Affiliation:
1Department of Medicine, Sana’a University, Sana’a 12268, Yemen
Email: balsharafi@hotmail.com
ORCID: https://orcid.org/0000-0002-0520-8164
Affiliation:
2Thi Qar Specialized Diabetes Endocrine and Metabolism Center, College of Medicine, University of Sumer, Thi Qar 61004, Iraq
ORCID: https://orcid.org/0000-0002-7212-4358
Explor Endocr Metab Dis. 2025;2:101430 DOI: https://doi.org/10.37349/eemd.2025.101430
Received: January 17, 2025 Accepted: April 03, 2025 Published: April 28, 2025
Academic Editor: Tzong-Shyuan Lee, National Taiwan University, Taiwan, China
The article belongs to the special issue Metabolic Syndrome in Menopause
Hormone replacement therapy (HRT) is essential for alleviating menopausal symptoms and mitigating complications linked to type 2 diabetes mellitus (T2DM) in postmenopausal women. Studies indicate that HRT may contribute to better glycemic control, improved lipid metabolism, and enhanced kidney function, which could help lower the risk of diabetes-related complications. Early initiation of HRT near menopause has shown cognitive and metabolic benefits, while transdermal estrogen is preferred for women with cardiovascular risk due to its safer profile. However, HRT is associated with risks, including thromboembolic events and increased risks of breast and endometrial cancers, necessitating individualized evaluations. Despite its potential, significant gaps remain regarding HRT’s long-term safety and efficacy, its interaction with modern diabetes therapies, and its impact on diverse populations. The optimization of estrogen replacement therapy (ERT) strategies requires further exploration, particularly regarding patient screening, individualized treatment regimens, and personalization of care based on risk factors such as metabolic status, cardiovascular health, and diabetes severity. Current evidence supports the cautious use of HRT in strictly screened postmenopausal women with T2DM, adhering to key principles including early intervention (within 10 years of menopause), low-dose and short-duration therapy (usually < 5 years), and integration of lifestyle interventions (e.g., diet, exercise). Future research should focus on defining clear screening criteria, optimizing treatment regimens, and personalizing ERT to maximize benefits while mitigating risks. This review examines the benefits, risks, and optimization strategies of HRT in postmenopausal women with T2DM. It focuses on its metabolic, cardiovascular, cognitive, and renal effects and emphasizes personalized treatment approaches.
Menopause, characterized by the permanent cessation of menstruation, leads to a substantial decline in estradiol and progesterone levels, impacting millions of women globally [1]. This shift in hormone levels often leads to symptoms like hot flashes, night sweats, and vaginal dryness. Hormone replacement therapy (HRT) is widely recognized as the most effective option for managing these symptoms [1]. Concurrently, type 2 diabetes mellitus (T2DM) has emerged as a global health challenge, with a prevalence of 10.5%, driven by rising obesity rates and aging populations [2].
HRT has shown potential in improving glycemic control and insulin sensitivity in postmenopausal women, with or without T2DM, and may delay diabetes onset [3]. However, the benefits of HRT are most pronounced when initiated within 10 years of menopause, as suggested by the “timing hypothesis” [4]. Evidence from cohort studies indicates that early initiation of HRT in this window can reduce insulin resistance, preserve pancreatic beta-cell function, and lower cardiovascular risks [5, 6]. On the other hand, starting HRT beyond this timeframe may increase the likelihood of thromboembolic events and negative cardiovascular effects [7].
Since its introduction in the 1930s, HRT has evolved significantly. Early formulations carried risks such as endometrial cancer with unopposed estrogen, leading to the addition of progesterone to reduce this risk [8]. Misinterpretations of findings from the Women’s Health Initiative (WHI) trial caused a decline in HRT use, particularly among women with T2DM, despite its potential benefits [8].
The development of individualized HRT plans should involve a multidisciplinary approach, integrating endocrinologists, cardiologists, and gynecologists to assess patient-specific risks and ensure safe and effective treatment [5, 6, 9].
Screening criteria should include cardiovascular evaluation, metabolic profiling, and an assessment of the patient’s risk for thromboembolic events before initiating therapy [10].
