Affiliation:
Department of Medicine and Pharmaceutical Sciences, University of Tennessee Health Science Center, Memphis, TN 38163, USA
Email: gmeduri@uthsc.edu
ORCID: https://orcid.org/0000-0002-4848-8928
Explor Endocr Metab Dis. 2025;2:101432 DOI: https://doi.org/10.37349/eemd.2025.101432
Received: January 26, 2025 Accepted: April 27, 2025 Published: May 14, 2025
Academic Editor: Karel Pacak, AKESO Holding, Czech Republic
The glucocorticoid receptor (GR) signaling pathway is essential for supporting the integrity of the intestinal barrier, regulating the gut microbiome, and preserving systemic homeostasis in critically ill patients. GR signaling limits bacterial translocation and systemic inflammation by suppressing pro-inflammatory cytokines, reinforcing tight junction proteins, and promoting epithelial renewal. Additionally, physiological levels of glucocorticoids (GCs) stimulate glutamine and proline metabolism, supporting intestinal maturation, with potential clinical relevance. GR signaling modulates inter-organ communication via the gut-lung and gut-brain axes, improving outcomes. Probiotics enhance GC therapy by restoring microbial balance, increasing short-chain fatty acid (SCFA) production, and modulating immune responses. Vitamins A, C, D, and E contribute to gut resilience by stabilizing tight junctions, mitigating oxidative stress, and strengthening mucosal immunity. Specifically, vitamin D balances T-cell subsets and promotes antimicrobial peptides; vitamin C supports collagen synthesis, antioxidant defenses, and immune function; vitamin A promotes immune tolerance and epithelial regeneration; and vitamin E mitigates oxidative damage and excessive cytokine release. GCs, probiotics, and vitamins counteract key drivers of critical illness, including hyperinflammation and dysbiosis, while maintaining strong safety profiles. This integrative approach leverages these interventions’ distinct yet complementary roles to provide a multi-layered defense against gut dysfunction. GCs reduce excessive inflammation and restore immune balance; probiotics enhance microbial diversity and strengthen gut-associated immunity; and vitamins support epithelial integrity and antioxidant defenses. Targeting multiple pathways simultaneously protects the gut barrier and modulates systemic immunity, potentially reducing complications such as sepsis, multiple organ dysfunction syndrome (MODS), and prolonged intensive care unit (ICU) stays. Incorporating these elements into critical care practice offers a novel strategy to mitigate gut dysfunction, reduce systemic inflammation, and enhance immune resilience. This approach may lower infection rates, decrease the incidence of sepsis and MODS, and accelerate recovery by targeting GR signaling, restoring microbial homeostasis, and reinforcing epithelial integrity.
The integrity of the intestinal barrier is central to maintaining systemic homeostasis, particularly in critically ill patients. Its disruption triggers systemic inflammation, immune dysregulation, and multi-organ failure. This manuscript comprehensively reviews the structure and function of the intestinal barrier, its regulation by the glucocorticoid receptor (GR) signaling pathway, and the roles of probiotics and essential vitamins in preserving barrier integrity, microbial balance, and immune resilience.
The intestinal barrier is a specialized, complex, multi-layered interface that upholds gut health by regulating the interactions between gut contents and the body’s internal environment. This barrier consists of several key components: the epithelial layer with tight junctions (TJs), the mucus layer, the enteric nervous system (ENS), blood circulation, lymphatic vessels, immune cells, antimicrobial peptides (AMPs), and a diverse resident gut flora microbiota. Table 1 elucidates the intestinal barrier’s critical features, functions, and clinical implications.
Key components and functions of the intestinal barrier
| Component | Description | Function | Clinical implications |
|---|---|---|---|
| Epithelial layer [1, 9, 14, 71, 72] | A single layer of tightly joined intestinal epithelial cells connected by tight junctions (TJs) | - Acts as a physical barrier preventing translocation of pathogens and toxins.- Regulates nutrient and water absorption.- Maintains paracellular permeability. | - Increased permeability contributes to inflammation, IBD, and systemic conditions.- Critical in maintaining barrier integrity during sepsis and ARDS. |
| Mucus layer [73, 74] | Composed of mucins, AMPs, and IgA | - Traps pathogens and toxins.- Neutralizes microbes.- Facilitates intestinal content movement. | - Impaired mucus production increases infection and inflammation risks. |
| Enteric nervous system [75–77] | A network of neurons and glial cells regulating motility, secretion, and local immune responses | - Coordinates digestion and nutrient absorption.- Supports blood flow and barrier integrity.- Modulates inflammation and immune responses. | - ENS dysfunction impairs gut motility and barrier integrity, worsening systemic inflammation and organ dysfunction during critical illness. |
| Enteric glial cells [2, 3] | Enteric glial cells, previously considered passive support cells, are critical regulators of intestinal motility, vascular tone, and gut homeostasis | - Coordinate neuron communication involving nitric oxide (NO) and glutamate.- Support mucosal barrier integrity, regulate neuroeffector junctions. | - Dysfunction disrupts motility, vascular regulation, and barrier integrity.- Exacerbates intestinal and systemic inflammation. |
| Blood circulation [78] | Delivers nutrients and oxygen, removes waste | - Maintains epithelial integrity.- Facilitates nutrient transport into systemic circulation. | - Hypoperfusion weakens barrier function, increasing permeability and systemic inflammation. |
| Lymphatic vessels [78] | Transports fats and immune cells | - Facilitates nutrient absorption.- Filters lymph and supports immune responses. | - Dysfunction impairs nutrient absorption and immune surveillance, raising infection risk. |
| Immune cells [4] | Immune cells in the gut mucosa | - Protect against pathogens.- Maintain immune tolerance and regulate inflammation. | - Dysregulation causes excessive inflammation, contributing to IBD. |
| AMPs | Small proteins in the mucus layer [73] | - Neutralize pathogens and toxins.- Link innate and adaptive immunity via chemokine and Toll-like receptor modulation [74]. | - Impaired AMP activity compromises barrier defenses and increases infection risks. |
| Resident gut microbiota [7] | The community of microorganisms in the intestinal tract | - Competes with pathogens, aids in digestion, produces CFAs and nutrients.- Regulates lipid metabolism and immune responses. | - Dysbiosis increases inflammation, pathogen overgrowth, and susceptibility to infections, especially during critical illness. |
| Disruption of the intestinal bassier in critical illness [8, 10] | Hyperinflammatory states disrupt barrier components [9] | - Disrupts TJs, reduces mucus production, and impairs epithelial function.- Facilitates bacterial translocation and systemic inflammation [11–13]. | - Worsens MODS and nosocomial infections.- ICU interventions like antimicrobials exacerbate dysbiosis, prolonging recovery. |
ARDS: acute respiratory distress syndrome; ENS: enteric nervous system; IgA: immunoglobulin A; MODS: multiple organ dysfunction syndrome; ZO: zonula occludens; AMPs: antimicrobial peptides; IBD: inflammatory bowel disease; ICU: intensive care unit
In critical illness, conditions such as sepsis and acute respiratory distress syndrome (ARDS) severely compromise intestinal barrier function, leading to increased permeability (“leaky gut”), impaired epithelial regeneration, mucus depletion, immune dysfunction, and marked dysbiosis. This breakdown facilitates bacterial translocation and systemic inflammation, exacerbating disease severity and complicating clinical management.
