Affiliation:
1Department of Social Economy and Family Management, HTTTC, University of Buea, Kumba P.O. Box 249, Cameroon
Email: silapeuxkamda@gmail.com
ORCID: https://orcid.org/0000-0003-4943-8065
Affiliation:
2Laboratory of Process Engineering, University of Douala, Douala P.O. Box 2701, Cameroon
Affiliation:
2Laboratory of Process Engineering, University of Douala, Douala P.O. Box 2701, Cameroon
Affiliation:
3Laboratory for Food Sciences and Metabolism, Department of Biochemistry, University of Yaoundé I, Yaoundé P.O. Box 812, Cameroon
Affiliation:
3Laboratory for Food Sciences and Metabolism, Department of Biochemistry, University of Yaoundé I, Yaoundé P.O. Box 812, Cameroon
Affiliation:
2Laboratory of Process Engineering, University of Douala, Douala P.O. Box 2701, Cameroon
Affiliation:
4Department of Agriculture, livestock and derivated product, National Advanced School of Engineering of Maroua, University of Maroua, Maroua P.O. Box 46, Cameroon
Affiliation:
3Laboratory for Food Sciences and Metabolism, Department of Biochemistry, University of Yaoundé I, Yaoundé P.O. Box 812, Cameroon
Affiliation:
3Laboratory for Food Sciences and Metabolism, Department of Biochemistry, University of Yaoundé I, Yaoundé P.O. Box 812, Cameroon
Explor Foods Foodomics. 2025;3:101096 DOI: https://doi.org/10.37349/eff.2025.101096
Received: April 08, 2025 Accepted: July 03, 2025 Published: August 21, 2025
Academic Editor: Zuhaib F Bhat, SKUAST-Jammu, India
Aim: Underexploited local plant resources, such as tiger nuts, have significant nutritional potential. Tiger nuts can be used to produce a plant-based yoghurt that would enable people suffering from lactose intolerance to enjoy its benefits. This study aimed to evaluate the sensory acceptability, physicochemical, and nutritional properties of a yoghurt-like product made with tiger nuts pretreated by various methods (soaking, drying, roasting, boiling, and germination).
Methods: Six types of vegetable yoghurt were produced from the milk obtained after different pretreatment methods (soaking, germination, roasting, drying, and boiling) of tiger nuts. A control sample was made of untreated tiger nuts. Nutritional analyses were carried out using conventional methods. Quantifying bioactive and antinutrient compounds was conducted via spectrophotometry and titration methods. Physicochemical analysis of samples was also carried out. A consumer preference test was conducted using an untrained panel.
Results: Yoghurt samples made with tiger nuts, pretreated through roasting, showed the best sensory characteristics and overall acceptability. Drying of tiger nuts resulted in a significantly higher energy value, as did the protein content (5.46%) in the germinated yoghurt and the fibre content (2.80%) in the boiled yoghurt. Syneresis and water holding capacity decrease slightly during all the pretreatment methods applied. With regards to bioactive compounds, phenolic compounds [393.39 mg GAE (gallic acid equivalent)/100 g DM (dry matter)] were more abundant in the yoghurt whose seeds were boiled, while the content of alkaloids [1,178.08 mg QE (quinine equivalent)/100 g DM] was higher in the products made with roasted tiger nuts. Roasting and germination were respectively the most effective pretreatment methods for the reduction of the amount of phytate and saponin.
Conclusions: Production of yoghurt with roasted tiger nuts appears to be the best option in terms of its sensory attributes and its nutritional properties. Its consumption will contribute significantly to improving the nutrient and bioactive compounds intake for those suffering from lactose intolerance.