This review seeks to examine the benefits and safety aspects of HRT in managing postmenopausal women with T2DM. It examines HRT’s effects on glycemic control, cardiovascular health, cognitive function, and renal outcomes while highlighting the need for personalized treatment approaches, this review focuses on risk assessment, appropriate timing, and the careful selection of therapy to ensure optimal outcomes.
Menopausal hormone therapy has demonstrated multiple mechanisms by which it positively influences glucose metabolism. However, personalization and optimization of therapy are crucial.
Estrogen enhances insulin sensitivity by modulating insulin receptor expression and reducing insulin resistance [3, 6]. Additionally, estrogen improves pancreatic beta-cell function, enhancing insulin secretion and protecting against beta-cell apoptosis, which is critical for glycemic control [9]. Furthermore, HRT has been shown to reduce systemic inflammation by lowering pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which contribute to insulin resistance [6, 11].
Studies have shown that HRT in women without T2DM can lower insulin resistance, as measured by the homeostatic model assessment for insulin resistance (HOMA-IR), by 13% and decrease the incidence of T2DM by 30% [3, 9]. In women already diagnosed with T2DM, the improvements are even more pronounced, with a reported 36% reduction in fasting blood glucose levels and HOMA-IR, likely due to improved insulin signaling and anti-inflammatory effects [3, 9].
Despite these benefits, lifestyle modifications—including adopting healthy eating habits, engaging in regular physical activity, achieving weight loss, and quitting smoking—remain the cornerstone of T2DM prevention [12]. Evidence suggests that the metabolic benefits of HRT are significantly enhanced when combined with structured lifestyle interventions [13]. For instance, adding a sugar-controlled diet and regular physical activity has been shown to amplify improvements in insulin sensitivity and lipid profiles among postmenopausal women undergoing HRT [14]. Clinicians should emphasize a multidisciplinary approach, incorporating dietary counseling, exercise programs, and behavioral interventions to maximize therapeutic outcomes while mitigating potential side effects [15]. Menopausal hormone therapy is not recommended as a primary preventive measure for T2DM [12, 16].
A meta-analysis of more than 191,000 women found a correlation between early menopause and a heightened risk of developing T2DM [11]. Furthermore, research indicates that women who experience surgically induced menopause face an increased risk of developing metabolic syndrome [5]. Another meta-analysis reported a significant reduction in glycated hemoglobin (HbA1c) levels by approximately 0.56% in women with T2DM receiving HRT. The analysis also showed a notable decrease in fasting glucose levels, although no significant changes were observed in postprandial glucose levels. Notably, estrogen’s influence on glucose metabolism appears to be time-dependent, with earlier initiation yielding more favorable metabolic outcomes, emphasizing the significance of timely HRT use. However, this review did not reach definitive conclusions regarding the impact of hormone therapy on individuals with type 1 diabetes mellitus [10].
T2DM significantly increases the risk of dementia and brain atrophy in older women [17, 18]. Some studies suggest that elevated estradiol levels may further exacerbate these risks. The WHI Memory Study (WHIMS) found a correlation between diabetes, reduced brain volume, and poorer cognitive function in older women [19]. The brain atrophy observed in women with T2DM is believed to result from heightened inflammation, glucose dysregulation, and impaired circulation [20–22].
Estrogen is essential for maintaining energy metabolism in the brain by enhancing aerobic glycolysis and facilitating glucose transport [20, 23]. Additionally, estrogen suppresses reliance on alternative energy sources, such as fatty acid and ketone metabolism [20, 24]. In older women with diabetes, this suppression might contribute to increased gray matter atrophy [20]. Studies observed an association with lower gray matter volumes in postmenopausal women with T2DM who had been on HRT for 4–6 years [17, 20].
