Affiliation:
Biocytogen Pharmaceuticals (Beijing) Co., Ltd, Beijing 102629, China
†These authors contributed equally to this work.
Email: zhangrf1992@163.com
ORCID: https://orcid.org/0000-0001-7379-6597
Affiliation:
Biocytogen Pharmaceuticals (Beijing) Co., Ltd, Beijing 102629, China
†These authors contributed equally to this work.
Affiliation:
Biocytogen Pharmaceuticals (Beijing) Co., Ltd, Beijing 102629, China
†These authors contributed equally to this work.
Explor Endocr Metab Dis. 2025;2:101433 DOI: https://doi.org/10.37349/eemd.2025.101433
Received: March 17, 2025 Accepted: May 15, 2025 Published: June 24, 2025
Academic Editor: Tilman D Rachner, Technische Universität Dresden, Germany
Aim: This study aimed to evaluate and compare the therapeutic effects of resmetirom, semaglutide, and obeticholic acid (OCA) on non-alcoholic fatty liver disease (NAFLD) activity score (NAS) and fibrosis progression across three distinct metabolic dysfunction-associated steatohepatitis (MASH) models. A secondary objective was to assess model-specific variations in drug efficacy to inform future preclinical model selection for MASH research.
Methods: The Gubra-Amylin NASH (GAN) diet-induced obesity (DIO)-MASH model was induced by the GAN diet in C57BL/6 mice for 24 weeks, followed by semaglutide and resmetirom treatment for 4 weeks. The ob/ob-MASH model was induced by the GAN diet in ob/ob mice for 6 weeks, followed by semaglutide and resmetirom treatment for 4 weeks. GAN-carbon tetrachloride (CCL4) MASH model was induced by 10 weeks of GAN diet and followed by 4 weeks of CCL4 in C57BL/6 mice, resmetirom and OCA were given in the last 4 weeks. Body weights, serum biochemical markers [alanine aminotransferase (ALT), aspartate aminotransferase (AST), lipids], histopathological NAS scores, fibrosis staging, and α-smooth muscle actin (α-SMA) expression were analyzed.
Results: In the GAN DIO-MASH model, both semaglutide and resmetirom reduced NAS significantly, and resmetirom but not semaglutide reduced α-SMA expression. In the ob/ob MASH model, treatment with semaglutide and resmetirom reduced NAS. Semaglutide significantly reduced α-SMA expression. In the GAN-CCL4 MASH model, both resmetirom and OCA significantly reduced MASH progression, resmetirom reduced liver fibrosis and α-SMA expression while OCA reduced α-SMA expression only.
Conclusions: Resmetirom, semaglutide, and OCA exhibited model-dependent efficacy in attenuating MASH progression. Although all agents improved the NAS, their antifibrotic effects diverged significantly: resmetirom demonstrated pan-model efficacy, semaglutide selectively reduced α-SMA expression in leptin-deficient models, and OCA showed minimal impact on fibrosis biomarkers. These observations highlight the critical importance of preclinical model selection for MASH therapeutic development, particularly when assessing fibrosis-targeted interventions.
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a growing global health concern, which is pathological hepatic steatosis (≥ 5% liver fat content) in the absence of significant alcohol consumption. It's closely associated with conditions such as obesity, diabetes, high cholesterol or high triglycerides (TGs) in blood. MAFLD is considered one of the most prevalent forms of chronic liver disease worldwide and its prevalence has been increasing rapidly due to rising rates of obesity and type 2 diabetes [1–6].
Significant progress has been made in clinical trials for potential treatments, including obeticholic acid (OCA), elafibranor, resmetirom, semaglutide and pegozafermin. OCA, a farnesoid X receptor (FXR) agonist, has shown promise in phase III trials by improving metabolic abnormalities and reducing fibrosis levels in MAFLD patients [7, 8]. Elafibranor, a dual peroxisome proliferator-activator receptors alpha/delta (PPARα/δ) agonist currently under phase II trial has demonstrated significant improvements in metabolic dysfunction-associated steatohepatitis (MASH) without worsening of fibrosis [9]. Resmetirom (MGL-3196), a thyroid hormone receptor beta selective agonist is also under investigation. It showed reduction of hepatic fat content and improved liver enzymes with a good tolerability profile in phase IIb studies [10–12] and improved hepatic steatosis and fibrosis in the phase III study [13]. Semaglutide, an injectable glucagon-like peptide-1 receptor (GLP1R) agonist used for type 2 diabetes management was found to significantly increase the percentage of patients with MASH resolution than placebo but didn’t show improvement in liver fibrosis [14, 15]. Fibroblast growth factor 21 (FGF21), a member of the FGF superfamily, which is a key regulator of energy balance and metabolism in mammals. A phase 2b study showed that treatment with an FGF21 analog pegozafermin led to improvements in MASH and fibrosis [16]. While these results are promising, more research is needed to fully understand their long-term efficacy and safety profiles [6, 17].