Yoghurts are dairy products made by fermenting cows’ milk in the presence of lactic acid bacteria such as Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus salivarius subsp. thermophilus [1]. The scarcity and high cost of cows’ milk, coupled with the problems of lactose intolerance that affect around 70% of the world’s population [2], have led researchers in the field of food science to develop plant-based yoghurts. Numerous plant-based yoghurts have since been developed from Bambara seed [3], soybeans [4], coconut cream and modified maize starch or cashew “milk” and tapioca starch [5]. Yoghurts made from plant sources are cheaper and have considerable nutritional and therapeutic potential. Tiger nuts, also known as yellow nutsedge is one of the most underutilized plant resources in Africa, yet it is rich in amino acids [6], has high levels of essential fatty acids [7], dietary fibres, vitamins, minerals [8], and phytochemicals such as phenolics, flavonoids, terpenoids, alkaloids, and saponins [9]. Recent studies have demonstrated some biological effects of tiger nuts, such as antidiabetic, cholesterol-lowering, hepatoprotective, aphrodisiac [10], antibacterial, and galactogenic [11]. In Cameroon, tiger nuts are processed locally into milk and then into yoghurt in small production units using the traditional method of extracting milk from seeds that have been soaked for at least 24 hours. This sector is slow to expand because the sensory qualities of the yoghurts produced are not always appreciated by consumers. Numerous studies have reported the effect of pretreatment methods like soaking, germination, drying, boiling, and roasting to be effective in improving the physicochemical and physiological properties of processed foods from plants [12–14]. Adekanmi et al. [15] and Frank et al. [16] have shown that soaking increases some nutrients by acting on chemically bound compounds, thereby making them readily available. Like soaking, boiling, germination, roasting, pregelatinization, and drying of plant products significantly reduce the content of antinutritional factors (oxalate, phytates, saponins, trypsin inhibitors), and have a positive effect on the sensory characteristics of the final product [17–19]. Studies conducted by Ketharin et al. [20] and Chen et al. [21] have revealed that there is an increase in the content of phenolic compounds after roasting and soaking of tiger nuts, cashew nuts and almonds. Fermentation of milk from plants such as Bambara groundnuts, tiger nuts, or coconuts is of particular interest because of the prospects for generating lactose-free yoghurt-like products with improved microbial stability and extended shelf life with acceptable sensory values [22, 23]. Such fermented systems could hold promise as a valuable alternative source of nutrients, particularly in many developing countries where children, adults, and the elderly have a high prevalence of lactose intolerance with limited access to nutritious foods [24]. Although plant-based vegetable yoghurt is so important, studies on the effects of pretreatment methods (soaking, drying, roasting, boiling, and germination) on the sensory and nutritional properties of tiger nut yoghurts are scarce. The aim of this work was to evaluate the sensory, physicochemical, and nutritional properties of a yoghurt-like product made from tiger nuts pretreated using various methods (soaking, drying, roasting, boiling, and germination).
Dried and mature tiger nuts (Cyperus esculentus L.) and starter culture were purchased at the Mokolo market (Yaoundé, Cameroon). Once at the Laboratory of Food Sciences and Metabolism (LabSAM) of the University of Yaoundé I, where the experiments took place, those nuts were sorted to get rid of any foreign bodies and/or deteriorated ones. The starter culture required was two weeks old, homemade plain yoghurt, kept in a refrigerator (4°C), and made of a mixture of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus salivarius subsp. thermophilus.
Six kinds of vegetable yoghurt were produced from the milk obtained after different pretreatment methods of the tiger nuts. The pretreatment methods used were soaking, germination, roasting, drying, and boiling. The control sample encoded “TeYS” was prepared with tiger nuts that had not undergone any pretreatment. For soaking pretreatment, approximately 500 g of tiger nuts were soaked for 24 h in 3 L of distilled water. The water was changed every 3 h to avoid spontaneous fermentation. Germination consists of spreading out 500 g of seeds on jute bags, which were moistened every 12 h for 72 h to stimulate germination. For roasting, 500 g of dried nuts were pan-roasted (open roasting method) at about 200°C for 15 min. For drying, 500 g of nuts were dried in an oven at 45°C for 48 h, and for boiling, 500 g of nuts were introduced into 3 L of boiling water for 30 min. Milk extraction was carried out according to the modified protocol of Bertrand-Harb et al. [24] (Figure 1A). The various milk samples obtained previously were pasteurised at 65°C for 15 min before being cooled to a temperature of approximately 40°C. One litre of pasteurised milk was homogenised using a mixer before being inoculated with the starter at a concentration of 3% (w/v) (Figure 1B). The resulting preparation was incubated at 45°C for 8 h. The yoghurts obtained were refrigerated in sterile plastic bottles and coded.
The codes TYS, EYS, SYS, GYS, and RYS were assigned to the yoghurt samples produced from tiger nuts pretreated with the following methods, respectively: soaking, boiling, drying, germination, and roasting.
The pH value was determined by direct reading with the digital pH metre (Hanna HI-98128) as given in KC and Rai [25].
Titratable acidity was determined by neutralising the acid present in 10 g of the yoghurt samples using 0.11 M NaOH solution. The titration was performed using 10 drops of phenolphthalein as an indicator until a pink endpoint was reached. The quantity of NaOH used to neutralise the solutions was divided by 10 in order to obtain the titratable acidity.