Furthermore, higher estradiol levels were linked to an increased risk of dementia among women with diabetes, though this effect was not observed in women without diabetes [17, 25]. However, initiating HRT closer to the onset of menopause does not appear to increase long-term cognitive risks, suggesting a potential protective window for its use [25]. Interestingly, the “timing hypothesis” suggests that initiating HRT closer to menopause may mitigate long-term cognitive risks associated with diabetes, potentially leveraging a neuroprotective window [26]. This aligns with findings showing that delayed initiation of HRT, particularly in older postmenopausal women, may exacerbate cognitive decline due to advanced vascular and metabolic dysfunction [25].
While these findings support the potential cognitive benefits of early HRT initiation, the long-term impact in women with pre-existing cardiovascular conditions remains unclear. Given that diabetes and cardiovascular diseases (CVD) share overlapping risk factors and that oral estrogen formulations may increase thromboembolic risk, future research should focus on the following:
There have been longitudinal studies examining cognitive outcomes in women with T2DM and CVD who initiate HRT early versus late [25, 26].
Compare the effects of transdermal vs. oral estrogen on cerebrovascular function and dementia risk in diabetic women with cardiovascular risk factors [20, 25].
The interplay between HRT and diabetes therapies, such as glucagon-like peptide 1 (GLP-1) receptor agonists and sodium-glucose co-transporter 2 (SGLT2) inhibitors, has impacted neuroprotection and metabolic regulation [25, 26].
Until further research clarifies these long-term effects, HRT should be carefully individualized, with transdermal estrogen preferred for women at higher cardiovascular risk [20, 25].
The relationship between HRT and cardiovascular risk remains a critical concern, particularly in women with T2DM, who already face an elevated baseline risk of CVD [7, 12, 27]. The choice of HRT regimen plays a major role in safety outcomes, necessitating individualized risk assessments before initiation.
Studies indicate that oral estrogen-based HRT can increase the risk of venous thromboembolism (VTE) and stroke, particularly in older women or those with pre-existing cardiovascular conditions [7]. However, transdermal estrogen has shown a lower thromboembolic risk and a neutral or potentially beneficial effect on cardiovascular health, making it the preferred option for women with T2DM at moderate cardiovascular risk [12, 27].
A Taiwanese cohort study comparing women with T2DM using conjugated equine estrogen (CEE) versus non-users found a significantly lower stroke risk in the HRT group, suggesting a potential protective effect when therapy is carefully selected [28]. Similarly, Swedish research noted no significant increase in stroke risk when HRT was initiated within five years of menopause, reinforcing the “timing hypothesis” for safer cardiovascular outcomes [4]. The cardiovascular risk is the parameter to be considered for the recommended route for the HRT as shown in Table 1.
HRT recommendations based on cardiovascular risk levels
| Cardiovascular risk category | Recommended routes | Clinical considerations |
|---|---|---|
| Low cardiovascular risk | Oral or transdermal HRT | Preference for transdermal formulations to minimize thromboembolic complications [3, 12, 27] |
| Moderate cardiovascular risk (e.g., hypertension, obesity, mild atherosclerosis) | Transdermal estrogen ≤ 50 mcg | Associated with lower stroke and VTE risks compared to oral options [7, 12, 13, 29] |
| High cardiovascular risk (established CVD, history of stroke, uncontrolled hypertension) | Avoid HRT, consider non-hormonal therapies | HRT is contraindicated due to increased cardiovascular risks [27, 30]. Vaginal estrogen or non-hormonal options should be considered [3, 12, 29] |
CVD: Cardiovascular diseases; HRT: Hormonal replacement therapy; VTE: venous thromboembolism
Optimizing HRT for postmenopausal women with T2DM requires careful consideration of timing, administration route, and patient-specific factors. Estrogen enhances insulin sensitivity by modulating insulin receptors, reducing inflammation, and supporting beta-cell function [3, 6]. Transdermal estrogen bypasses hepatic metabolism and lowers thromboembolic risk, making it a safer option for women with cardiovascular concerns [17, 20]. However, the precise molecular mechanisms underlying its metabolic benefits remain unclear. Further research is needed to explore its interactions with modern diabetes therapies and long-term safety [18, 21].
These findings highlight the necessity of carefully choosing the type and timing of HRT to optimize its metabolic advantages while minimizing cardiovascular risks.