An integrative hypothesis proposed here suggests that combining glucocorticoids (GCs), probiotics, and vitamins provides synergistic benefits that simultaneously address multiple pathological processes. GR signaling maintains barrier integrity by controlling inflammation, enhancing epithelial repair, and modulating gut-brain and gut-lung communication. Probiotics complement GCs by re-establishing microbial diversity, boosting short-chain fatty acid (SCFA) production, and reinforcing mucosal immunity. Vitamins A, C, D, and E strengthen barrier resilience by stabilizing TJs, reducing oxidative stress, and promoting balanced immune responses. This integrative approach holds transformative potential as a low-risk, high-benefit treatment strategy to enhance outcomes and recovery in critically ill patients.
This manuscript aims to address the following core research questions: How does GR signaling preserve and restore intestinal barrier integrity in critically ill patients? In what ways do probiotics enhance gut resilience by modulating microbial diversity and immune responses and reducing systemic inflammation? How do essential vitamins—specifically A, C, D, and E—work with GR signaling to stabilize TJs, reduce oxidative stress, and support mucosal immunity? What are the pathophysiological consequences of intestinal barrier disruption and microbiome dysbiosis in critical illness, and how do they contribute to complications such as sepsis and multiple organ dysfunction syndrome (MODS)? Can an integrative therapeutic approach that combines GCs, probiotics, and vitamins provide a safe and effective strategy to alleviate gut dysfunction and enhance systemic outcomes? Finally, how might this combined intervention affect the gut-brain and gut-lung axes to reduce neuroinflammation, promote recovery, and improve resilience in critically ill patients?
The epithelial layer is the first line of intestinal defense, comprising tightly joined intestinal columnar epithelial cells interconnected by TJs. These junctions contain structural proteins such as claudins, occludin, and zonula occludens (ZO), creating a selective barrier that regulates paracellular transport from the gut lumen to underlying tissues and systemic circulation. The integrity of TJs prevents the translocation of pathogens and toxins while permitting controlled absorption of nutrients and water, preserving gut homeostasis. Disruption of TJ integrity increases epithelial permeability, facilitating the translocation of luminal pathogens and antigens, contributing to local inflammation and systemic immune activation. This phenomenon underlies the concept of “leaky gut syndrome”. Continuous epithelial renewal, driven by intestinal stem cells and coordinated intestinal motility regulated by enteric glia, is essential for barrier maintenance. Rapid epithelial turnover eliminates damaged or infected cells, while effective motility patterns prevent harmful microbial accumulation in the gut lumen, reinforcing barrier integrity and overall gut health. For a comprehensive and updated review, see [1].
The mucus layer complements the epithelial layer, primarily consisting of mucins from goblet cells. It provides additional protection by trapping pathogens and particles, preventing their direct epithelial contact, and facilitating the movement of intestinal contents. Embedded within this layer are AMPs and secretory immunoglobulin A (IgA; sIgA), both critical for neutralizing toxins and microbes, thus enhancing the barrier’s defenses. AMPs, secreted by Paneth cells, epithelial cells, and immune cells, neutralize pathogens by disrupting their membranes and inhibiting their replication. Additionally, AMPs bridge innate and adaptive immunity through interaction with chemokines and Toll-like receptors, modulating immune cell responses and microbial homeostasis. Reduced mucus production or impaired AMP activity can weaken barrier integrity, increasing susceptibility to infections and inflammation.
The ENS maintains intestinal barrier integrity by autonomously regulating gastrointestinal (GI) motility, secretion, and local blood flow through complex neuronal networks. These coordinated actions optimize digestion, nutrient absorption, and barrier structure. Additionally, ENS neurons communicate with epithelial and immune cells, modulating immune responses and inflammation to preserve gut homeostasis and effectively manage infections or tissue injury.
Enteric glial cells, once thought to be mere support cells, are now recognized as crucial regulators of intestinal motility, vascular tone, and gut homeostasis. Through bidirectional communication with neurons, involving neurotransmitters such as nitric oxide (NO) and glutamate, enteric glia actively coordinates motility and blood flow, thereby supporting mucosal barrier integrity. Embedded within intrinsic gut neural circuits, these cells maintain homeostasis in the neuronal microenvironment and regulate neuroeffector junctions. Collectively, enteric glia contributes to a dynamic network within the ENS, vital for responding to physiological demands and challenges related to pathological conditions [2, 3].
Blood and lymphatic circulation are crucial for maintaining intestinal barrier integrity. Efficient blood circulation, regulated by the ENS, delivers essential nutrients and oxygen, removes metabolic waste, and transports absorbed nutrients into systemic circulation. Deficits in mesenteric perfusion compromise barrier function, increasing gut permeability and systemic inflammation. Gut endothelial cells (ECs), interconnected by adherens junctions (AJs), TJs, cadherins, and catenins, further regulate vascular permeability and barrier integrity. Lymphatic vessels transport absorbed fats and fat-soluble vitamins, while supporting immune surveillance by filtering lymph fluid and presenting pathogens to immune cells. Collectively, these vascular networks ensure optimal nutrient absorption, immune regulation, and overall gut health.
Immune cells, including macrophages, dendritic cells, and lymphocytes, reside in the intestinal mucosa and play a crucial role in detecting and responding to pathogenic invasions. This function maintains immune surveillance and promotes tolerance to harmless antigens. These immune cells collaborate with AMPs to provide a first-line defense against pathogens and modulate immune responses, ensuring a balanced and tolerant immune environment. The gut-associated lymphoid tissue (GALT), which contains immune cells such as dendritic cells, macrophages, T cells, and B cells, maintains a delicate balance between immune defense and tolerance, regulated by cytokines and chemokines. Dysregulation of these immune functions can lead to excessive inflammation, contributing to inflammatory bowel diseases (IBDs) and disturbing the immune balance within the gut [4].
The human gut microbiome comprises bacteria, viruses, fungi, and archaea, containing over 232,000 unique genomes. These microbes support metabolism by breaking down complex carbohydrates and dietary fibers into SCFAs, essential for intestinal barrier integrity and immune regulation through modulation of T cell activity [5, 6]. The gut microbiome synthesizes essential nutrients such as B vitamins (B1, B2, B5, B6, B7, and B9), vitamin K, and essential amino acids not independently produced by humans [7]. It regulates lipid metabolism by modifying bile acids and influences energy storage and balance. Moreover, it plays a crucial role in modulating immune responses, preventing chronic inflammation, and maintaining metabolic health. Additionally, gut microbes detoxify harmful substances, promote metabolic stability, and interact with hormones such as insulin, leptin, and ghrelin to regulate appetite and energy homeostasis. This diverse microbial community is essential for maintaining overall health, immune resilience, and protection against metabolic disorders.
