphous calcium phosphate demonstrates superior remineralizing potential compared to calcium sodium phosphosilicate, making it the preferred choice for remineralizing early enamel carious lesions [182]. This compound shows promise as a safe anticariogenic agent with protective properties for orthodontic treatments [183]. Fractured teeth can be reattached and repaired using remineralizing agents like CN phosphopeptide-amorphous calcium phosphate [184]. A novel magnetic composite (Fe3O4@PDA-PEI-Fe3+) was developed for enriching phosphopeptides from skim milk and human saliva, enhancing mass spectrometry detection [185]. The nanomechanical properties and structures of κ-CN nanofibrils were examined during thermally induced amyloidogenesis for potential material science applications. Disulfide bonds of κ-CN from bovine milk, reduced with dithiothreitol at temperatures from 37 to 95°C, resulted in twisted mature nanofibrils formed at 95°C with rapid growth kinetics. Atomic force microscopy revealed elevated Youngʼs moduli (2.6 GPa) for mature fibrils compared to oligomers and protofibrils (0.6 and 2.2 GPa), attributed to twisted β-sheet stack cores [186]. CN serves as an effective agent in urease-induced carbonate precipitation treatment, generating biocemented precipitates with higher compressive strength (2 MPa) and low calcium carbonate content [187]. Surface modification of polylactic acid (PLA) with CN enhances compatibility with cellulose nanofibrils, crucial for fabricating 3D-shaped cellulose-based packaging materials via thermoforming [188]. Food packaging aims to prevent spoilage, extend shelf life, and attract consumers, with bio-composites offering sustainable alternatives to reduce plastic pollution [189]. Active packaging using a chitosan-CN phosphopeptides coating on low-density polyethylene (LDPE) film innovatively delays the softening of freshly cut fruit by inhibiting the activity of cell wall-degrading enzymes, thereby extending shelf life [190]. These can be coupled with antimicrobial and antioxidant agents for smart packaging.
Natural proteins, such as CN, are challenging to process into fibrous forms without additives; however, with polyethylene oxide (PEO) or polyvinyl alcohol (PVA), CN can be electrospun into ultra-thin fibrous membranes [191]. Electrospinning pure CN requires high ethanol content (> 40% v/v), room temperature (> 20°C), and high pH (> 9), necessitating the dissociation of CN micelles [192]. CN-treated cotton fabrics showed flame resistance, suggesting potential industrial applications in continuous processing [193]. Flame retardancy is achieved by applying phosphorus-rich CN with eco-friendly chemicals to cotton fabrics. Ammonium polyphosphate and CN enhance PLAʼs biodegradability while improving flame retardancy [194]. CN-modified rigid polyurethane foam was prepared using a one-step foaming method. The foam containing 6 wt% CN exhibited the highest initial decomposition temperature, superior flame retardant and smoke suppressant properties, and the lowest peak and total heat release rates [195].
CN possesses properties ideal for biomedical materials. CN and chitosan polyelectrolytes were layered on PLA/poly-ε-caprolactone substrates, facilitating efficient drug release [196]. A novel CN-based nanocomposite was developed by incorporating organic montmorillonite (OMMT) and ethylene glycol diglycidyl ether (EGDE). FTIR spectroscopy confirmed the reaction of EGDE with CN, forming a network structure. X-ray diffraction (XRD) and TEM indicated a shift in OMMT structure from exfoliated to intercalated states with increased OMMT content. SEM revealed the nanocompositeʼs high porosity at low OMMT content. The incorporation of OMMT and EGDE enhanced the physical properties, resulting in a reduced swelling ratio, improved thermal stability, and stronger mechanical properties compared to neat CN. A cell counting kit-8 (CCK-8) assay demonstrated the biocompatibility of the nanocomposite films [197]. Lactic acid CN films plasticized with sorbitol exhibited effective mechanical and barrier properties [198]. The incorporation of gelatin-coupled cellulose microgel into the CN matrix enhanced tensile strength and Youngʼs modulus by 6 and 3.5 times, respectively, due to the strong hydrogen bonds between cellulose and CN [199]. A 3D composite scaffold, designed using bioprinting technology, utilizes the synergistic hemostatic action of CN, cellulose nanofibrils, and chitosan to manage blood loss in trauma situations [200].