In corresponding with clinical trials. Preclinical animal studies have been instrumental in understanding the potential MASH drugs. OCA has shown promising results of MASH resolution and anti-fibrotic effects with a dose of 30 mg/kg by reducing hepatic stellate cell activation and collagen deposition in mice [18, 19]. Elafibranor showed efficacy in rodent models of MAFLD/MASH, improving steatosis, inflammation, ballooning degeneration and fibrosis while also improving insulin sensitivity and lipid profile [20]. Resmetirom was further evaluated at doses of 3 mg/kg and 5 mg/kg in diet-induced obesity (DIO)-MASH models, where it reduced hepatic steatosis significantly along with improvements in metabolic parameters [21, 22]. For the best known GLP1R agonist analogues have shown beneficial effects on weight loss and glycaemic control, there are also a large number of studies demonstrating their pharmacodynamic effects in MASH, such as liraglutide [23], dulaglutide [24], semaglutide [25] and cotadutide [26, 27].
In this study, we validated the three most promising drugs for the treatment of MASH in several different MASH models and comprehensively compared the efficacy data of the different drugs. It is hoped that this work will provide a reference for preclinical studies of MASH drugs and improve the translational success of new drugs.
Male C57BL/6 and leptin-deficient ob/ob mice (with exons 2 and 3 of the leptin gene deleted) were obtained from Biocytogen Pharmaceuticals (Beijing, China). All procedures were conducted in accordance with the guidelines approved by the Animal Care and Use Committee of Biocytogen Pharmaceuticals, the Animal Use Protocol approval number is BAP-BJ-PS-10. Mice were housed under specific pathogen-free (SPF) conditions with ad libitum access to food and water, a controlled temperature (22° ± 2°C), and a 12-hour light/dark cycle. Alcaine anesthesia was used for visceral blood collection. Euthanasia was performed via CO2 inhalation at the endpoint of experiments.
Gubra-Amylin NASH (GAN) DIO-MASH Model: Six-week-old male C57BL/6 mice were fed either a standard chow diet or a GAN diet (Research Diets, D09100310; 40% kcal from fat, 22% fructose, 2% cholesterol) for 28 weeks. Semaglutide or resmetirom was administered during the final 4 weeks.
ob/ob-MASH Model: Eight-week-old ob/ob mice received either standard chow or the GAN diet for 10 weeks, with semaglutide or resmetirom treatment initiated during the last 4 weeks.
GAN-CCL4 MASH Model: Six-week-old male C57BL/6 mice were fed the GAN diet or standard chow for 10 weeks, followed by intraperitoneal CCL4 injections (0.25 μL/g body weight, twice weekly) for 4 weeks. Resmetirom or OCA treatment commenced concurrently with CCL4 administration.
Semaglutide (HY-114118), resmetirom (HY-12216) and OCA (HY-12222) were pruchased from MedChemExpress (MCE). In all of the MASH models above, semaglutide was administered by subcutaneous injection at a dose of 30 nM/kg once daily. Resmetirom was administered orally at a dose of 5 mg/kg once daily. OCA was administered orally at a dose of 30 mg/kg once daily.
Mouse whole blood was allowed to stand for 20 minutes at room temperature and then centrifuged at 3,000 rpm for 10 minutes to separate the serum for subsequent assays. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), triglycerides (TGs), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured using an automated biochemistry instrument (Hitachi 3100, HITT-TC) and the corresponding kits (Maccura, China).
Liver tissues were fixed in 10% neutral-buffered formalin overnight, paraffin-embedded, and sectioned (4 μm thickness). Hematoxylin and eosin (H&E) staining was performed using a commercial kit (Beyotime, China). A blinded pathologist evaluated non-alcoholic fatty liver disease (NAFLD) activity score (NAS), including steatosis, lobular inflammation, and hepatocellular ballooning.
Sirius red staining was performed using a Picro-Sirius red staining kit (G-CLONE, Beijing, China) according to the manufacturer’s instructions. Fibrosis score were assessed by a pathologist blinded to this study according to the Ishak staging system for fibrosis (score 0—no fibrosis; 1—fibrous expansion of some portal areas, with or without short fibrous septa; 2—fibrous expansion of most portal areas, with or without short fibrous septa; 3 fibrous expansion of most portal areas with occasional portal to portal bridging; 4—fibrous expansion of portal areas with marked bridging (portal to portal as well as portal to central); 5—marked bridging with occasional nodules (incomplete cirrhosis); and 6—cirrhosis, probable or definite) [28].