The degree of syneresis was measured by a method used by Lee and Lucey [26]. A 100 g sample of yoghurt was placed on a filter paper resting on the top of a funnel. After 10 min of drainage under vacuum conditions, the quantity of the remained yoghurt was weighed and syneresis was calculated as follows:
The method described by Silva and O’Mahony [27] was followed for the analysis of water holding capacity (WHC). In brief, 20 g of yoghurt sample was placed in 50 mL tubes (Thermo Fisher Scientific, Waltham, MA, USA) and centrifuged at 640 × g for 20 min at 4°C in a Sorvall RC 5C Plus centrifuge, equipped with a Sorvall SS-34 rotor (Du Pont Instruments, Wilmington, DE, USA), after which the supernatant was collected and weighed. WHC was calculated according to the equation below:
The method described by AFNOR [28] was adopted for the determination of the moisture content of the vegetable yoghurt sample. A clean crucible was dried to a constant weight in an air oven at 110°C, cooled in a desiccator, and weighed (W1). Two grams of the yoghurt sample were accurately weighed into the previously labelled crucible and reweighed (W2). The crucible containing the sample was dried in the same oven to constant weight (W3). The percentage moisture content was calculated thus:
The methodology set by AFNOR [28] was used for the determination of ash content. The porcelain crucible was dried in an oven at 100°C for 10 min, cooled in a desiccator, and weighed (W1). Two grams of the vegetable yoghurt sample was placed into a previously weighed porcelain crucible and reweighed (W2). It was first ignited and then transferred into a furnace, which was set at 550°C. The sample was left in the furnace for eight hours to ensure proper ashing. The crucible containing the ash was then removed, cooled in a desiccator, and weighed (W3). The percentage ash content was calculated as follows:
The determination of crude lipid content was done with a Soxhlet apparatus according to the method described by Bourely [29]. A clean, dried 500 cm3 round-bottom flask containing a few anti-bumping granules was weighed (W1) with 300 cm3 of hexane for extraction poured into the flask, and filled with a Soxhlet extraction unit. The extractor thimble weighing twenty grams was fixed into the Soxhlet unit. The round-bottom flask and a condenser were connected to the Soxhlet extractor, and cold water circulation was connected/put on. The heating mantle was switched on, and the heating rate adjusted until the solvent was refluxing at a steady rate. Extraction was carried out for 6 hours. The solvent was recovered, and the oil was dried in an oven set at 70°C for 1 hour. The round-bottom flask and oil were then weighed (W2). The lipid content was calculated thus:
The determination of crude fibre was done according to the method described by AOAC [30]. The defatted yoghurt sample (1 g) was weighed into a round-bottom flask, 100 cm3 of sulphuric acid solution (0.25 M) was added, and the mixture was boiled under reflux for 30 min. The hot solution was quickly filtered under suction. The insoluble matter was washed several times with hot water until it was acid-free. It was quantitatively transferred into the flask, and 100 cm3 of hot 0.31 M NaOH solution was added. The mixture was boiled under reflux for 30 min and filtered under suction. The residue was washed with boiling water until it was base-free, dried to constant weight in an oven at 100°C, cooled in a desiccator, and weighed (C1). The weighed sample (C1) was then incinerated in a muffle furnace at 550°C for 2 h, cooled in a desiccator, and reweighed (C2). Calculation: the loss in weight on incineration = C1 – C2
Crude protein content was determined following the Kjeldahl method [31] for the mineralisation of nitrogen, and the method of Devani et al. [32] for the titration of nitrogen.
The ground defatted sample (91.5 g) in an ashless filter study was dropped into a 300 cm3 Kjeldahl flask. The flask was then transferred to the Kjeldahl digestion apparatus. The sample was digested unit a clear green color was obtained. The digest was cooled and diluted with 100 cm3 of distilled water.
Distillation of the digest: into a 500 cm3 Kjeldahl flask containing antibumping chips and 40 cm3 of 40% NaOH was slowly added to a flask containing mixture of 50 cm3 2% boric acid and 3 drops of mixed indicator was used to trap the ammonia being liberated. The conical flask and the Kjeldahl flask were then placed on the Kjeldahl distillation apparatus, with the tubes inserted into the conical flask. Heat was applied to distil out the NH3 evolved, with the distillate collected into the boric acid solution. The distillate was then titrated with 0.1 M HCl. Calculation:
where,
M = actual molarity of acid, V100 = titre value (volume) of HCl used, Vt = total volume of diluted digest.
Carbohydrate contents were determined according to the Association of Official Analytical Chemists [33]. The carbohydrate value was expressed as the difference of the protein, fat, crude fibre, total ash, and moisture content of the sample from 100%.
The total phenolic content and the total flavonoid content were determined according to the methods described by Chandra et al. [34]. For total phenolic compounds, Folin-Ciocalteu’s reagent (10%) and Na2CO3 (7%) were used. Gallic acid solutions in methanol (5–500 mg/L) were prepared for the standard curve, and total phenolic content was calculated as mg gallic acid equivalent per gram (mg GAE/g) of fresh weight of the sample. Total flavonoid content of the extract was investigated using the aluminium chloride colourimetric method. The standard calibration curve was prepared for quercetin; the total flavonoid content was expressed as milligrams quercetin equivalent per gram of extracted sample (mg QCE/g sample) based on a standard curve of quercetin.
The total alkaloid content in ethanolic extracts of yoghurt was determined according to the method employed by Singh et al. [35], using quinine as a standard. The total alkaloid content of the samples was measured using 1,10-phenanthroline. The reaction mixture contained 1 mL of yoghurt extract, 1 mL of 0.025 M ferric chloride in 0.5 M hydrochloric acid, and 1 mL of 0.05 M 1,10-phenantroline in ethanol. The mixture was incubated for 30 minutes in a hot water bath with a maintained temperature of 70°C. The absorbance of red colored complex was measured at 510 nm against the reagent blank. Alkaloid contents were estimated, and it was calculated with the help of a standard curve of quinine.