CN is used to modify the gel structure of whey protein, resulting in decreased sensory firmness and fracturability while increasing cohesiveness and preventing excessive moisture release [201]. Heating mixtures of CN with starch, sucrose, or glucose at temperatures ranging from 37 to 300°C results in up to a 99% loss of chemically available lysine and arginine. This indicates varying susceptibilities to heat-induced damage depending on the carbohydrate present [202]. CN/whey protein mixtures heated at 95°C for 5 min form heat-induced aggregates from CN micelles and heat-denatured whey proteins [203].
Complex formation between whey proteins and κ-CN during milk heat treatment notably influences protein organization in the colloidal CN and serum phases, particularly between the CN micelle and lactoserum [204]. These complexes impact functional properties, especially during the acid gelation of milk in dairy applications [205]. Technological advancements have enabled the production of micellar CNs and milk-derived whey proteins from skim milk using microfiltration, providing unique functional and nutritional benefits for cheesemaking [206]. Cheese yield correlates with milk CN content, particularly the CN to total protein ratio (CN number).
The β-LG genotype is a key variant for optimizing the efficiency of milk protein conversion into cheese [207]. In river buffalo milk, whole CN was separated using cation-exchange chromatography and analyzed with sodium dodecyl sulfate-PAGE. A protein fragment with an estimated molecular weight of 15.3 kDa was identified using matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) peptide mass mapping, offering a potential molecular marker for Mozzarella cheese production [208]. Pre-acidification, or adding acid to milk before starter culture, is utilized in Mozzarella cheese production to reduce calcium levels, which crosslink CN proteins and enhance texture and melting properties [209].
Heat-induced interactions of hemp protein particles with milk proteins, specifically whey proteins and CNs, at 95°C for 20 min demonstrated that CN binding was reversible due to its chaperone-like properties, while whey proteins formed irreversible disulfide bonds with hemp protein particles [210]. A novel micro-foaming extrusion process was developed for producing innovative cheeses, involving the injection of nitrogen at concentrations of 0, 0.05, 0.10, and 0.15% w/w into a hot, plasticized CN melt. This melt is sheared at screw speeds of 250, 300, and 350 rpm during high-moisture extrusion [211].
Chlortetracycline is an antibiotic that spontaneously binds to CN through hydrogen bonding and hydrophobic interactions involving the N(CH3)2 group. This binding extends the half-life of chlortetracycline by 48%, thereby altering its susceptibility to thermal degradation [212]. A stable oil-in-water emulsion utilizing CN-caffeic acid–glucose ternary conjugates has been developed to improve the oral bioavailability of astaxanthin, a lipophilic bioactive. This innovation facilitates the design of functional beverages, pharmaceuticals, and nutritional supplements that can effectively deliver lipophilic bioactives under harsh gastric conditions [213].
An emulsion polymerization strategy has been employed to synthesize biphasic hybrid particles with controlled morphology by adding an ethanol solution of zein as a supporting biomaterial to an aqueous solution of CN. Zein, the major storage protein of corn (45–50%), forms a core surrounded by a methacrylic copolymer, making protein-acrylic hybrid films that are suitable for coating applications [214]. The incorporation of biomaterials can serve to decrease the amount of petroleum-based polymers in the formulation and can also contribute to enhancing the physical properties of the resulting bio-composites [215].
CN/starch composites serve as effective binders for activated carbon black electrodes in capacitive deionization or saltwater desalination. Optimal performance metrics include a salt adsorption capacity of 12.7 mg/g, charge efficiency of 50%, and specific energy consumption of 1.01 W h/g. This performance is credited to the hydrophilic mesoporous network (3.0–4.7 nm pore diameter), which exhibits anion and cation-exchange membrane characteristics [216]. Piezoelectric quartz sensors incorporating MCC demonstrate increased selectivity for detecting highly volatile organic compounds (alcohols, acids, aldehydes, and esters) over water vapor [217]. CN phosphatidylcholine functions as a natural emulsifier, enhancing transmembrane resistance and remodeling lipid homeostasis in lipopolysaccharide-induced intestinal barrier dysfunctions, underscoring its superior biosafety [218].
CN, a naturally-derived, amine-rich protein, is effective in surface functionalization to enhance hydrophilicity and cell adhesion. It constitutes approximately 80% of the total protein content in milk. Thermoplastics have been developed from CN and soybean proteins, incorporating Al2O3 ceramic and tricalcium phosphate to improve mechanical performance, water absorption, and bioactivity in injection molding applications [219]. CN isolated from cow milk using acetic acid and sodium acetate-initiated acid coagulation demonstrated superior heat stability compared to commercial CN [220].