For the immunohistochemical analysis of α-smooth muscle actin (α-SMA), paraffin-embedded liver sections were stained with α-SMA antibody (ab320058, Abcam, Cambridge, MA, USA). Quantitative analysis of positive signaling was performed using HALO image analysis software (HALOTM, Indica Labs, Inc., Corrales, NM, USA).
Liver TG content was measured using the Tissue Triglyceride Assay Kit (Applygen, E1015). Liver TC content of liver was measured using the Tissue Total Cholesterol Assay Kit (Applygen, E1013). Briefly, liver tissues were added with an appropriate amount of lysate and then homogenized. The supernatant was collected by centrifugation and the TG and TC content of the homogenate was determined by enzymatic reaction while the protein in the supernatant was quantified by BCA method. TG and TC contents in liver were normalized to protein levels.
Data are expressed as means ± SEM. For comparisons among three or more groups at a single time point, ordinary one-way ANOVA was performed followed by Tukey’s multiple comparison test. When analyzing three or more groups across multiple time points, a two-way ANOVA was conducted with Tukey’s multiple comparisons test for post hoc analyses. A significance threshold of P < 0.05 was applied for all statistical evaluations.
Firstly, we tested the efficacy of semaglutide and resmetirom in a GAN DIO-MASH model, in which mice usually have typical MASH features and are in the early stages of fibrosis initiation and development after 24 weeks of GAN diet induction. After 4 weeks of treatment with semaglutide and resmetirom (Figure 1A), the mice showed a significant reduction (P < 0.0001) in body weight compared to vehicle groups (Figure 1B and C). GAN DIO-MASH mice exhibited significantly higher liver weight and liver index compared to the standard diet group, whereas both semaglutide and resmetirom treatments were able to significantly reduce liver weight and liver index (Figure 1D and E). On further analysis of the serum transaminase activities, we found that semaglutide was able to significantly reduce the GAN diet-induced elevation of ALT and AST activities. Intriguingly, we found that ALT and AST activities were significantly higher after resmetirom treatment at a dose of 5 mg/kg than in the standard diet control and GAN DIO-MASH groups (Figure 1F and G). When analyzing serum TG levels, we found that semaglutide had a tendency to lower TGs, whereas resmetirom was able to do so significantly (Figure H). The results of the serum cholesterol assay showed that both semaglutide and resmetirom were able to significantly reduce TC, LDL-C and HDL-C levels, whereas resmetirom was able to reduce them to a greater extent compared to semaglutide (Figure 1I–K).
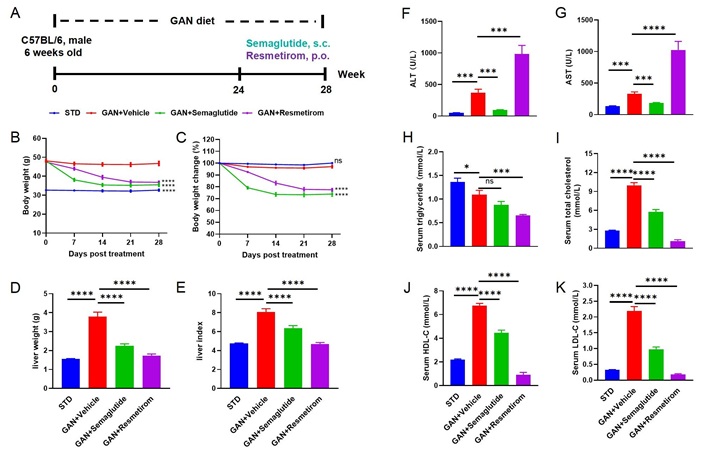
Metabolic effect of Semaglutide and Resmetirom treatment in GAN DIO-MASH model. (A) Experimental design showing modeling and treatment of GAN DIO-MASH mice; (B, C) body weight and percent body weight change of different groups after treatment; (D, E) liver weight and Liver Index of different groups at study termination; (F, G) ALT and AST activities of different groups at study termination; (H–K) serum TG, TC, HDL-C, LDL-C levels of different groups at study termination. Data are expressed as mean ± SEM. n = 10 mice per group. ns: no significance; * P < 0.05; *** P < 0.001; **** P < 0.0001. GAN: Gubra-Amylin NASH; STD: standard diet; ALT: alanine aminotransferase; AST: aspartate aminotransferase
At the 28-week time point of GAN diet induction, mouse liver samples were analyzed and liver pathological findings showed that the GAN DIO-MASH mice exhibit significant MASH symptoms, including steatosis, inflammation and ballooning, while semaglutide treatment significantly reduced the NAS total score and steatosis score, and resmetirom improved the NAS to a greater extent (Figure 2A–E). Semaglutide treatment significantly reduced NAS and steatosis score, while resmetirom improved NAS to a greater extent and improved both steatosis score and ballooning score (Figure 2C and D), while semaglutide and resmetirom did not have a significant improvement in inflammation score compared to the GAN DIO-MASH modelling group (Figure 2E). Further, liver homogenates were obtained to measure TGs and TC in the liver, and it was found that both were significantly elevated in the GAN DIO-MASH group, and semaglutide treatment lowered cholesterol but not TGs in the liver. Resmetirom, on the other hand, significantly reduced liver TG and TC concentrations to levels similar to the standard diet model group (Figure 2F and G).