Together, these reviewed elements form a dynamic and resilient intestinal barrier that prevents pathogen invasion, supports efficient nutrient absorption, and maintains immune homeostasis. The barrier reduces the risk of chronic inflammation and autoimmune conditions by carefully regulating immune responses and promoting tolerance toward beneficial microbes while defending against harmful pathogens. Moreover, barrier integrity profoundly influences systemic health by modulating metabolic functions, immune function, and neurological activities through the gut-brain axis. Thus, the integrated actions of the epithelial layer, mucus, ENS, circulation, lymphatics, immune cells, AMPs, and gut microbiota create an effective interface between the internal and external environment, providing essential protection against infections and ensuring optimal nutrient assimilation and immune function. Figure 1 schematically illustrates the coordinated interaction among the gut lumen (microbiota), mucus layer (AMPs, sIgA), epithelial barrier (TJ proteins including claudins, occludin, and ZO-1), and the underlying lamina propria (immune cells). Together, these components preserve epithelial integrity and regulate immune responses to maintain intestinal homeostasis.
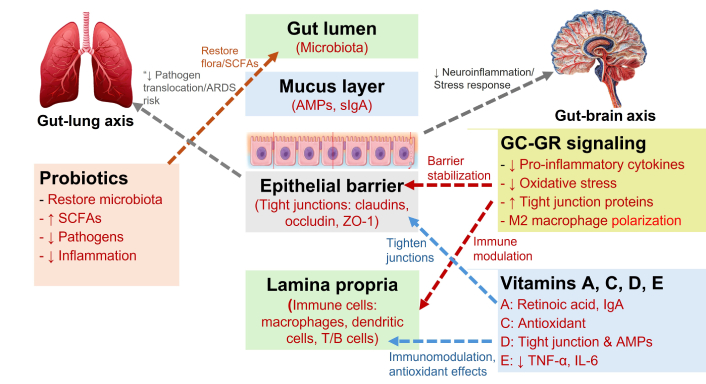
Integrated gut-lung-brain interactions: role of probiotics, nutrients, and GC-GR signaling in barrier function and immune modulation. This schematic illustrates how the gut lumen (microbiota), mucus layer (AMPs, sIgA), epithelial barrier (TJs: claudins, occludin, and ZO-1), and lamina propria (immune cells) work together to maintain intestinal integrity and modulate immune responses. Probiotics aid in restoring healthy microbial communities, boosting SCFAs, and decreasing pathogens and inflammation. Vitamins A, C, D, and E support gut barrier function and immune balance through their antioxidant, tight-junction-reinforcing, and cytokine-suppressing properties. The GC-GR signaling reduces proinflammatory cytokines and oxidative stress, enhances TJ proteins, and promotes M2 macrophage polarization. These processes collectively reinforce the epithelial barrier, limit pathogen translocation, and lower the risk of acute respiratory distress syndrome (ARDS) via the gu-lung axis while reducing neuroinflammation and stress responses through the gut-brain axis. SCFAs: short-chain fatty acids; AMPs: antimicrobial peptides; sIgA: secretory immunoglobulin A; TJs: tight junctions; TNF-α: tumor necrosis factor-alpha; IL-6: interleukin-6; ZO-1: zonula occludens-1; GC: glucocorticoids; GR: glucocorticoid receptor. Adapted from Servier Medical Art (https://smart.servier.com), licensed under Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/)
In critical illness, disruptions to the components of the intestinal barrier severely compromise its integrity, triggering systemic inflammatory responses and contributing to worse patient outcomes. Conditions like sepsis and ARDS are marked by dysregulated systemic inflammation [8], a hyperinflammatory state that disrupts TJ structure, reduces mucus production, and impairs epithelial cell function and endothelial integrity. Inflammatory cytokines, neutrophil activity, and the enzymatic degradation of barrier components drive this damage. This pathogenic mechanism also underlies IBD like Crohn’s disease and ulcerative colitis [9]. The complex interactions between the intestinal epithelium and immune cells during mucosal inflammation are discussed in reference [10].
These disruptions facilitate the translocation of bacteria (see dysbiosis below) and endotoxins from the gut lumen into the bloodstream through hematogenous or lymphatic pathways, worsening systemic inflammation, exacerbating MODS, and increasing the risk of hospital-acquired infections [11–13]. Preserving the integrity of TJs, maintaining mucus production, and ensuring the proper function of epithelial and ECs are vital for protecting the intestinal barrier. This is crucial not only under normal physiological conditions but also for mitigating the harmful effects of critical illness [14]. Notably, probiotics may influence endothelial health through several mechanisms, including the modulation of NO production, reduction of inflammation, and improvement of gut microbiota balance [15].
The gut microbiome is crucial for maintaining overall health. However, its balance is often severely disrupted during critical illnesses, sometimes within hours of intensive care unit (ICU) admission. Critically ill patients experience significant dysbiosis, marked by the loss of commensal bacteria and an overgrowth of potential pathogens such as Staphylococcus aureus, Acinetobacter spp., and Pseudomonas aeruginosa, whose growth is amplified by pro-inflammatory cytokines. This disruption, often exacerbated by ICU interventions like antimicrobials, can cause lasting damage to the gut microbiome. The consequences of this altered microbiome include increased susceptibility to infections, organ dysfunction, and prolonged recovery times, significantly affecting patient outcomes in intensive care settings. Therapeutic potential of probiotics gut microbiota-targeted therapies, including fecal microbiota transplantation (FMT) and probiotics, represent promising strategies to restore microbial balance and decrease systemic inflammation associated with dysbiosis. In critically ill patients, probiotics and synbiotics have demonstrated the potential to reduce systemic inflammation by stabilizing gut microbiota, minimizing postoperative infectious complications, and lowering the risk of ventilator-associated pneumonia (VAP). The prophylactic use of probiotics or synbiotics may offer a valuable approach to preventing infectious complications and modulating immune responses in these patients. Furthermore, probiotics have reduced VAP, nosocomial infections, infection rates, mechanical ventilation duration, and antibiotic use in critically ill populations. Nonetheless, more well-designed multicenter trials are necessary to establish evidence-based guidelines for their clinical application [16].
GRs are crucial for maintaining GI homeostasis by regulating the balance between pro- and anti-inflammatory factors, local immune responses, metabolic pathways, and epithelial integrity, especially during stressful situations such as critical illness. GRs are widely distributed throughout the GI tract, with the highest concentrations found in the duodenum, followed by the jejunum, ileum, stomach, and colon, while being notably absent in the esophageal mucosa. This distribution reflects each GI region’s unique physiological demands and stress responses.
GRs are essential for mitigating colitis and its associated risks in intestinal epithelial cells, including colorectal cancer. The lack of GRs in these cells worsens clinical symptoms, increases tissue damage, compromises epithelial barrier integrity, and accelerates tumorigenesis in the colon [17]. Notably, epithelial cells within the intestinal crypts serve as a significant source of locally synthesized GCs, which regulate activation in the GI tract. Unlike GCs derived from the adrenal glands, locally produced GCs reflect an adaptation to the unique needs of the intestine [18, 19]. Tumor necrosis factor (TNF) has been shown to stimulate GC production in the intestine, thus suppressing inflammation through localized steroidogenesis [20].
Intestinal epithelial cells have been recognized as a significant source of GCs. The synthesis of GCs in these cells differs from that in adrenal cells, likely due to adaptations to the local environment. Locally produced GCs are vital for regulating immune responses against viruses and chronic inflammatory conditions such as ulcerative colitis, Crohn’s disease, and related disorders. They are also crucial for proper macrophage activation and effective immune responses against infections like Helicobacter pylori, a known risk factor for gastric cancer [21].