T2DM is a major contributor to diabetic nephropathy, and several studies have explored the potential role of HRT in mitigating renal decline [12, 31, 32]. Observational data suggest that HRT, particularly transdermal estrogen, may offer renal protection by improving endothelial function, reducing proteinuria, and stabilizing glomerular filtration rates [32].
A small clinical study of hypertensive postmenopausal women with diabetic nephropathy found that estradiol-based HRT significantly reduced proteinuria and improved creatinine clearance over 3.5 months [32]. Additionally, a cohort study of over 700,000 women with chronic kidney disease (CKD) demonstrated improvements in kidney function markers among HRT users, particularly in those receiving early therapy [31].
Guidelines for balancing benefits versus risks (Figure 1):
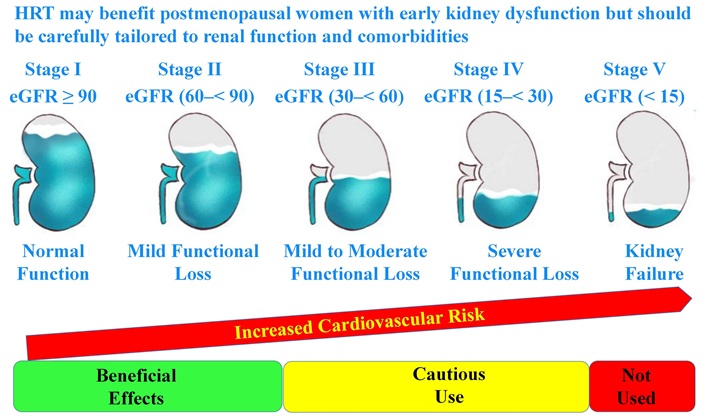
HRT considerations across different stages of renal functional decline
Note. Adapted with permission from [https://www.kidney.org/kidney-topics/stages-chronic-kidney-disease-ckd], cited 2025 Mar 27, © 2025 National Kidney Foundation, Inc.
For women with early-stage CKD or mild renal impairment, transdermal estrogen may be considered to slow disease progression, given its anti-inflammatory and endothelial-stabilizing effects [12, 32].
For women with moderate-to-advanced CKD or significant proteinuria, HRT should be approached cautiously due to potential fluid retention effects, and non-hormonal alternatives should be explored [31].
For women with end-stage renal disease (ESRD), HRT is not recommended due to uncertain long-term safety and increased cardiovascular risks [12, 32].
These findings suggest that HRT may provide renal benefits in select postmenopausal women with early kidney dysfunction, but its use should be carefully individualized based on renal function and comorbidities.
Menopause triggers significant lipid metabolism changes, increasing CVD risk. These include elevated total cholesterol, low-density lipoprotein (LDL) cholesterol, and apolipoprotein B levels, particularly in women undergoing surgical or early menopause [3, 14, 15]. HRT has shown potential in mitigating these effects, with outcomes varying by administration route.
Oral estrogens improve lipid profiles more effectively than transdermal options by increasing high-density lipoprotein (HDL) cholesterol and reducing LDL cholesterol. However, oral estrogens also raise VTE risk, making them suitable for women with low cardiovascular risk. Transdermal estrogen is preferred for moderate-risk patients due to its beneficial effects on triglycerides and inflammatory markers, with a lower thromboembolic risk [3, 29].
A study of 20 postmenopausal women with T2DM and 20 without diabetes found ERT reduced total and LDL cholesterol while increasing HDL cholesterol. Adding medroxyprogesterone neutralized the HDL improvement without affecting other lipids [33]. Another study found that transdermal estradiol had no significant effect on lipid parameters, while oral estrogen increased HDL and triglycerides but left LDL and total cholesterol unchanged [13].
HRT is contraindicated for women with existing CVD or high risk. However, it may benefit women under 60 or within 10 years of menopause, as it demonstrates neutral to favorable effects on lipid profiles and cardiovascular risk [12, 34].