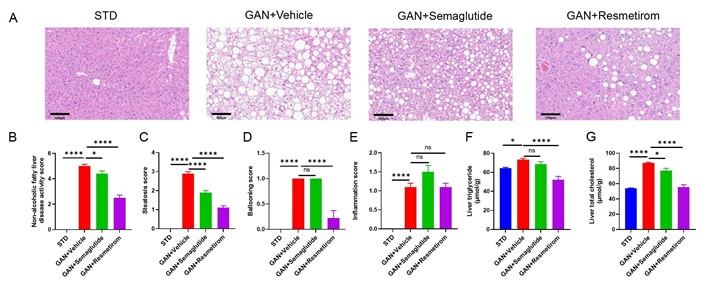
Effect of Semaglutide and Resmetirom on Liver histomorphology in GAN DIO-MASH model. (A) Representative pictures of H&E staining showing degree of MASH; (B–E) histological assessment of the NAS, steatosis score, ballooning score and inflammation score; (F, G) TG and TC content in liver at study termination. Data are expressed as mean ± SEM. n = 10 mice per group. ns: no significance, * P < 0.05,**** P < 0.0001. STD: standard diet; GAN: Gubra-Amylin NASH
Finally, Sirius red staining and analysis of the degree of fibrosis were performed after 28 weeks of GAN diet induction, and it was found that the GAN DIO-MASH group exhibited mild liver fibrosis, whereas semaglutide and resmetirom did not have a significant ameliorative effect on fibrosis scores (Figure 3A and B). However, analysis of α-SMA expression levels by immunohistochemistry showed that the resmetirom treatment group was able to significantly reduce α-SMA expression levels (Figure 3C and D). This suggests that resmetirom has a certain inhibitory effect on the formation process of liver fibrosis.
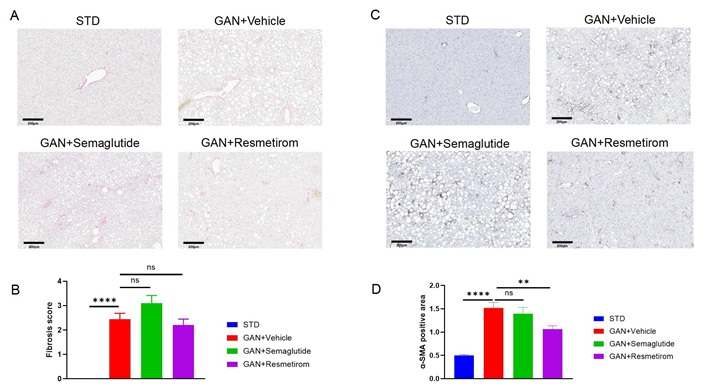
Effect of Semaglutide and Resmetirom on liver fibrosis in GAN DIO-MASH model. (A) Representative pictures of Sirius red staining showing degree of liver fibrosis; (B) liver fibrosis score assessed according Sirius red staining; (C) representative pictures of IHC staining showing α-SMA expression; (D) quantitively data of α-SMA expression. Data are expressed as mean ± SEM. n = 10 mice per group. ns: no significance, ** P < 0.01, **** P < 0.0001. STD: standard diet; GAN: Gubra-Amylin NASH
ob/ob mice are genetically modified animals that develop obesity due to leptin deficiency. When fed on a GAN diet, they exhibit exacerbated symptoms similar to those seen in human metabolic syndrome including severe hepatic steatosis, inflammation and fibrosis [29]. In the ob/ob-MASH model, we also tested the efficacy of semaglutide and resmetirom (Figure 4A), and the results showed that both semaglutide and resmetirom were able to significantly reduce the body weight (P < 0.0001) of the mice (Figure 4B and C), compared to the ob/ob-MASH model mice. For liver weight and liver index, both semaglutide and resmetirom showed similar reductions (Figure 4D and E). Both semaglutide and resmetirom were able to significantly reduce ALT and AST activity (Figure 4F and G). The serum TG results illustrate that semaglutide and resmetirom were both able to significantly reduce their levels and there was no significant difference between them (Figure 4H). Serum cholesterol levels were analyzed and it was found that both semaglutide and resmetirom significantly reduced TC, HDL-C and LDL-C levels (Figure 4I–K).