A biphasic calcium phosphate-CN composite incorporating Cassia occidentalis extract acts as an osteo-inductive material, promoting proliferation and increasing alkaline phosphatase activity in SaOS-2 cells [221]. Environmentally friendly techniques have been developed to produce CN fibers from waste milk, overcoming traditional spinning challenges [222]. These fibers exhibit superior moisture absorption, a smooth texture, UV resistance, and antibacterial properties, expanding their applicability in the medical industry [223].
More recently, the applications of re-assembled casein micelles (rCMs) have been investigated; these include their use as nanocarriers of bioactive molecules. The formulation of rCMs, their physico-chemical properties, and their behavior under different heating or pressure treatments have been reviewed, along with their industrial production for use as nanocarriers of bioactive molecules [224]. PVA/CN blend films, prepared by solution casting with varying glycerol concentrations, have demonstrated improved water vapor barrier properties [225]. CN from commercial β-CN-rich skim milk (A2 milk) was chemically modified with methacrylic anhydride and integrated with PVA and polyvinyl pyrrolidone to develop a biodegradable biomaterial for cardiac tissue engineering [226].
Acid CN significantly influenced the hygrothermal and mechanical properties of hemp concrete. Adding 5% dissolved CN enhanced the bonding between the lime binder and hemp shives. Using 3% to 5% by weight of acid CN reduced the need for mixing water due to the biopolymerʼs liquefying properties. A 1% CN admixture decreased thermal conductivity by 1% to 8% [227].
Electrospun composites of polycaprolactone, gelatin, and CN were fabricated into fibrous scaffolds with uniform diameters ranging from 0.1 to 1 µm for cartilage tissue engineering [228]. Artificial CN micelles could revolutionize animal-free food production using recombinant CN. These micelles are larger, more polydisperse, and more mineralized than bovine micelles yet exhibit similar coagulation behaviors and can be readily plasticized [229].
CN-based hollow polymeric spheres were fabricated via emulsifier-free polymerization combined with alkali swelling, enabling tunable visible-light transmittance and anti-ultraviolet properties by adjusting the monomer content. These spheres can form films at room temperature, opening new possibilities for opaque coatings in biomedical, leather, and packaging applications [230].
Organic dyes, often toxic and carcinogenic with low biodegradability, pose risks of bioaccumulation and biomagnification in aquatic ecosystems. A novel flocculant was synthesized from CN-based formulations using PAAm through microwave irradiation. This flocculant effectively treats textile effluent, significantly reducing dye color and heavy metal content [231].
Various dairy products, including skim milk, whole liquid milk, powdered milk, and infant formula, have been identified to contain nanoplastics of differing sizes, shapes, and concentrations. These nanoplastics can interact with proteins, carbohydrates, and fats, negatively affecting the digestion and absorption of these crucial nutrients by the body. Furthermore, the presence of nanoplastics within the gastrointestinal tract may alter the metabolism of lipids, proteins, glucose, and energy, thus heightening the risk of developing multiple health conditions [232]. A new decontamination study successfully removed 94% of polystyrene nanospheres (diameter = 80 nm) by adding just 20 mg of CN powder into 1.6 mL of water initially containing 36 mg of nanoplastics [233]. Decontamination through the addition of CN was evaluated using hydrophobic polystyrene nanospheres, each with a diameter of 80 nm, suspended as a colloidal solution in water. The reduction in nanoplastic concentration, as illustrated in Figure 2a, indicates that some nanoplastics were precipitated upon the introduction of CN. This finding suggests that CN induces aggregation of nanoplastics, forming larger particles that can be efficiently removed via sedimentation and centrifugation. As shown in Figure 2b, a removal efficiency of 92% was achieved by introducing 15 mg of CN powder into 1.6 mL of water, initially containing 36 mg of nanoplastics. Following an additional 5 mg of CN to the water sample, the removal efficiency increased to 94%. The aggregation kinetics of nanoplastics in aquatic environments are affected by several factors, including their adsorption affinity, cation bridging, electrical double layer compression, electrostatic interactions, pH, protein corona formation, precipitation, salt concentration, screening effects, steric hindrance, surface charge, and zeta potential [234].