While short-term studies highlight HRT’s lipid benefits, long-term data are scarce. It remains unclear whether initial improvements in lipid profiles reduce cardiovascular events or introduce risks like cancer or cognitive decline with prolonged use. Future research should address these gaps, particularly in women with T2DM, who face elevated baseline CVD risks [12, 34].
The association between HRT and cancer risk is complex, particularly in women with T2DM, who may already have an elevated baseline risk of hormone-sensitive malignancies such as breast and endometrial cancers [12, 34–36]. The choice of HRT regimen and duration of use significantly influence cancer outcomes.
Breast Cancer: Combined estrogen-progestin therapy (EPT) has been associated with a higher risk of invasive breast cancer, particularly with extended use exceeding five years [34, 36]. However, estrogen-only therapy (ET), commonly prescribed for women who have undergone a hysterectomy, appears to carry a lower risk of breast cancer [12, 37].
Endometrial Cancer: Unopposed estrogen therapy raises the risk of endometrial hyperplasia and cancer, making it essential to include progestin for women with an intact uterus to mitigate these risks [12, 35].
Guidelines for balancing benefits vs. risks:
For women with a history of breast or endometrial cancer, HRT is contraindicated, and non-hormonal therapies should be prioritized [35, 38].
For women with T2DM but no history of cancer, ET may be preferable in hysterectomized patients. At the same time, combined HRT should be used cautiously and for the shortest duration necessary [12, 36].
Routine cancer screening, including mammograms and endometrial monitoring, is essential for all women considering or using HRT [12, 34, 38].
While HRT offers metabolic benefits and symptom relief, its use in women with T2DM necessitates thorough cancer risk evaluation and vigilant clinical monitoring.
The method of HRT administration plays a crucial role in determining safety outcomes, particularly in women with T2DM [3, 12, 13, 29].
Different formulations and delivery routes vary in their impact on metabolism, thromboembolic risk, and cardiovascular safety as shown in Table 2.
Comparison of hormonal replacement therapy delivery routes in terms of safety profiles and clinical considerations
| Delivery route | Safety profile | Clinical considerations |
|---|---|---|
| Oral estrogen | Increases venous thromboembolism risk due to first-pass hepatic metabolism, leading to procoagulant effects [3, 29] | More effective in improving lipid profiles but may worsen triglyceride levels [13] |
| Transdermal estrogen | Bypasses hepatic metabolism, reducing thromboembolic and stroke risks [12, 29] | Preferred option for women with metabolic syndrome or cardiovascular risk factors [12, 29]; has neutral effects on triglycerides and may offer safer cardiovascular outcomes in women with type 2 diabetes [3, 12, 29] |
| Vaginal estrogen | Minimal systemic absorption, primarily used for urogenital symptoms [3, 13] | Safer alternative for localized symptoms in women with high systemic risks [3, 13] |
This comparative evaluation highlights that transdermal estrogen is generally the safest option for postmenopausal women with T2DM, while oral formulations should be used selectively
Modern diabetes therapies, including GLP-1 receptor agonists and SGLT2 inhibitors, have transformed the management of T2DM by improving glycemic control, promoting weight loss, and providing cardiovascular and renal benefits [39, 40]. However, their interactions with HRT remain underexplored, particularly regarding metabolic and cardiovascular outcomes in postmenopausal women.
GLP-1 receptor agonists (e.g., semaglutide, liraglutide) enhance insulin sensitivity and facilitate weight loss, counteracting the potential weight gain associated with certain HRT regimens [39]. HRT, particularly estrogen therapy, improves insulin action and reduces visceral fat accumulation; however, the extent to which these effects complement or conflict with GLP-1 therapies remains unclear [39]. Some evidence suggests that GLP-1 receptor agonists may amplify estrogen’s cardiovascular protective effects by reducing inflammation and arterial stiffness, potentially lowering the risk of atherosclerosis [39]. However, since both therapies influence lipid metabolism and glucose uptake, further clinical studies are needed to evaluate their combined impact on long-term metabolic health [39].