The titration method described by Aina et al. [36] was used to determine the oxalate content of the sample. One gram of SYS was macerated with 75 mL of 3 M H2SO4 in a conical flask. The mixture was stirred with a magnetic stirrer for 1 h and filtered. The filtrate (25 mL) was collected and heated to 80°–90°C, then maintained at 70°C. The hot aliquot was titrated continuously with 0.05 M KMnO4 until the endpoint of a light pink color, which persisted for 15 s. The oxalate content was calculated by taking 1 mL of 0.05 M KMnO4 as equivalent to 2.2 mg oxalate.
The phytic acid concentration was determined using Wade reagents of 0.03% FeCl3·6H2O and 0.3% sulfosalicylic acid. A standard phytic acid curve was constructed under the same conditions, and results were expressed as phytic acid mg/100 g of fresh weight of the sample [37].
Saponin content was determined by weight difference after extraction in solvent [38]. To a conical flask containing 50 mL of 20% aqueous ethanol, 2 g each of the samples was added. With continuous stirring, the samples were heated over a hot water bath (at about 55°C) for 4 hours, then filtered. The residue was re-extracted with another 50 mL of 20% ethanol. This was followed by the reduction of combined extracts to 40 mL over a water bath at 90°C. Twenty millilitres of diethyl ether was added and shaken vigorously after the concentrates were transferred into a 250 mL separator funnel. The purification process was repeated after recovery of the aqueous layer and discarding of the ether layer. This is followed by the addition of 60 mL of n-butanol extract, then washing twice of the combined n-butanol (extracted) in 10 mL of aqueous sodium chloride. The remaining solution was heated in a water bath. After evaporation, the sample was dried in the oven to a constant weight after evaporation. The saponin content was calculated as a percentage.
Where B = weight of Whatmann filter paper, A = weight of Whatmann filter paper with sample, and S = sample weight.
The condensed tannin content of samples was determined by Ndhlala et al. [39]. Three millilitres of butanol-HCl reagent (95:5, v/v) was added to 500 μL of each extract, followed by 100 μL ferric reagent (2% ferric ammonium sulphate in 2 M HCl). The test combination was vortexed and placed in a water bath (100°C) for 60 min. The absorbance was then read at 550 nm against a blank prepared in a similar way, but without heating. Each extract had three replicates. Condensed tannin (%) was calculated as leucocyanidin equivalents using the formula developed by Porter et al. [40]:
With A550, the absorbance of the sample at 550 nm and Rd is the extraction yield.
A consumer acceptance test was done with 60 untrained panellists of both genders who were all consumers of tiger nuts. The panellists aged between 18 and 36 years old were recruited among the students at the University of Yaoundé I. A preference test was used to measure factors such as aroma, taste, color, texture, and general acceptability by scoring them on a nine-point hedonic scale (1 means the yoghurt is extremely unpleasant and 9 means it is extremely pleasant). The souchet yoghurt evaluation was repeated three times in sessions of 2.5 h per day in the morning for three days [41]. Yoghurts with pretreated seeds and the control were presented to each panellist and labelled with a random three-letter code. Operationally, 50 mL of each sample of fresh tiger nuts yoghurt contained in opaque jars was presented simultaneously to panellists who had been fasting for at least 6 h. The panellists were instructed to rinse their mouths thoroughly with mineral water between samples. The evaluations were conducted in a well-lit room with white fluorescent lights.
The statistical analysis was carried out using a one-way analysis of variance (ANOVA) for chemical composition and sensory acceptability data. The experiments were run in triplicate. Means were separated using Turkey’s (HSD) test, and p-values < 0.05 at a 95 percent confidence interval were considered significant. Principal component analysis (PCA) was designed to further elucidate the effects of different pretreatment methods on the proximate contents, general acceptability, and bioactive compounds. All the figures were organised using the XLSTAT (2016) software.
In this study, the quality control of vegetable yoghurt samples was carried out by determining some of their physicochemical characteristics. Table 1 below shows the physicochemical characteristics (pH, total titratable acidity, syneresis, and WHC) of the yoghurt samples immediately after manufacture.