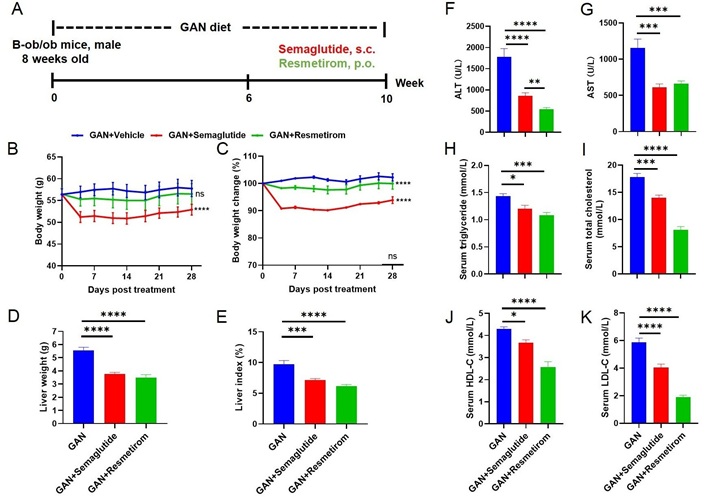
Metabolic effect of Semaglutide and Resmetirom treatment in ob/ob-MASH model. (A) Experimental design showing modeling and treatment of ob/ob-MASH model; (B, C) body weight and percent body weight change of different groups after treatment; (D, E) liver weight and Liver Index of different groups at study termination; (F, G) ALT and AST activities of different groups at study termination; (H–K) serum TG, TC, HDL-C, LDL-C levels of different groups at study termination. Data are expressed as mean ± SEM. n = 9 mice per group. ns: no significance, * P <0.05, ** P < 0.01, *** P < 0.001,**** P < 0.0001. GAN: Gubra-Amylin NASH; ALT: alanine aminotransferase; AST: aspartate aminotransferase
Pathological analysis of the livers from ob/ob-MASH model mice revealed that GAN diet-induced ob/ob mice exhibited distinct MASH features, and that both semaglutide and resmetirom were able to significantly reduce NAS, steatosis scores, and ballooning scores following treatment with the drugs (Figure 5A–D). While no significant improvement in inflammation scores was observed with either drug (Figure 5E). In ob/ob mice, 10 weeks of GAN diet induction showed only a slight degree of fibrosis (Figure S1A and B), on the basis of which semaglutide was able to significantly reduce the level of α-SMA expression, while resmetirom showed no significant improvement in fibrosis score and α-SMA expression (Figure S1C and D). Taken together, these results suggest that in the ob/ob-MASH model, semaglutide and resmetirom were equally effective in improving MASH symptoms, with semaglutide having an ameliorative effect on liver fibrosis while resmetirom did not.
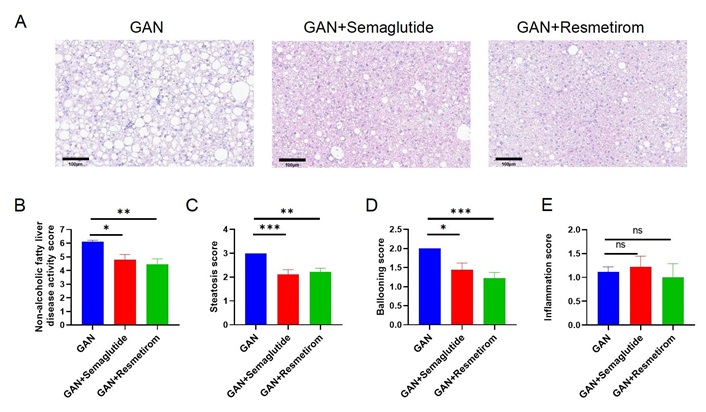
Effect of Semaglutide and Resmetirom on Liver histomorphology in ob/ob-MASH model. (A) Representative pictures of H&E staining showing degree of MASH; (B–E) Histological assessment of the NAS, steatosis score, ballooning score and inflammation score. Data are expressed as mean ± SEM. n = 9 mice per group. ns: no significance, * P < 0.05, ** P < 0.01,*** P < 0.001. GAN: Gubra-Amylin NASH
Lastly, we tested the efficacy of resmetirom and OCA in another commonly used MASH model, the GAN-CCL4 MASH model, in which the amylin diet is combined with carbon tetrachloride (CCL4), a hepatotoxic chemical that induces liver damage mimicking advanced stages of MAFLD, such as steatohepatitis and fibrosis (Figure 6A). The results showed that resmetirom was able to reduce liver weight and liver index, whereas OCA elevated liver weight and liver index compared to GAN-CCL4 MASH model mice (Figure 6B and C). CCL4 induction significantly elevated ALT and AST activities, whereas both resmetirom and OCA significantly alleviated ALT and AST elevations (Figure 6D and E), with resmetirom showing a greater improvement. Serum TG levels were significantly elevated in the GAN-CCL4 MASH model mice compared to the standard diet and GAN diet controls, while the resmetirom and OCA treatment groups significantly reduced serum TG levels compared to the GAN-CCL4 MASH model group (Figure 6F). Analysis of serum cholesterol levels showed that resmetirom significantly reduced TC, HDL-C and LDL-C levels, whereas OCA only significantly reduced TC levels (Figure 6G–I).