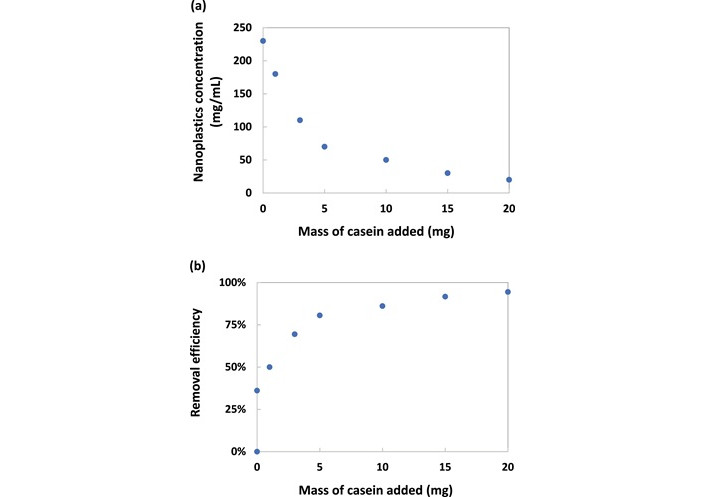
Effect of casein addition on polystyrene nanospheres (diameter = 80 nm) in water. (a) Concentration of nanoplastics remaining in supernatant after addition of casein; (b) efficiency of nanoplastics removal by addition of casein. Taken from [233] without modification. © 2024 The Authors. Licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives (CC-BY-NC-ND) license number 6057960193591.
CNs, as versatile biopolymers, are applied in films, hydrogels, and fibers across various industries [235]. They enhance the biocompatibility of superparamagnetic iron oxide nanoparticles [236] and are widely used in drug delivery systems for effective encapsulation of drugs within nanoparticles [237, 238]. Porous LaMnO3 perovskite has been successfully fabricated using CN as a template to control phase structure, microstructure, morphology, and narrow band gap, with characterization via XRD, SEM, and energy-dispersive X-ray spectroscopy (EDS) [239]. Uniform CN/CaCO3 microspheres have been fabricated with well-tuned properties suitable for bone tissue engineering due to their biocompatibility and ease of fabrication. The vaterite microspheres are formed by mixing CaCl2 and Na2CO3 solutions under stirring with CN, resulting in microspheres composed mainly of nanosized crystals, illustrating CNʼs role in vaterite formation and stabilization [240].
A CN-graft-dextran copolymer, synthesized via the Maillard reaction, achieves molecular solubility in neutral aqueous solutions, despite the insolubility of β-carotene. During dialysis, the solubility dynamics create nanoparticles with a CN/β-carotene core and a dextran shell [241]. Additionally, a click reaction between rod-shaped nanocrystalline cellulose (bearing azide groups) and spherical β-CN micelles (bearing acetylene groups) yields mushroom-like nanoparticles as promising building blocks for advanced materials [242]. The electrostatic adsorption of κ-carrageenan with these micelles forms large particle-sized complexes, influencing the gelʼs rheological properties towards a more uniform and dense gel network [243]. Micellar CN and whey protein ultrafiltration retentates serve as alternative carriers for curcumin, offering functional properties as active food ingredients [244]. Ultrasound-assisted pH shifting of micellar CN alters particle size and zeta potential, enhancing its hydrophobic interactions and bioavailability [245].
CN is extensively used to encapsulate bioactive substances, protecting them from degradation [246]. Crocin/CN nanocomplexes enhance the stability and bioavailability of crocin, benefiting therapeutic applications [247]. Bio-nanocomposites with natural biopolymers and layered silicates improve mechanical properties and thermal stability without compromising biodegradability. CN micro- and nanoparticles facilitate drug and dietary supplement encapsulation through techniques such as gelation and coacervation [248]. Micelles effectively carry oral pharmaceuticals like celecoxib, ensuring stability and bioavailability without additives [249]. The β-CN-derived peptide BCCY-1 mitigates necrotizing enterocolitis by modulating immune responses and enhancing barrier integrity [250].