Experimental studies further emphasize the significance of GRs in maintaining colonic homeostasis, highlighting their protective role in preserving epithelial integrity and immune balance. GCs modulate repair mechanisms and nuclear factor-kappa B (NF-κB) activity in the intestinal epithelium in a dose-dependent manner, promoting cell proliferation and restitution at lower doses while inhibiting these processes and increasing apoptosis at higher doses. These findings underscore the vital roles of GRs and GCs in supporting GI health by mitigating the effects of stress, inflammation, and infection across various regions of the GI tract.
Table 2 underscores the essential roles of GCs in maintaining gut homeostasis by reducing inflammation, strengthening epithelial barrier integrity, and restoring microbial balance. These effects are achieved through their ability to suppress inflammatory pathways, upregulate TJ proteins, promote epithelial regeneration, and modulate immune responses to prevent excessive inflammation and ensure immune tolerance. Moreover, GCs regulate gut microbiota composition, enhance digestive enzyme activity, and strengthen antimicrobial defenses. They also facilitate communication between interconnected systems, such as the brain-gut and lung-gut axes, ensuring systemic and intestinal health maintenance.
Functions of GCs in maintaining gut homeostasis
| Function category | Description | Key mechanisms/examples |
|---|---|---|
| Anti-inflammatory and antioxidant effects | Suppression of inflammatory responses and attenuation of oxidative stress to alleviate gut inflammation, protect against tissue injury, and preserve cellular viability. | - Inhibition of pro-inflammatory cytokines (e.g., IL-1β, IL-6) and induction of anti-inflammatory mediators IL-10 and Annexin A1 [64]- Upregulation of antioxidant enzymes superoxide dismutase and catalase [23, 79] |
| Preservation of epithelial barrier integrity | GRs are widely distributed throughout the GI tract [80]. GC-GR signaling strengthen gut epithelial function by enhancing epithelial tight junction structure and function to prevent pathogen invasion and maintain nutrient absorption. | - Upregulation of tight junction proteins (e.g., occludin, claudins, ZO-1) [71]- Annexin A1 as a crucial mediator for maintaining epithelial integrity [22]- Modulation of chemokine expression and control of leukocyte recruitment, resulting in decrease in gut permeability [17]- Promotion of epithelial cell regeneration |
| Modulation of microbiota composition | Support microbial diversity and homeostasis to maintain gut integrity and modulate immune responses. | - Promotion of beneficial bacterial growth [81]- Inhibition of pathogenic microbial species- Restoration of microbial balance post-dysbiosis |
| Immune system regulation | Balances immune activity to defend against pathogens while preventing excessive inflammation. | - Regulation of chemokines and leukocyte recruitment 18- Modulation of antigen presentation- Maintenance of immune tolerance |
| Interaction with the gut-brain-axis | Facilitates bidirectional interactions between the gut and the brain, impacting stress responses and neuroimmune functions [42]. | - Endothelial GRα helps regulate vascular homeostasis and supports blood-brain barrier (BBB) integrity [82]- Control of neuroinflammation, enhancing neuronal recovery, and bolstering stress resilience [46] |
| Cross-talk with lung and other organs | Support bidirectional interactions between the gut and lung which share microbial sources [50].Compromised gut barrier integrity allows microbial translocation to the lungs [51]. | - Modulation of the lung-gut axis in conditions such as asthma and inflammatory bowel disease (IBD)- GCs suppress systemic inflammatory cascades, reduce lung inflammation caused by gut-derived endotoxemia, and enhance barrier integrity in both the intestine and lungs |
| Regulation of intestinal enzymes and metabolic pathways | Control enzyme expression involved in digestion and nutrient absorption, and metabolic processes within the gut, including the conversion of amino acids into essential metabolites.Enhance glucose uptake by upregulating genes involved in glucose transport leading to increased blood glucose levels. | - Regulation of digestive enzyme expression and amino acid metabolism (e.g., arginase, glutaminase) [60]- Modulation of fucosyltransferases (FUTs) for fucosylatio- Influence on microbial metabolic pathways [31]- Upregulation of sodium-coupled glucose transporter 1 (SGLT1) in enterocytes [83] |
| Influence on gut motility and secretion | Modulation of intestinal motility and secretion of mucus and other substances essential for digestion and barrier function. Also regulate electrolyte and fluid secretion in the intestine. | - Enhance cholinergic neuromuscular transmission within the ENS [62]- Stabilize mucosal barrier [84]- Increase the electrical potential difference and Na+/K+- ATPase activity—key processes for effective ion transport [63]- Control of electrolyte and fluid secretion in the gut |
| Intestinal epithelial cells synthesis of GCs | Regulate immune responses against viruses and chronic inflammatory conditions, including macrophage activation in infections such as Helicobacter pylori. | - Regulation immune responses against viruses and chronic inflammatory conditions [17, 85, 86]- Macrophage activation for immune responses against infections like Helicobacter pylori [21] |
The GC-GR signaling plays a pivotal role in suppressing inflammation and mitigating oxidative stress. It inhibits the production of pro-inflammatory cytokines such as TNF-α, interleukin-1β (IL-1β), and IL-6 while enhancing anti-inflammatory mediators such as IL-10 and Annexin A1 [8]. The GC-GR signaling pathway regulates immune responses in intestinal epithelial cells by modulating chemokine expression and controlling leukocyte recruitment, ensuring a balanced inflammatory reaction [8, 17].
Evidence suggests that GC treatment effectively reduces intestinal inflammation by preventing neutrophil-driven epithelial damage and promoting macrophage polarization towards anti-inflammatory phenotypes. Annexin A1 emerges as a crucial mediator for managing intestinal inflammation and maintaining epithelial integrity [22]. This dual action lowers inflammation. It supports tissue healing, making GCs particularly effective in treating IBD conditions. In addition to its anti-inflammatory properties, GC mitigates oxidative stress by upregulating antioxidant enzymes such as superoxide dismutase and catalase. These enzymes protect epithelial cells from damage caused by reactive oxygen species (ROS)—induced apoptosis, preserving cellular viability. Studies in septic models demonstrate that GC therapy reduces markers of oxidative damage, prevents epithelial apoptosis, and bolsters mitochondrial antioxidant defenses, safeguarding epithelial viability during oxidative stress [23, 24]. Macrophage polarization is crucial for resolving intestinal inflammation. M1-like macrophages are pro-inflammatory and contribute to tissue damage, while M2-like macrophages possess anti-inflammatory properties and aid in tissue repair [8]. GC treatment facilitates a significant shift from the M1 to the M2 phenotype, promoting inflammation resolution and improving healing outcomes [25]. This macrophage reprogramming highlights the various benefits of GC therapy in restoring intestinal homeostasis and supporting recovery.
GCs play a pivotal role in maintaining intestinal epithelial barrier integrity by modulating claudin expression via the MAPK phosphatase-1 (MKP-1) pathway, a mechanism particularly relevant to conditions like IBD and collagenous colitis, where barrier dysfunction is a hallmark [26–28]. Studies demonstrate that GCs enhance transepithelial electrical resistance (TEER) in a time- and dose-dependent manner via GR activation, thereby strengthening the barrier without affecting the flux of larger solutes or compromising TJ architecture [26, 27]. This effect is attributed mainly to regulating claudin proteins, with GCs downregulating claudin-2, a pore-forming protein, while upregulating claudin-4, which fortifies TJs and barrier function [27, 28].