pH, total titratable acidity, syneresis, and water holding capacity of tiger nut yoghurts
| Samples | pH | Total titratable acidity (%) | Syneresis (%) | Water holding capacity (%) |
|---|---|---|---|---|
| RYS | 4.75 ± 0.01d | 0.89 ± 0.00a | 18.18 ± 1.64a | 39.03 ± 1.05b |
| TYS | 4.21 ± 0.02a | 1.49 ± 0.01e | 20.08 ± 1.82b | 39.06 ± 1.72b |
| EYS | 4.51 ± 0.02c | 1.08 ± 0.01b | 18.38 ± 1.57a | 38.12 ± 0.34a |
| SYS | 4.37 ± 0.03b | 1.28 ± 0.01c | 18.53 ± 1.62ab | 38.32 ± 1.69ab |
| GYS | 4.23 ± 0.02a | 1.42 ± 0.02d | 19.00 ± 2.78b | 38.73 ± 1.37b |
| TeYS | 4.11 ± 0.01a | 1.88 ± 0.01f | 20.32 ± 1.07b | 39.73 ± 1.35b |
RYS (roasting); TYS (soaking); EYS (boiling); SYS (drying); GYS (germination); TeYS (control). Values are expressed as mean ± standard deviation (n = 3). Values marked with different letters in the same column are significantly different (p < 0.05)
The pH values of the manufactured products range from 4.11 in the TeYS to 4.75 in the RYS. Conversely, the titratable acidity varies from 0.89% in RYS to 1.88% in TeYS.
With regard to syneresis and WHC, no significant differences were observed between the different products obtained. This suggests that fermentation did not have any significant effect on these parameters. Among these products, RYS have the lowest syneresis values (18.18%), while TeYS (39.73%) have the highest WHC.
Table 2 presents the proximate composition of yoghurt samples made from pretreated tiger nuts. A significant variation (p < 0.05) in the nutrient composition of the different yoghurts was recorded as a function of the pretreatments applied to the tiger nuts. Pretreatment of tiger nuts by drying resulted in a significantly higher fat content [8.89 g/100 g DM (dry matter)], as did the protein content (5.46 g/100 g DM) in the GYS. The ash content (1.67%), which represents the total mineral content, was higher in the RYS, while boiling of tiger nuts led to a yoghurt sample with the highest fibre content (2.80%). The higher level of ash in RYS could provide more minerals to the organism. The protein content ranged between 4.01% to 5.46% while the fibre content ranged between 0.55% to 2.8 %. The drying of tiger nuts for the formulation of yoghurt gives it a higher energy value compared with other pretreatments applied to tiger nuts, 165.81 ± 7.85, which could be explained by the high lipid content of this yoghurt. The germination of tiger nuts results in a higher protein content in the yoghurt. Moisture contents ranged between 67.54% and 75.98% and were relatively higher than those of cow milk yoghurt.
Macronutrient content of yoghurts
| Pretreatments | Fat content (g/100 g DM) | Protein content (g/100 g DM) | Carbohydrate content (g/100 g DM) | Ash content (g/100 g DM) | Fibre content (g/100 g DM) | Moisture (g/100 g DM) | Energy (kcal) |
|---|---|---|---|---|---|---|---|
| RYS | 6.81 ± 0.07d | 4.01 ± 0,08a | 18.12 ± 0.04a | 1.67 ± 0.00e | 1.39 ± 0.03d | 67.74 ± 0.15a | 149.81 ± 1.11d |
| TeYS | 4.98 ± 0.15a | 4.68 ± 0.08cd | 12.37 ± 0.14e | 1.47 ± 0.04d | 0.55 ± 0.02a | 75.98 ± 0.13c | 113.02 ± 2.23a |
| TYS | 6.78 ± 0.24d | 4.35 ± 0.00b | 12.65 ± 0.18c | 1.03 ± 0.06c | 1.67 ± 0.06c | 73.52 ± 0.16b | 129.02 ± 2.88c |
| EYS | 5.53 ± 0.30c | 4.58 ± 0.08c | 12.86 ± 0.50c | 0.83 ± 0.03a | 2.80 ± 0.15e | 73.60 ± 0.45b | 119.53 ± 5.02ab |
| SYS | 8.89 ± 0.61e | 4.78 ± 0.08c | 16.67 ± 0.51a | 0.99 ± 0.00bc | 1.13 ± 0.07b | 67.54 ± 0.13a | 165.81 ± 7.85e |
| GYS | 3.79 ± 0.30b | 5.46 ± 0.08e | 15.37 ± 0.31d | 0.87 ± 0.03ab | 1.05 ± 0.08b | 73.47 ± 0.18b | 117.43 ± 4.26a |
RYS (roasting); TYS (soaking); EYS (boiling); SYS (drying); GYS (germination); TeYS (control). Values are expressed as mean ± standard deviation (n = 3). Values marked with different letters on the same line are significantly different (p < 0.05). DM: dry matter
The bioactive compounds in the yoghurts are shown in Figure 2. A large significant variation (p < 0.05) in the concentration of bioactive compounds in the different yoghurts was recorded in relation to the pretreatments. The concentrations of phenolic compounds (Figure 2A), flavonoids (Figure 2B), and alkaloids (Figure 2C) were significantly affected by the pretreatment methods. Boiling tiger nuts had the greatest significant influence (p < 0.05) on the concentration of phenolic compounds in the yoghurt samples, whereas roasting those nuts gave the highest concentration of flavonoids and alkaloids compared with the other pretreatment methods and the control. In fact, boiling the tiger nuts to formulate the yoghurt resulted in the highest content of phenolic compounds (393.39 ± 1.65 mg GAE/100 g DM).