SGLT2 inhibitors (e.g., dapagliflozin, empagliflozin) have demonstrated significant cardiovascular and renal protective effects by reducing glucose reabsorption, decreasing blood pressure, and promoting weight loss [40]. HRT, particularly transdermal estrogen, has been shown to stabilize renal function and reduce proteinuria in postmenopausal women, potentially complementing the nephroprotective effects of SGLT2 inhibitors [12, 32, 40]. However, the diuretic effect of SGLT2 inhibitors could exacerbate fluid balance alterations in postmenopausal women on HRT, necessitating careful monitoring of electrolyte levels and hydration status [40]. Given that both therapies impact cardiovascular risk factors, combining them may require adjustments in antihypertensive or lipid-lowering medications to prevent hypotension or excessive lipid modulation [40].
For postmenopausal women with obesity or metabolic syndrome, GLP-1 receptor agonists may provide additional benefits alongside HRT by mitigating estrogen-related weight changes and optimizing metabolic outcomes [39]. For women with diabetic nephropathy or cardiovascular comorbidities, SGLT2 inhibitors may enhance the renal and cardiovascular benefits of HRT, but fluid status should be carefully monitored [40]. Further randomized controlled trials (RCTs) are needed to determine the most effective and safest combinations of HRT and modern diabetes therapies, particularly cardiovascular outcomes and long-term metabolic stability [39, 40].
HRT is effective in relieving menopausal symptoms such as hot flashes and night sweats; however, its overall impact on quality of life (QoL) in women with T2DM remains insufficiently studied [3, 12]. Managing multiple comorbidities often heightens physical, emotional, and social challenges for postmenopausal women with T2DM.
Preliminary evidence suggests that HRT improves physical well-being by enhancing glycemic control, reducing inflammation, and supporting cardiovascular health, potentially alleviating diabetes-related complications [12, 34]. Emotionally, HRT may reduce depressive symptoms and improve sleep quality, while better symptom management can enhance social functioning and interpersonal relationships [12, 41]. However, longitudinal studies assessing these benefits across diverse populations, particularly women with advanced disease, are lacking. Further research is crucial to determine HRT’s comprehensive role in enhancing the QoL for this high-risk group [12, 34].
The majority of HRT studies have been conducted in predominantly white populations, limiting the generalizability of findings to racially and ethnically diverse groups who may exhibit different metabolic and cardiovascular responses to HRT [12, 34, 38, 42]. Emerging evidence suggests that genetic variations, cultural practices, and disparities in healthcare access significantly influence HRT metabolism, cardiovascular outcomes, and diabetes management across racial and ethnic groups [38, 42].
Genetic polymorphisms in estrogen metabolism pathways, such as variations in cytochrome P450 1A1 (CYP1A1) and CYP3A4 enzymes, influence how different ethnic groups metabolize estrogen [38, 42]. African American and Hispanic women have been found to exhibit slower estrogen metabolism, potentially leading to prolonged estrogen exposure and increased thromboembolic risks [38]. Conversely, East Asian populations may have a higher prevalence of genetic variations associated with reduced estrogen receptor sensitivity, potentially affecting the efficacy of HRT in symptom relief and metabolic regulation [38].
Cardiovascular risks associated with HRT use may be modulated by ethnicity. African American and South Asian women have a higher baseline risk of hypertension and insulin resistance, which could make oral estrogen formulations particularly unfavorable due to their impact on clotting factors and lipid profiles [12, 34]. Studies suggest that Hispanic and African American women are at increased risk of postmenopausal metabolic syndrome, which may necessitate closer monitoring when initiating HRT to prevent worsening insulin resistance [42]. In contrast, East Asian women tend to have lower rates of CVD but may still experience metabolic disruptions from HRT, particularly in the presence of diabetes [38].
Disparities in healthcare access contribute to differences in HRT use. Minority women are less likely to receive HRT due to lower screening rates, socioeconomic barriers, and cultural attitudes toward menopause and medical interventions [34, 38]. Some ethnic groups may prefer alternative therapies, including traditional medicine, which may impact adherence to HRT and diabetes management plans [38].