In addition to regulating claudin, GCs promote the synthesis of polyamines from amino acids such as proline and arginine, further supporting intestinal maturation and reducing paracellular cation flux to enhance barrier integrity [29]. These outcomes depend on the upregulation of MKP-1, which suppresses mitogen-activated protein kinase (MAPK) signaling pathways that influence TEER and TJ protein composition. Inhibition of MKP-1 negates these benefits, underscoring its critical role in GC-driven barrier enhancement [26–28].
GR signaling also engages the PI3K/Akt/NF-κB pathway, which exerts anti-inflammatory effects while preserving epithelial integrity [30]. This dual mechanism highlights GCs’ unique ability to reduce inflammation and restore barrier function simultaneously. By reshaping the TJ protein landscape, modulating inflammatory pathways, and stabilizing epithelial cells, GCs offer a comprehensive therapeutic strategy for improving epithelial barrier integrity in inflammatory bowel conditions [26–28].
GC-GR signaling plays a critical role in microbiome regulation, beginning early in life to shape intestinal development and maintain homeostasis. Experimental studies have revealed that GCs and gut microbiota collaboratively regulate the expression of intestinal fucosyltransferase 2 (Fut2), an enzyme vital for an enzyme vital for producing fucosylated glycans. These glycans support commensal microbiota, strengthen the intestinal barrier, and protect against inflammation and infections. Disruption of this GC-microbiota-Fut2 axis, particularly during early life, leads to dysbiosis and impaired gut health [31]. Additionally, the gut microbiota modulates local GC production, influencing intestinal responses to stress and inflammation. This intricate interplay between the microbiota, local steroidogenesis, and immune modulation directly impacts immune cell activity, inflammation resolution, and overall intestinal homeostasis [32].
The evidence further underscores this dynamic in autoimmune and inflammatory conditions. In systemic lupus erythematosus (SLE) GC treatment restored gut microbiota composition to resemble that of healthy individuals, supporting homeostasis [33]. Similarly, in immune thrombocytopenia (ITP), distinct microbial profiles in corticosteroid-resistant and corticosteroid-sensitive patients highlighted the microbiota’s influence on therapeutic outcomes [34]. However, prolonged GC use may induce dysbiosis, reducing microbial diversity and altering bacterial populations, which contributes to side effects like metabolic disorders [35]. Notably, commensal gut bacteria appear to mediate anti-inflammatory effects in the large intestine following GC exposure, further emphasizing their collaborative role.
The interaction between GCs and probiotics presents significant therapeutic potential for managing inflammatory diseases. Combining GCs with probiotics enhances outcomes by reducing inflammation and restoring microbial balance. In Crohn’s disease, this combination decreased levels of pathogenic species like yeast and enterococci while increasing Lactobacillus populations, surpassing sulfasalazine in efficiency and infection prevention [36]. Similarly, probiotics with GCs inhibited NF-κB activation in ulcerative colitis, reduced pro-inflammatory cytokines, and preserved mucosal integrity, effectively preventing disease relapse [37]. GCs also influence microbial diversity and populations, affecting inflammation, bone health, and drug metabolism [38–40]. In animal models, GCs like dexamethasone reduced colonic inflammation while promoting beneficial microbial populations. Remarkably, transferring these microbiome changes to other mice conferred anti-inflammatory benefits, suggesting that the microbiome mediates some of the therapeutic effects of GCs [38].
Furthermore, in autoimmune hepatitis (AIH), Lactobacillus enhances the therapeutic effects of prednisone by modulating gut microbiota and reducing follicular helper T (Tfh) cells along with their associated cytokines, which are critical drivers of disease progression. These findings highlight the potential of combining probiotics with GCs to amplify therapeutic efficacy in immune-mediated diseases. Interestingly, GCs also modulate the gut microbiome in non-human systems. For example, a study involving wild birds demonstrated that GC treatment altered the GI microbiome, reducing both pathogenic and beneficial microbes. While the precise implications of this dual effect remain unclear, it underscores the complex interplay between GCs and microbial ecosystems, which may vary across species and environmental contexts. These findings highlight the bidirectional relationship between gut microbiota and GC signaling, emphasizing their collective role in modulating immune responses, shaping therapeutic outcomes, and maintaining systemic health equilibrium. Conversely, the gut microbiota can influence GC metabolism in the GI tract. Certain bacterial species enzymatically degrade or modify GCs, impacting their bioavailability and efficacy. Dysbiosis exacerbates these effects, which may explain the variable outcomes of GC therapy in conditions like IBD and critical illness. Administering GCs intravenously bypasses first-pass metabolism, avoiding microbiota-mediated degradation and ensuring effective systemic drug delivery.
Maintaining a healthy microbiome is essential for minimizing complications associated with GC therapy, including osteoporosis and osteonecrosis. GC-induced osteoporosis (GIO) often results from prolonged GC use, with studies showing that GCs alter gut microbiota composition, which plays a critical role in bone density regulation, essential for regulating bone density. Notably, reducing gut microbiota due to antibiotic use prevented GC-induced bone loss, highlighting the microbiota’s involvement in GIO pathogenesis. Probiotic treatment with Lactobacillus reuteri halted bone loss, suggesting potential therapeutic strategies focusing on the gut microbiota to reduce GIO [39]. Probiotics and dietary interventions can preserve gut health and reduce complications, including bone loss and other adverse effects of prolonged GC therapy [39].
GCs significantly influence intestinal immune responses by modulating inflammation, T-cell activation, and stabilizing the mucosal barrier, which are critical for maintaining gut homeostasis. Their local synthesis in the intestine plays a dual role in sustaining immune homeostasis. Although research on the role of GCs in critical illness-associated gut inflammation and dysbiosis remains limited, experimental models of intestinal inflammation, such as colitis, provide valuable insights into potential mechanisms and therapeutic strategies for critically ill patients. In the absence of GR signaling in intestinal epithelial cells, the clinical severity of colitis increases, leading to heightened tissue damage, compromised epithelial barrier integrity, and an elevated risk of colon tumorigenesis. GR signaling in intestinal epithelial cells plays a protective role by suppressing chemokine expression and modulating leukocyte recruitment, stabilizing the mucosal barrier, and reducing tissue injury [17]. This anti-inflammatory activity is further supported by the interaction of GR with molecular pathways such as the PI3K/Akt/NF-κB axis, which enhances epithelial barrier integrity, reduces oxidative stress, and limits the amplification of inflammatory responses. Future research focusing on the interplay between GR and other signaling pathways and the impact of GC-based interventions on the gut microbiome will be essential for translating these findings into effective clinical treatments.
Combining GCs and probiotics in critical illness offers a promising therapeutic strategy for addressing inflammation, dysbiosis, and immune dysregulation, providing a multifaceted approach to improve patient outcomes. GCs, known for their potent anti-inflammatory and immunomodulatory properties, effectively reduce the hyperinflammatory response typical of critical illness by suppressing pro-inflammatory cytokine production. Additionally, they strengthen epithelial barrier integrity by promoting TJ proteins, thereby lessening intestinal permeability and safeguarding against systemic complications. However, prolonged GC use may unintentionally disturb the gut microbiome, raising the risk of dysbiosis and secondary complications.