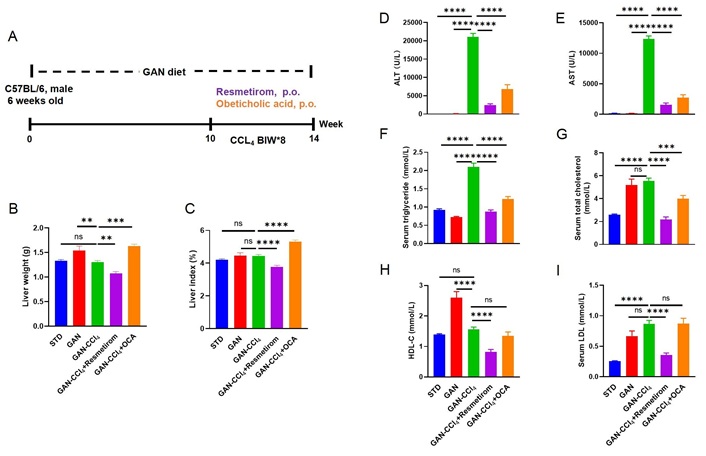
Metabolic effect of Resmetirom and obeticholic acid treatment in GAN-CCL4 MASH model. (A) Experimental design showing modeling and treatment of GAN-CCL4 MASH model; (B, C) liver weight and Liver Index of different groups at study termination; (D, E) ALT and AST activities of different groups at study termination; (F–I) serum TG, TC, HDL-C, LDL-C levels of different groups at study termination. Data are expressed as mean ± SEM. n = 9 mice per group. ns: no significance, ** P < 0.01, *** P < 0.001,**** P < 0.0001. GAN: Gubra-Amylin NASH; CCL4: carbon tetrachloride; STD: standard diet; ALT: alanine aminotransferase; AST: aspartate aminotransferase
Pathological analysis of the liver showed that resmetirom and OCA reduced the NAS score (Figure 7A and B), steatosis score (Figure 7C) and ballooning score (Figure 7D) to the same extent and did not significantly improve the inflammation score (Figure 7E). The results of liver TG and TC content showed that resmetirom and OCA significantly reduced liver TG and TC content, and the magnitude of the reduction was basically the same (Figure 7F and G). CCL4 is known to be a strong stimulator of liver inflammation and fibrosis. Therefore, increased fibrosis was observed in the livers of GAN-CCL4 MASH mice. After 4 weeks of treatment, significant reductions in liver fibrosis scores were observed by resmetirom but not OCA (Figure 8A and B). And α-SMA expression levels were significantly reduced in both resmetirom and OCA treated groups (Figure 8C and D). These results suggest that in the GAN-CCL4 MASH model, resmetirom and OCA had the same ameliorative effect on MASH, while resmetirom was better than OCA in improving liver fibrosis.