Growing consumer awareness of health and nutrition has increased demand for functional foods and nutraceuticals enriched with bioactive compounds [251]. CN-based ingredients have a longstanding history in diverse applications. CN nano-formulations, developed via enzymatic crosslinking, graft copolymerization, heat gelation, and ionic complexation, show potential for controlled drug delivery [252]. Enhancements through the Maillard reaction with various oligosaccharides decrease particle size, reduce hydrophobicity, and improve thermal stability [253]. Exfoliated or intercalated nanocomposites with sodium caseinate improve drug release properties for applications as tablet coatings [254]. CN/gliadin nanoparticles effectively encapsulate amphotericin B, maintaining stability in gastrointestinal fluids with reduced cytotoxicity [255]. Adjusting pH and xanthan gum concentration enhances CN-based complexesʼ stability for novel food formulations [256]. CN alginate macro-capsules show promise as carriers for lipophilic compounds [257]. CN micelles protect β-carotene during industrial processing [258]. Advances in encapsulating fucoxanthin support its use in food and nutraceutical industries [259]. Microbial fermentation releases functional peptides from milk proteins with therapeutic potential, highlighting CNʼs multifaceted roles. Peptides from αs1- and β-CNs exhibit antihypertensive effects [260]. Cow milk fermented with Lactobacillus rhamnosus and Lactobacillus plantarum demonstrates angiotensin-I-converting enzyme (ACE) inhibition activity of up to 90% [261]. Bioactive peptides generated from lactoferrin, a glycoprotein derived from both bovine and human milk, exhibit inhibitory potential against ACE. Given their functional similarities, bovine lactoferrin could serve as a promising alternative to human lactoferrin [262]. Encapsulating probiotics in CN-based microparticles ensures high bacterial survival [263]. Hydrolyzed CN peptide tablets significantly lower blood pressure in hypertensive patients, demonstrating their potential as functional foods [264]. In these nano-formulations, CN plays a central role in determining the chemical compositions and molecular structures. This categorization breaks down the applications and research avenues in CN nano-formulations, offering detailed insights into their central roles, processes, and benefits across various scientific and industrial contexts.
Natural polymeric and biodegradable compounds are pivotal in the development of biomaterials, including packaging films, implanted devices, and carriers for bioactive ingredients. Their scientific merit stems from their ability to degrade and be metabolically absorbed after use. Key biodegradable natural polymers include polysaccharides such as alginate, chitin, chitosan, and starch, as well as proteins. CNs, a primary biopolymer in milk, exhibit a wide range of physical and biological properties applicable in the food, material, and pharmaceutical industries, as summarized in Figure 3.
Physical and biological properties of casein as applicable in food materials and pharmaceutical sciences.
Most CN applications typically involve soft materials such as hydrogels; however, conjugation of CN with hard polymers presents the possibility of creating biodegradable materials suitable for novel applications. The absence of a tertiary structure in CN permits flexible binding with various polymers, but transforming CN into hard materials necessitates a comprehensive understanding of the chemical groups on hard material surfaces to facilitate effective linkages. The prevalent method involves grafting methacrylate polymers onto the CN backbone, commonly employing glycidyl methacrylate (GMA) to inhibit homopolymer formation and enable targeted polymerization. While it is relatively straightforward to create non-specific linkages, current efforts are directed toward achieving site-specific linkages to enhance material properties and functions. CN proteins are abundant in amino, carbonyl, carboxyl, hydroxyl, and phosphate functional groups, which enable property enhancement through controlled chemical coupling with agents such as glycerol or stearic acid [265]. The mechanical and thermal properties of the conjugates can be fine-tuned by adjusting polymer grafting. Protein–polymer conjugates integrate the mechanical strength of polymers with the bioactive functionality of proteins, thereby offering a wide array of biological properties. Due to these enhanced properties, such conjugates are utilized as biocompatible components in diverse biomedical applications [266]. Emerging research is focused on improving the mechanical robustness, tensile strength, and water vapor permeability of CN-based biomaterials to match or surpass the performance of petroleum-based polymers. This pursuit could profoundly impact industries that seek sustainable material alternatives. Continued exploration of synthesis techniques and scalability is crucial for future advancements. Future research directions could focus on advanced analytical techniques for structural analysis [267], clinical trials to assess health impacts [268, 269], and innovative processing technologies to optimize CN for diverse applications [270].
ACE: angiotensin-I-converting enzyme
BCM-7: β-casomorphin-7
CN: casein
EGDE: ethylene glycol diglycidyl ether
FPLC: fast protein liquid chromatography
FTIR: Fourier transform infrared
GNPs: gold nanoparticles
MCC: micellar casein concentrate
OMMT: organic montmorillonite
PAAm: polyacrylamide
PAGE: polyacrylamide gel electrophoresis
PLA: polylactic acid
PVA: polyvinyl alcohol
rCMs: re-assembled casein micelles
SEM: scanning electron microscopy
TEM: transmission electron microscopy
XRD: X-ray diffraction
α-LA: α-lactalbumin
β-LG: β-lactoglobulin
AI-Assisted Work Statement: During the preparation of this work, EPCL used GovAI.com for sourcing scientific literature to explore the scope of casein chemistry. After using the tool, EPCL reviewed the findings as appropriate and takes full responsibility for the content of the publication.
EPCL: Conceptualization, Investigation, Visualization, Writing—original draft, Writing—review & editing. AT: Investigation, Visualization, Writing—review & editing. Both authors read and approved the submitted version.
Both authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