GCs, known for their potent anti-inflammatory and immunomodulatory effects, effectively reduce the hyper-inflammatory response typical of critical illness by inhibiting pro-inflammatory cytokine production [41]. Furthermore, they improve epithelial barrier integrity by increasing TJ proteins, thus lowering intestinal permeability and safeguarding against systemic complications. However, prolonged GC usage may unintentionally disrupt the gut microbiome, heightening the risk of dysbiosis and secondary complications, including infections.
Probiotics are a valuable addition to GCs, restoring microbial diversity, fostering the growth of beneficial bacteria, and inhibiting pathogenic species. By enhancing mucosal immunity, strengthening epithelial barriers, and reducing intestinal permeability, probiotics decrease bacterial translocation and systemic inflammation, offering further protection against complications such as MODS and prolonged recovery times. Reducing systemic inflammation is particularly crucial, as inflammation disrupts the intestinal environment by damaging the epithelial barrier, altering gut motility, and causing oxidative stress, all contributing to dysbiosis. Probiotics address these issues by reestablishing microbial balance, regulating immune responses, and reducing oxidative stress, complementing GCs in sustaining gut and systemic health.
The synergy between GCs and probiotics addresses the immediate challenges of inflammation and dysbiosis while creating a positive feedback loop that improves gut microbiota composition and function. By reducing systemic inflammation, GCs support the restoration of a stable microbiome, and probiotics enhance GC efficacy by strengthening the gut’s defense mechanisms. Together, these therapies boost intestinal resilience, protect against secondary complications, and promote recovery in critically ill patients. While emerging evidence highlights the potential of this combined approach, further well-designed clinical studies are necessary to establish the optimal strains, dosages, and treatment protocols. These investigations will be crucial in maximizing the efficacy and safety of this dual therapy.
The interactions between the gut and the brain are critical for maintaining overall health and homeostasis. At the core of this interplay lies the microbiota-gut-brain (MGB) axis, which facilitates bidirectional communication through neural, endocrine, immune, and metabolic pathways. This system is essential for regulating neurodevelopment, cognitive functions, and stress responses, underscoring its significant role in preserving physiological balance [42]. Central to the MGB axis is the interplay between gut microbiota and the hypothalamic-pituitary-adrenal (HPA) axis, whose coordinated activity is vital for adaptive responses and homeostatic maintenance. The gut microbiota is a key mediator that connects the central nervous system (CNS), autonomic nervous system, and endocrine system. Communication occurs through direct neural pathways, including the vagus nerve, and indirect routes involving cytokines, neuropeptides, and neuroactive compounds. Notably, dysregulation within the MGB axis has been linked to a wide range of neuropsychiatric and systemic disorders, highlighting the critical role of gut microorganisms in sustaining proper HPA axis balance [43].
Animal models have further illuminated how gut microbes influence neural processes such as neurogenesis and myelination by activating microglia—the brain’s resident immune cells—thereby shaping brain development and function. Experimental research supports the significance of this gut-brain connection. Investigations using germ-free mice reveal elevated expression of GR-related genes, establishing a direct link between gut microbial status and brain function via GR signaling [44]. In this context, probiotics have emerged as promising modulators of HPA axis responsiveness. By preserving intestinal barrier integrity and minimizing circulating lipopolysaccharide (LPS)—a potent inflammatory trigger—probiotics can reduce the physiological burden of stress [45].
In critical care, the gut-brain axis is integral to neuroprotection through its roles in controlling neuroinflammation, enhancing neuronal recovery, and bolstering stress resilience [46]. GCs are central to these protective effects: the endothelial GRα helps regulate vascular homeostasis and supports blood-brain barrier (BBB) integrity [8]. Furthermore, GCs confer neuroprotection by suppressing lipid peroxidation and stimulating neurotrophin receptors, thus favoring neuronal survival and recovery [47].
In certain conditions such as post-traumatic stress disorder (PTSD)—often observed after critical illness—the reduced GR hormone-binding capacity is implicated in persistent stress-related symptoms [48]. However, prolonged GC therapy in critically ill patients has shown promise in diminishing PTSD symptom severity post-recovery, potentially by impairing the retrieval of traumatic memories during treatment [24, 49]. Neurological complications, including delirium and cognitive decline, are frequent in critically ill individuals, emphasizing the importance of strategies that target both central and peripheral mechanisms. In this context, GCs help stabilize the BBB, mitigate neuroinflammation, and facilitate neuronal recovery. Probiotics, in turn, enhance gut barrier function, reduce systemic inflammation, and promote the generation of neuroactive molecules. These observations suggest a potential synergistic approach: co-administration of probiotics and GCs may offer enhanced benefits for neuroprotection and recovery in critical illness. While the evidence is promising, additional research is required to elucidate and optimize this combined therapeutic strategy fully.
The gut-lung axis is an emerging research area that explores the bidirectional interactions between the gut and lung microbiomes, particularly in critically ill patients. It has shown a strong correlation between the gut and lung microbiomes, with common microbial species like Enterococcus faecium and Corynebacterium striatum identified in both sites. This overlap suggests a shared microbial source and reinforces the concept of the gut-lung axis [50]. These interactions occur primarily through the portal venous and mesenteric lymphatic systems, particularly when gut barrier integrity is compromised, allowing microbial translocation to the lung [51].
Gut-derived metabolites, such as SCFAs, play a critical role in modulating immune responses and maintaining homeostasis in the airways [52]. SCFAs, including butyrate and propionate, exhibit systemic anti-inflammatory effects by influencing immune cell activity, primarily T and dendritic cells [5, 6]. These metabolites suppress the expression of adhesion molecules and chemokines in response to inflammatory stimuli, thereby reducing the recruitment of monocytes, macrophages, and neutrophils [5, 6]. Butyrate alleviates excessive inflammation by enhancing the functions of M2 macrophages and regulatory T cells while inhibiting neutrophil infiltration. Elevated levels of SCFA—especially propionate—are associated with reduced lung inflammation, underscoring their potential to preserve immune homeostasis in the lungs. Higher levels of SCFAs, especially propionate, are associated with reduced lung inflammation, underscoring their potential role in preserving immune homeostasis in the lungs [53]. Additionally, tryptophan metabolites produced by intestinal bacteria interact with the aryl hydrocarbon receptor (AhR) in lung epithelial and immune cells, promoting epithelial repair and mitigating inflammation. The interaction between tryptophan metabolites and AhR is not limited to the lungs. Still, it extends to other systems, such as the CNS [54] and GI tract [55], which influence inflammation and immune responses.
Various therapeutic interventions—including antibiotics, sedatives, analgesics, nutritional support, body positioning, and invasive mechanical ventilation—can disrupt microbiota balance and amplify the inflammatory response in critically ill patients. Mechanically ventilated patients often experience respiratory microbiome dysbiosis, characterized by decreased microbial diversity and a shift toward dominant bacterial pathogens such as Proteobacteria. This dysbiosis is particularly pronounced in conditions like VAP and ARDS, where gut-associated bacteria such as Enterobacteriaceae are concentrated in the lungs. Although the exact role of this dysbiosis in ARDS pathogenesis is not fully understood, it is believed to worsen disease severity and progression [56]. Mendelian randomization analysis further supports the essential role of gut microbiota-inflammation interactions in ARDS pathogenesis [57].