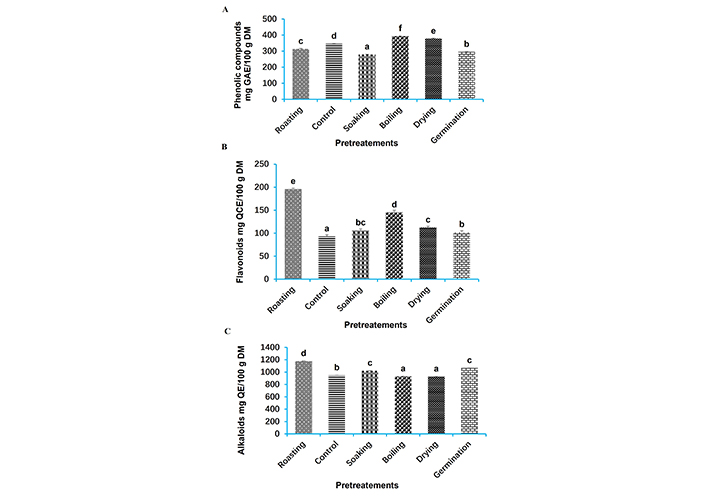
Levels of bioactive compounds in tiger nut yoghurts. (A): Phenolic compounds, (B): flavonoids, (C): alkaloids. Pretreatment values marked with different letters on the bar chart are significantly different (p < 0.05). DM: dry matter; GAE: gallic acid equivalentr; QCE: quercetin equivalent; QE: quinine equivalent
A significant decrease (p < 0.05) in all the antinutrients assessed was observed after pretreatment of the tiger nuts (Table 3). The rate of decrease was greatest in the yoghurt for saponins (68%; 17.84 mg/100 g DM) with germinated tiger nuts, for phytates (43%; 0.37 mg/100 g DM) with roasted tiger nuts, and tannins (69%; 7.83 mg/100 g DM) with dried tiger nuts.
Levels of antinutrients in tiger nut milk from different pretreatments
| Pretreatments | Oxalate content mg/100 g DM | Phytate content mg/100 g DM | Saponin content mg/100 g DM | Tanins content mg/100 g DM |
|---|---|---|---|---|
| RYS | 0.37 ± 0.02c | 0.37 ± 0.00a | 47.51 ± 4.18c | 13.57 ± 1.04b |
| TeYS | 0.63 ± 0.03d | 0.65 ± 0.00f | 55.51 ± 2.75d | 25.04 ± 0.00d |
| TYS | 0.32 ± 0.01b | 0.46 ± 0.00d | 44.09 ± 4.25c | 22.40 ± 0.90d |
| EYS | 0.26 ± 0.01b | 0.41 ± 0.01c | 24.18 ± 1.17ab | 19.30 ± 0.91c |
| SYS | 0.39 ± 0.01c | 0.58 ± 0.00e | 26.11 ± 0.00b | 7.83 ± 1.57b |
| GYS | 0.26 ± 0.00a | 0.39 ± 0.00b | 17.84 ± 0.00a | 15.65 ± 0.00b |
RYS (roasting); TYS (soaking); EYS (boiling); SYS (drying); GYS (germination); TeYS (control). Values are expressed as mean ± standard deviation (n = 3). Values marked with different letters on the same line are significantly different (p < 0.05). DM: dry matter
Table 4 below shows the average scores for the sensory attributes attributed by panellists following the preference test on the various samples of tiger nut yoghurt produced. This shows that, with the exception of the taste of TYS, the sensory attributes of all the other products received average scores of over 6 (a little pleasant). It should be noted that for each sensory attribute, the average score awarded is correlated with the pane’s assessment. The average scores attributed to smell (7.98) and taste (7.23) were higher for RYS. TYS received the highest average score for texture (7.98), while EYS received the highest average score for color (7.70).
Average scores for the sensory attributes
| Yoghurt | Color | Odor | Texture | Taste |
|---|---|---|---|---|
| RYS | 7.67 ± 0.18b | 7.98 ± 0.18b | 6.84 ± 0.32a | 7.23 ± 0.29c |
| TeYS | 7.49 ± 0.26b | 6.95 ± 0.22a | 7.60 ± 0.26b | 6.91 ± 0.25ab |
| TYS | 6.86 ± 0.22ab | 6.77 ± 0.23a | 7.98 ± 0.24c | 5.95 ± 0.27a |
| EYS | 7.70 ± 0.23b | 6.81 ± 0.25a | 7.35 ± 0.24ab | 6.81 ± 0.28ab |
| SYS | 6.53 ± 0.22a | 7.09 ± 0.19ab | 7.05 ± 0.29a | 6.93 ± 0.26ab |
| GYS | 7.30 ± 0.27ab | 6.79 ± 0.23a | 7.79 ± 0.34bc | 6.00 ± 0.30a |
RYS (roasting); TYS (soaking); EYS (boiling); SYS (drying); GYS (germination); TeYS (control). Values are expressed as mean ± standard deviation (n = 3). Values marked with different letters on the same line are significantly different (p < 0.05)
Figure 3 below shows the general acceptability of tiger nut yoghurt samples made by panellists. RYS had the highest score, followed by the TeYS, EYS, SYS, TYS, and GYS. Significant differences (p < 0.05) were found between these samples, namely GYS, TeYS, SYS, and EYS. However, more than 53.48% of panellists said they liked RYS, which is in line with the results. Taste and texture were the main contributors to this score, but odor and color were sensory attributes that had a negative impact on overall acceptability.