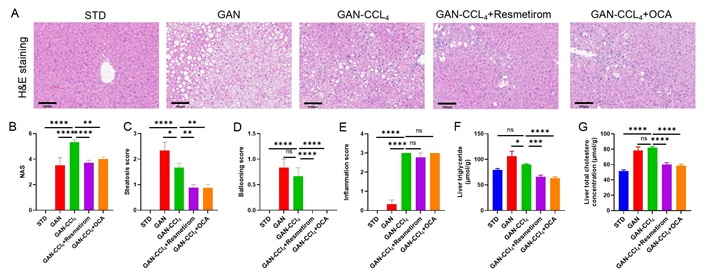
Effect of Resmetirom and obeticholic acid on Liver histomorphology in GAN-CCL4 MASH model. (A) Representative pictures of H&E staining showing degree of MASH; (B–E) histological assessment of the NAS, steatosis score, ballooning score and inflammation score; (F, G) TG and TC content in liver at study termination. Data are expressed as mean ± SEM. n = 9 mice per group. ns: no significance, * P < 0.05, ** P < 0.01,*** P < 0.001,**** P < 0.0001. H&E: hematoxylin and eosin; STD: standard diet; GAN: Gubra-Amylin NASH; CCL4: carbon tetrachloride; OCA: obeticholic acid; NAS: non-alcoholic fatty liver disease activity score
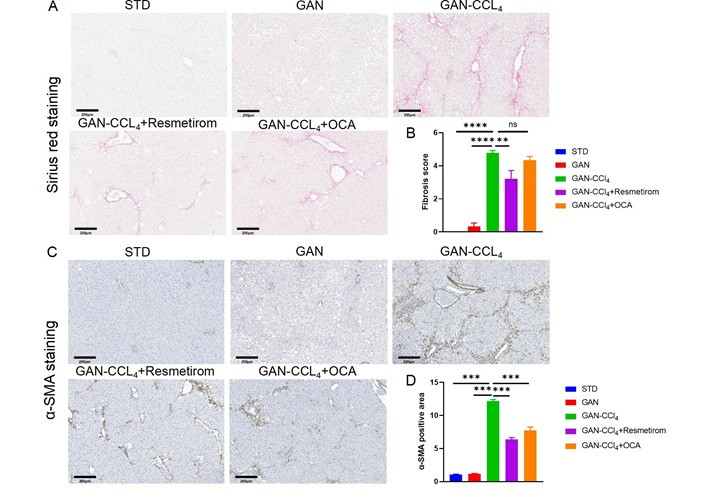
Effect of Resmetirom and obeticholic acid on liver fibrosis in GAN-CCL4 MASH model. (A) Representative pictures of Sirius red staining showing degree of liver fibrosis. (B) Liver fibrosis score assessed according Sirius red staining. (C) Representative pictures of IHC staining showing α-SMA expression. (D) Quantitively data of α-SMA expression. Data are expressed as mean ± SEM. n = 9 mice per group. ns: no significance, ** P < 0.01,*** P < 0.001,**** P < 0.0001. STD: standard diet; GAN: Gubra-Amylin NASH; CCL4: carbon tetrachloride; OCA: obeticholic acid; NAS: non-alcoholic fatty liver disease activity score; α-SMA: α-smooth muscle actin
The spectrum of MAFLD ranges from simple steatosis to MASH, which can progress to cirrhosis and hepatocellular carcinoma if left untreated. Despite its widespread prevalence, MAFLD often goes undiagnosed because it typically presents no symptoms until its later stages. Currently, there are no specific treatments for MAFLD, so drug development for MAFLD is imperative. Understanding the pathogenesis and natural history of this disease continues to be an area of intense study among scientists globally given its significant public health implications.
Mouse models of MAFLD/MASH are essential tools in the study of MAFLD pathogenesis and therapeutic development. There are various MASH models, all with their own advantages and disadvantages [30–33]. Currently, there is no perfect animal model of MASH that can fully simulate the pathogenesis of clinical patients, so it is necessary to choose a suitable animal model according to the type of target and mechanism of action to improve the success rate of translation from preclinical research to the clinic.
In this study, we used three different MASH mouse models to validate the efficacy of resmetirom, OCA and semaglutide. liver damage index and lipid contents, NAS scores and liver fibrosis were determined.
GAN DIO-MASH model replicates many features of human MAFLD including obesity, insulin resistance, dyslipidemia, inflammation, and fibrosis [34]. In the DIO-MASH model, resmetirom treatment significantly reduced NAS scores and α-SMA expression but did not improve fibrosis scores, which is consistent with the effect of 8 weeks of resmetirom treatment on MASH and fibrosis in previous studies [21]. Semaglutide treatment in this model significantly reduced NAS scores but did not improve fibrosis scores and α-SMA expression. Previous studies have similarly shown that in the DIO-MASH model, semaglutide improves NAS scores but not fibrosis scores [25].
Several drugs have been tested for efficacy in the ob/ob-MASH model, including liraglutide, elafibranor, and OCA. Of these, Liraglutide showed little or no improvement in NAS scores and fibrosis scores, elafibranor improved both NAS and fibrosis scores, and OCA was able to improve NAS but not fibrosis score [23, 35]. In our ob/ob-MASH model, we found that semaglutide showed significant improvement in NAS score and SMA expression. The efficacy of resmetirom in the ob/ob-MASH model is poorly reported, and our results suggest that resmetirom improved the NAS score but not the fibrosis score.