Systemic immune signaling plays a significant role in the dynamics of the gut-lung axis. Microbial dysbiosis raises pro-inflammatory cytokine levels, exacerbating pulmonary injuries such as ARDS [58] and VAP [59]. Healthy intestinal microbiota supports regulatory T cells (Tregs) that suppress excessive lung inflammation, maintain immune balance, and aid in the resolution of inflammation through anti-inflammatory cytokines like IL-10 and TGF-β. In contrast, intestinal barrier dysfunction allows microbial translocation and the release of endotoxins into the systemic circulation, triggering pulmonary inflammation. GC therapy is crucial in modulating the gut-lung axis during critical illness. By activating GR signaling, GCs suppress systemic inflammatory cascades, reduce lung inflammation caused by gut-derived endotoxemia, and enhance barrier integrity in both the intestine and lungs. This dual action minimizes microbial translocation and lowers the risk of secondary infections, providing a promising therapeutic strategy for alleviating complications related to the gut-lung axis.
GCs are crucial in regulating intestinal enzymes and metabolic pathways, particularly concerning amino acid metabolism, glucose uptake, and immune regulation within the intestinal microenvironment. These effects are mediated through complex signaling networks, highlighting the multifaceted role of GCs in intestinal physiology and pathology.
GCs play a crucial role in enhancing the metabolism of amino acids, such as arginine and glutamine, in the intestines, especially during physiological stress. For instance, they upregulate the activity of enzymes like arginase and pyrroline-5-carboxylate synthase, which facilitate the conversion of amino acids into essential metabolites. This process supports cellular energy needs and helps maintain metabolic homeostasis under stress [60]. These adaptive mechanisms highlight the importance of GCs in fostering resilience during challenging physiological stress conditions.
GCs are crucial regulators of glucose metabolism in the intestine. They enhance glucose uptake by upregulating genes involved in glucose transport, such as sodium-coupled glucose transporter 1 (SGLT1). This upregulation occurs via GR signaling within enterocytes, increasing blood glucose levels. While this mechanism is essential for meeting systemic energy demands during stress, chronic activation may result in hyperglycemia and related metabolic disturbances.
Enteric glia residing within the ENS, particularly in the myenteric and submucosal plexuses, play a critical role in regulating the neuronal circuits responsible for the coordinated contraction and relaxation of intestinal smooth muscles. These glial cells release signaling molecules such as glutamate, ATP, and NO, modulating neuronal activity to ensure the synchronized contractions necessary for peristalsis [61]. Calcium signaling within enteric glia further harmonizes neuronal responses, facilitating efficient smooth muscle function.
GCs enhance cholinergic neuromuscular transmission within the ENS, an effect mediated by GRs. This interaction increases the proportion of choline acetyltransferase (ChAT)-immunoreactive neurons and acetylcholine levels, thereby influencing colonic motility and promoting coordinated intestinal movements [62].
In addition to their effects on motility, GCs significantly regulate electrolyte and fluid secretion in the intestine. By modulating ion transport channels and pumps, GCs enhance sodium and water absorption in the colon, increasing the electrical potential difference and Na⁺/K⁺-ATPase activity—key processes for effective ion transport. They also facilitate potassium secretion in the colon, although their influence on potassium absorption varies depending on the intestinal segment [63]. These combined actions of GCs on gut motility and secretion underscore their essential role in maintaining intestinal homeostasis and responding to physiological and pathological challenges.
Maintaining and restoring intestinal barrier homeostasis requires complex interactions among epithelial cells, immune components, and the gut microbiota. Vitamins D, A, C, and E are pivotal in reinforcing epithelial integrity, modulating immune responses, and reducing oxidative stress, thereby optimizing GR function within the gut microenvironment (Table 3). Their synergy is significant in critical illness, where epithelial damage, dysbiosis, and excessive inflammation often occur, and GR-mediated homeostatic corrections may be impaired [64].
Vitamins and their roles in GC receptor function and gut homeostasis
| Vitamin | Impact on GR function | Impact on epithelial barrier integrity | Impact on microbiome composition | Antioxidant activity | Anti-inflammatory effects | Immune modulation |
|---|---|---|---|---|---|---|
| Vitamin D | Augments GR signaling by reducing local inflammation and enhancing local GC pathways [64]. | Regulates tight junction proteins (e.g., ZO-1, claudins), reducing permeability and bacterial translocation [65, 66]. | Shapes gut microbiota by promoting beneficial genera (e.g., Akkermansia, Bifidobacterium) and mitigating dysbiosis [87, 88]. | Limited direct antioxidant effects | Suppresses pro-inflammatory Th1/Th17 cells, enhances Tregs, and induces antimicrobial peptides [65, 88] | Balances adaptive immunity by supporting regulatory T cells and controlling excessive inflammation [66] |
| Vitamin C | Preserves GR function by alleviating oxidative stress, which sustains GR sensitivity [68]. | Upregulates tight junction proteins (some evidence of synergy with vitamin D3), improving barrier integrity [67]. | May help shift gut microbiota toward beneficial species; can increase short-chain fatty acid-producing bacteria. | Strong antioxidant, neutralizes ROS [64, 68] | Modulates cytokines (IL-22, IL-6) to curb inflammation; aids epithelial repair [68, 69] | Enhances phagocytic cell function (chemotaxis, phagocytosis) and supports B-cell/T-cell proliferation [69] |
| Vitamin A | Deficiency alters GR binding capacity; retinoic acid normalizes receptor activity [89]. | Enhances tight junction expression; counters barrier disruption caused by inflammatory stimuli [87]. | Regulates microbial complexity and supports mucosal immunity for gut homeostasis. | Primarily immunoregulatory, with some antioxidant benefits (especially from carotenoid forms) | Reduces inflammatory mediators and promotes epithelial repair [87, 89] | Directs T-cell homing to gut, facilitates IgA production, and aids dendritic cell function [90] |
| Vitamin E | Indirectly supports GR-mediated anti-inflammatory pathways by lowering oxidative/inflammatory loads. | Stabilizes tight junction proteins and reduces colitis-associated epithelial injury [91]. | Modulates gut microbiota composition, diminishing pro-inflammatory taxa*. | Antioxidant; lowers ROS levels | Decreases TNF-α, IL-6, and other inflammatory mediators [91] | Contributes to mucosal healing by preserving barrier integrity |
ZO-1: zonula occludens-1; GR: glucocorticoid receptor; ROS: reactive oxygen species; TNF-α: tumor necrosis factor-alpha; IL: interleukin; Treg: regulatory T cell; GC: glucocorticoid; IgA: immunoglobulin A; Th: T helper. * Pro-inflammatory taxa refer to groups of gut microbes known to promote inflammation in the body (e.g., certain Proteobacteria or Enterobacteriaceae)
Vitamin D signals primarily through the vitamin D receptor (VDR) to regulate TJ proteins (e.g., ZO-1 and claudin-2), essential for preserving epithelial barrier integrity and minimizing bacterial translocation [65, 66]. These proteins are necessary to maintain epithelial barrier integrity, prevent the translocation of harmful bacteria, and reduce susceptibility to inflammation and infections [65]. Vitamin D deficiency disrupts this barrier function, heightening vulnerability to inflammation-related diseases such as IBD [65, 66].