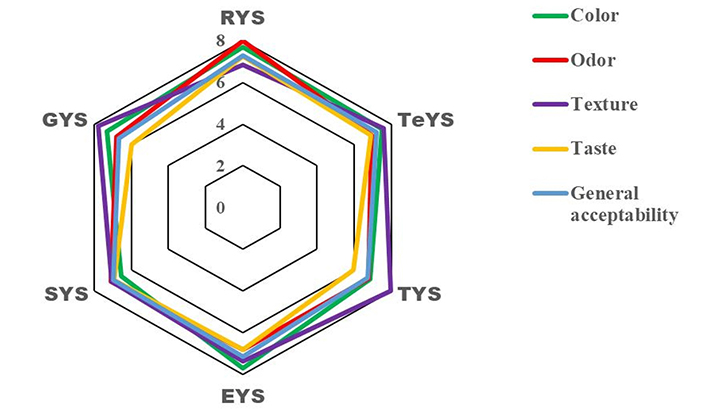
General acceptability of tiger nut yoghurts. RYS (roasting); TYS (soaking); EYS (boiling); SYS (drying); GYS (germination); TeYS (control)
The physicochemical characteristics (pH, total titratable acidity, syneresis, WHC) of the yoghurt samples represent not only the indicators of fermentation, but also the indicators of quality and the criteria of consumer preferences for this category of food product [26, 42, 43]. The value of the pH and titratable acidity of the yoghurt samples corroborate those obtained by Odejobi et al. [44], who found that the pH and titratable acidity of tiger nut yoghurts ranged from 3.93 to 5.06 and from 0.45 to 2.04% respectively. WHC is an important parameter of yoghurts, linked to their ability to form gels. In fact, acidification of the medium during fermentation leads to protein denaturation, aggregation, and network formation that captures water molecules [4]. This parameter is particularly important because it is responsible for the texture of yoghurt and essential for microbial growth [45–47]. Syneresis, on the other hand, is an undesirable parameter because it reflects the capacity of yoghurt to lose water. Vegetable yoghurts with low syneresis have a stable texture. This indicates that these products have good water retention capacity and a greater capacity to maintain the stability of a gel [48].
The higher level of ash in RYS was lower than the average ash content in tiger nuts, according to Muhammad et al. [49], which is 2.2%. These results of protein and crude fibre contents are similar to those found by Akoma et al. [50] and Matela et al. [51]. The fibre content of the yoghurt increased according to the type of pretreatment applied to the tiger nuts prior to the formulation of the yoghurt. Because of its fibre content, EYS, particularly, could facilitate digestion. Crude fibre or insoluble fibre speeds up the transit of food through the digestive system and, by so doing, promotes the regularity or evacuation of stools [52]. The higher content of protein in yoghurt samples obtained through the germination of tiger nuts is due to the activation of proteolytic enzymes during germination, such as proteases, which break down reserve proteins into amino acids that can then be used to synthesise new proteins [53, 54]. The value of the moisture content, which ranged between 67.54 and 75.98%, is in agreement with a similar study by Cheng [55], who revealed that high moisture in vegetable yoghurts such as soy yoghurt is present as free water that is not incorporated in the coagulated protein. This implies the occurrence of syneresis during coagulation. In addition, high moisture content in yoghurt is typical and essential for its refreshing and thirst-quenching properties. However, such a level of hydration requires that the product be stored in a cold environment to prevent microbial growth, and thus extend its shelf life [56]. The range of crude fat content was higher compared to that found by Bristone et al. [57] in tiger nut yoghurt from Nigeria. Fat is a major component in these samples aside from moisture content, contributing to the overall texture and mouthfeel of the yoghurt. Fats also provide essential fatty acids and aid in the absorption of fat-soluble vitamins [58]. The yoghurt with dried tiger nuts contained more lipids than the other yoghurts. This could be explained by the pretreatment method, i.e. drying, which makes the lipids contained in the nuts more available. Since fat contributes to energy, tiger nut yoghurt made from dry nuts could provide the body with a considerable amount of energy.