In the GAN-CCL4 model, the amylin diet is combined with CCL4, a hepatotoxic chemical that induces liver damage mimicking advanced stages of MAFLD, such as steatohepatitis and fibrosis [36, 37]. In this model, our results showed that 4 weeks of resmetirom and OCA treatment were able to reduce liver damage index, blood lipids, and NAS scores. The fibrosis score also decreased significantly after resmetirom but not OCA. The improvement in fibrosis by OCA was not as significant as reported in the literature, and it is speculated that a possible reason for this may be that 4 weeks of dosing is not enough time to have a large improvement in fibrosis [18, 36]. However, the reduction of α-SMA expression by OCA demonstrated a blockade of the fibrosis development process.α-SMA expression is a hallmark of activated hepatic stellate cells (HSCs), serving as a key biomarker for liver fibrosis progression. Its upregulation correlates directly with extracellular matrix (ECM) deposition and fibrosis formation. The observed greater improvement in α-SMA expression compared to fibrosis regression may be attributed to a preferential reduction in activated HSCs, whereas collagen degradation mechanisms or disruption of mature collagen cross-linking remained insufficiently engaged.
The selection of appropriate animal models is critical to the preclinical evaluation of drug efficacy, and validation of efficacy in 2 or more different models can significantly improve the translational success of a drug in clinical studies. This study systematically compared the efficacy of the current more promising MASH drug candidates on blood biochemistry and liver pathological effects in several different MASH mouse models. These findings not only demonstrate model-specific drug efficacy but also provide a roadmap for translating preclinical insights into clinical practice. By aligning patient stratification with biomarker profiles (e.g., leptin status, fibrosis stage) and selecting preclinical models that mirror target pathophysiology, future studies can optimize therapeutic discovery for MASH—a disease with heterogeneous drivers and clinical trajectories.
While this study provides valuable insights into the therapeutic potential of the investigated compound, several limitations should be acknowledged. First, the administration of the drug was restricted to a single treatment duration. Incorporating multiple time points could better elucidate the temporal dynamics of therapeutic efficacy. Second, the dose-response evaluation was limited to only one concentration. A broader range of dosages would enhance the characterization of dose-dependent effects, clarify the therapeutic window, and identify potential toxicity thresholds, thereby improving translational relevance. Third, the mechanistic underpinnings of the drug’s action remain incompletely resolved. Complementary approaches such as transcriptomic profiling, pathway-specific inhibition assays, or proteomic analyses could unravel molecular targets and signaling cascades modulated by the compounds, bridging the gap between phenotypic observations and mechanistic understanding. Addressing these limitations in future studies will strengthen the robustness of the conclusions and provide a more comprehensive framework for clinical development.
ALT: alanine aminotransferase
AST: aspartate aminotransferase
CCL4: carbon tetrachloride
DIO: diet-induced obesity
FGF21: fibroblast growth factor 21
GAN: Gubra-Amylin NASH
GLP1R: glucagon-like peptide-1 receptor
HDL-C: high-density lipoprotein cholesterol
HSCs: hepatic stellate cells
LDL-C: low-density lipoprotein cholesterol
MAFLD: metabolic dysfunction-associated fatty liver disease
MASH: metabolic dysfunction-associated steatohepatitis
NAFLD: non-alcoholic fatty liver disease
NAS: non-alcoholic fatty liver disease activity score
OCA: obeticholic acid
TC: total cholesterol
TGs: triglycerides
α-SMA: α-smooth muscle actin
The supplementary figure for this article is available at: https://www.explorationpub.com/uploads/Article/file/101433_sup_1.pdf.
X Li, X Liang, YK, XC, YN, LY: Investigation, Writing—original draft. RZ: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. DL, XZ, CS: Validation, Writing—review & editing, Supervision. All authors read and approved the submitted version.
All of the authors are employees of Biocytogen Pharmaceuticals. All experimental data, design and manuscript preparation in this article were performed by the authors. Some of the data are shown on the company’s official website. The release of all data has been approved by the business and legal departments of the company.
All procedures were conducted in accordance with the guidelines approved by the Animal Care and Use Committee of Biocytogen Pharmaceuticals, the Animal Use Protocol approval number is BAP-BJ-PS-10.
Not applicable.
Not applicable.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
All sources of funding for this study were provided by Biocytogen Pharmaceuticals (Beijing, China).
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