Beyond its structural role, vitamin D influences immune responses by enhancing Treg populations and suppressing pro-inflammatory T helper 1 (Th1)/Th17 subsets, thereby curbing epithelial inflammation. Additionally, vitamin D induces AMPs (e.g., cathelicidins and defensins) to strengthen barrier defense and plays a key role in shaping gut microbiota, promoting beneficial genera like Akkermansia and Bifidobacterium while mitigating dysbiosis. These combined actions directly support GR function by creating an anti-inflammatory microenvironment favorable for GR signaling, thus enhancing GR’s capacity to maintain epithelial homeostasis and immune regulation. Restoring microbial balance through vitamin D supplementation has shown potential to reduce inflammation and promote mucosal healing in IBD.
Vitamin C supports intestinal barrier integrity by enhancing the expression of TJ proteins, especially when co-administered with vitamin D3 [67]. As a potent antioxidant, vitamin C neutralizes ROS, thus minimizing oxidative stress-mediated epithelial damage in conditions such as IBD [68]. Furthermore, vitamin C modulates cytokine profiles (e.g., IL-22 and IL-6) to balance immune activity and promote phagocytic cell function for effective pathogen clearance [68, 69]. By controlling local oxidative stress and cytokine balance, vitamin C directly preserves GR sensitivity and function, which is crucial under the altered redox conditions characteristic of critical illness. Additionally, vitamin C supports various immune cell functions, including those of phagocytic cells like neutrophils, enhancing chemotaxis, phagocytosis, and microbial killing. It also aids in the differentiation and proliferation of B-cell and T-cells [69].
Through its active metabolite retinoic acid, vitamin A regulates the expression of epithelial TJ proteins and influences dendritic cell and T-lymphocyte functions, all of which uphold gut mucosal immunity. Retinoic acid receptor (RAR) signaling also facilitates T-cell homing to the gut and supports IgA production, thus bolstering the mucosal defense against pathogens. A deficiency in vitamin A has been linked to altered GR binding capacity. In contrast, retinoic acid supplementation normalizes receptor activity, underscoring vitamin A’s importance in fine-tuning GR-mediated immune regulation. Therefore, vitamin A directly modulates GR responsiveness, strengthening epithelial homeostasis and immune balance through receptor-level interactions.
Vitamin E, mainly alpha- and gamma-tocopherol, helps protect the intestinal epithelium by stabilizing the expression of TJ proteins and reducing oxidative damage. This activity lowers levels of pro-inflammatory cytokines such as TNF-α and IL-6, thereby protecting experimental models of colitis. Although direct interactions between vitamin E and GR signaling are less characterized, vitamin E’s antioxidant activity maintains the cellular redox state essential for optimal GR responsiveness, indirectly reinforcing GR’s barrier-protective and anti-inflammatory roles in the gut.
Supplementing vitamins D, A, C, and E—potentially in conjunction with GCs and probiotics—can stabilize the intestinal barrier, mitigate dysbiosis, and promote balanced immune responses in critically ill patients. Restoring barrier function reduces pathogen translocation, while these vitamins’ anti-inflammatory and antioxidant actions align with and enhance GR-mediated immunomodulation [64, 66]. In IBD, targeted vitamin supplementation may help restore epithelial integrity, modify pathogenic inflammation, and aid mucosal healing, improving clinical outcomes [65]. For a comprehensive review of micronutrient support for GR functions, the reader is referred to the supplementary material titled “The Temporal Phases of GRα Function and the Potential Impact of Micronutrients on Its Regulation” in [70] and a recent review [64].
Vitamins D, A, C, E, and selected B vitamins [7] each play distinct yet complementary roles in maintaining epithelial barrier function, regulating gut microbiota, reducing oxidative stress, and modulating immune and inflammatory pathways—all of which closely interact with GR-mediated processes. These synergistic effects highlight the potential therapeutic value of targeted vitamin supplementation, in conjunction with GCs and probiotics, for managing IBD, enteropathy associated with critical illness, and other conditions marked by impaired intestinal homeostasis. Further research is needed to identify optimal dosing strategies, timing, and vitamin combinations that most effectively enhance gut integrity and patient outcomes.
Emerging evidence highlights the therapeutic potential of integrating GCs, probiotics, and targeted vitamin supplementation to address key pathophysiological drivers of critical illness—namely hyperinflammation, dysbiosis, epithelial barrier disruption, and oxidative stress. Collectively, these interventions create a biologically coherent, synergistic framework: GCs modulate inflammation; probiotics restore microbial balance, and vitamins—particularly D, A, C, E, and B-complex—enhance barrier function, immune responses, and redox homeostasis. This integrated, low-risk strategy can potentially restore intestinal barrier function, reduce systemic inflammation, and improve clinical outcomes in critically ill patients.
To advance this approach, a comprehensive research agenda is needed. Mechanistic studies should explore how GR signaling interacts with the microbiota, epithelial cells, and microbial metabolites. Preclinical models of sepsis, ARDS, and intestinal injury models can evaluate combination therapies on inflammation, epithelial integrity, and organ function.
Future efforts should refine dosing regimens and treatment durations for GCs, probiotics, and each vitamin; identify the most effective probiotic strains with robust immunomodulatory and barrier-stabilizing properties; define optimal vitamin combinations (such as D, A, C, E, and select B vitamins); and determine timing to maximize synergy with GCs and probiotics. Well-designed, randomized controlled trials are essential across diverse populations of critically ill patients, including those with severe sepsis and ARDS, to evaluate the efficacy, safety, and long-term outcomes.
AIH: autoimmune hepatitis
AMPs: antimicrobial peptides
ARDS: acute respiratory distress syndrome
BBB: blood-brain barrier
ChAT: choline acetyltransferase
CNS: central nervous system
ECs: endothelial cells
ENS: enteric nervous system
FMT: fecal microbiota transplantation
FUTs: fucosyltransferases
GALT: gut-associated lymphoid tissue
GCs: glucocorticoids
GI: gastrointestinal
GIO: glucocorticoid-induced osteoporosis
GR: glucocorticoid receptor
HPA: hypothalamic-pituitary-adrenal
IBD: inflammatory bowel disease
ICU: intensive care unit
IgA: immunoglobulin A
IL-6: interleukin-6
ITP: immune thrombocytopenia
LPS: lipopolysaccharide
MAPK: mitogen-activated protein kinase
MGB: microbiota-gut-brain
MKP-1: mitogen-activated protein kinase phosphatase-1
MODS: multiple organ dysfunction syndrome
NF-κB: nuclear factor-kappa B
NO: nitric oxide
PTSD: post-traumatic stress disorder
RAR: retinoic acid receptor
ROS: reactive oxygen species
SCFA: short-chain fatty acid
SGLT1: sodium-coupled glucose transporter 1
sIgA: secretory immunoglobulin A
SLE: systemic lupus erythematosus
TEER: transepithelial electrical resistance
Tfh: follicular helper T
Th: T helper
TJs: tight junctions
TNF-α: tumor necrosis factor-alpha
Tregs: regulatory T cells
VAP: ventilator-associated pneumonia
VDR: vitamin D receptor
ZO: zonula occludens
GUM: Conceptualization, Investigation, Writing—original draft, Writing—review & editing.
The author declares no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