EYS have the highest content of phenolic compounds (393.39 ± 1.65 mg GAE/100 g DM). This would be due to the heat that would allow the degradation of the cell walls of the tiger nut seeds, thus releasing the bound phytochemical compounds, and boiling can activate the enzymes responsible for the biosynthesis of these compounds, thus increasing their concentration [59, 60]. Roasting nutsedge seeds leads to an increase in flavonoids and alkaloids in nutsedge yoghurt compared with other pretreatments because, during the roasting process, the heat applied can cause Maillard reactions. In fact, during roasting, the Maillard reaction, a non-enzymatic browning reaction between amino acids and reducing sugars, significantly impacts flavonoid and phenol formation. This reaction leads to the degradation and transformation of initial flavonoids and phenols, as well as the formation of new, often volatile, aromatic compounds [61, 62].
Antinutrients interfere with digestion and reduce their nutritional contribution, leading to numerous undesirable effects in the body [63]. The reduction in oxalates and saponins is more significant in yoghurt, where the seeds have undergone germination and drying for tannins, because during germination, the natural enzymes in tiger nut seeds are activated, which can help break down antinutrients and improve nutrient digestibility [64]. Roasting, on the other hand, is the seed pretreatment that reduces the phytate content of yellow nutsedge yoghurt the most. During roasting, the heat degrades the phytates, making them less active, and the proteins present in the nutsedge seeds undergo structural changes that can lead to a reduction in phytate activity, such as enzyme and lectin inhibitors and their ability to form complexes with minerals and interfere with their absorption [65]. Tannins can bind to proteins and minerals, reducing their bioavailability [66]. While tannins have been noted for their antinutritional effects, they also offer health benefits, such as improving heart health by lowering blood pressure [67], having antibacterial properties [68], and potential neuroprotective effects that might slow down conditions like dementia and Parkinson’s disease [69]. According to Obiakor-Okeke and Oly-alawuba [70], the inconvenience with phytates is that they can chelate positively charged cations such as zinc, magnesium, iron and calcium and form insoluble complexes, making those minerals unavailable for absorption. However, phytates may also have some beneficial effects, such as acting as antioxidants, having antineoplastic properties and reducing pathological calcifications in blood vessels and organs [71, 72]. All yoghurt samples have low levels of oxalate, ranging from 0.26 mg/100 g DM in GYS to 0.63 mg/100 g DM in products made with untreated samples. High levels of oxalates can interfere with calcium absorption and lead to the formation of kidney stones [73]. However, the low levels found in the yoghurt samples are unlikely to pose a significant health risk.
PCA was carried out to classify the different yoghurts according to their general acceptability, their nutritional value and bioactive compounds. Figure 4 shows the correlation circle between the different variables, in particular general acceptability, nutritional value (ash, energy value, sugars, fat, protein, fibre), and bioactive compounds (phenolic compounds, flavonoids, and alkaloids). The figure shows that these variables contribute 66.37% to the formation of the (F1 × F2) axis system. The F1 axis alone explains 37.22% of the variables observed, while the second axis, F2, explains 29.15%.
Figure 5 shows that the yoghurts whose nuts had undergone germination and soaking showed a positive correlation with the proteins in the tiger nut yoghurt. The TeYS, on the other hand, showed a positive correlation with sugars.
The color of the yoghurt samples was among the sensory characteristics assessed by the panellists. EYS received the highest score for color. Munzur et al. [74] described that the color of the yoghurt depends on the color of the milk used. RYS had the highest score for general acceptability. This is perhaps due to the Maillard reaction that occurred during the roasting of dry tiger nuts, causing browning in the final product [75].
The aim of this work was to evaluate the sensory and nutritional properties of a yoghurt-like product made from tiger nuts pretreated by various methods (soaking, drying, roasting, boiling, and germination). Among all the pretreatment methods carried out in this study, the roasting, germination, and soaking of tiger nuts seem to be the most promising pretreatment techniques with regard to the sensory properties, the level of nutritional, bioactive, and antinutritional compounds. This transformation is a major innovation in the tiger nut yoghurt production process. The consumption of yoghurt made from plant products such as tiger nuts will contribute significantly to improving the nutrient and bioactive compounds intake for those suffering from lactose intolerance. However, further research is recommended to develop standardised processing protocols and to assess the functional properties of the tiger nut yoghurt. It will also be interesting to explore its long-term stability and shelf life.
DM: dry matter
EYS: boiling yoghurt sample
GAE: gallic acid equivalent
GYS: germination yoghurt sample
PCA: principal component analysis
RYS: roasting yoghurt sample
SYS: drying yoghurt sample
TeYS: control yoghurt sample
TYS: soaking yoghurt sample
WHC: water holding capacity
AGSK, GMM, CN, and MA: Conceptualization, Methodology, Resources, Formal analysis, Writing—original draft, Investigation. HMC and ARY: Conceptualization, Methodology, Visualization. ISB: Conceptualization, Formal analysis, Data curation. RP and EF: Supervision, Validation, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Informed consent to participate in the study was obtained from all participants. Participants were fully informed about the study’s purpose, the nature of the yoghurt, any potential risks, and the evaluation process. They are assured of their right to withdraw at any time without consequence. Participants know their role and rights to maintain ethical standards in the research.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