For African American and Hispanic women, transdermal estrogen may be preferable to minimize thromboembolic risks associated with slower estrogen metabolism [12, 42]. For South Asian and Middle Eastern populations with a high prevalence of insulin resistance, careful monitoring of lipid profiles and glucose control is essential when initiating HRT [38, 42]. Lower-dose formulations may be considered for East Asian women due to genetic differences in estrogen receptor sensitivity [38].
The most effective treatment for managing vasomotor symptoms is HRT, which remains the first-line option for menopausal women within 10 years of their final menstrual period. However, for women who are not suitable candidates for HRT due to contraindications (e.g., estrogen-dependent cancers, CVD, or history of thromboembolic events) or personal preference, healthcare providers must be knowledgeable about evidence-based non-hormonal treatment options for alleviating vasomotor symptoms [43].
The American and British Menopause Societies recommend several alternatives for women with hot flashes who cannot or choose not to use HRT as shown in Table 3 [43, 44].
Non-hormonal therapies for menopausal symptoms with their mechanisms of action and clinical evidence
| Non-hormonal therapy | Clinical evidence |
|---|---|
| Gabapentin | Modulates calcium channels in the central nervous system, reducing hot flashes [45]. Effective in reducing hot flashes and sleep quality, especially in women who cannot use estrogen therapy due to contraindications [43] |
| Selective serotonin reuptake inhibitors (SSRIs; e.g., paroxetine, citalopram) | Alter serotonin levels to regulate body temperature. Approved for managing vasomotor symptoms, providing relief in non-hormonal therapy settings [43, 44] |
| Oxybutynin | Anticholinergic effects are commonly used for overactive bladder. It reduces sweating and hot flashes in postmenopausal women [43] |
| Fezolinetant | Neurokinin B receptor antagonist, modulating hypothalamic temperature control. Represents a new class of medications with promising efficacy in clinical trials [43] |
| Selective estrogen receptor modulators (SERMs; e.g., bazedoxifene) | Estrogen receptor modulation to reduce symptoms without systemic estrogen exposure. Bazedoxifene, when combined with conjugated estrogens, has been shown to provide benefits in reducing vasomotor symptoms while offering endometrial protection, making it a viable option for some women contraindicated for standard HRT [43] |
| Lifestyle interventions (e.g., weight loss, exercise, dietary modifications) | Reduces body fat and stabilizes thermoregulatory mechanisms. Sustaining a healthy weight and engaging in physical activity can decrease hot flash frequency and intensity [44] |
| Cognitive behavioral therapy (CBT) and mindfulness-based interventions | Behavioral and psychological strategies for symptom management. Explored as effective non-hormonal strategies for alleviating menopausal symptoms [44] |
HRT: hormone replacement therapy
By integrating non-hormonal alternatives such as selective estrogen receptor modulators (SERMs) and lifestyle interventions into menopausal management strategies, healthcare providers can offer personalized options for women who cannot undergo HRT [43, 44]. These findings demonstrate the potential of HRT when initiated early and tailored to individual needs while identifying gaps in long-term safety, therapy interactions, and outcomes in diverse populations.
While this review provides a comprehensive assessment of HRT in postmenopausal women with T2DM, several limitations must be acknowledged.
The findings primarily rely on observational studies and meta-analyses, which, while valuable for evaluating long-term safety and real-world effectiveness, may introduce selection bias and confounding variables. RCTs continue to be the most reliable method for establishing causal relationships in medical research. Still, existing RCTs [e.g., WHI and Kronos Early Estrogen Prevention Study (KEEPS) trials] [8, 46] often exclude women with pre-existing diabetes, limiting direct applicability to T2DM populations.
There is a scarcity of RCTs investigating HRT’s effects in women with T2DM, especially regarding long-term metabolic, cardiovascular, and renal outcomes. Future trials should address how different HRT formulations impact glucose metabolism, cardiovascular health, and renal function in this high-risk group.
The review discusses oral, transdermal, and vaginal estrogen, yet direct head-to-head RCTs comparing these routes in T2DM patients remain limited [3, 12, 13, 29]. The choice of progestin component in combined HRT also influences thromboembolic risk and metabolic effects, requiring further research on the safest regimens.
Most large-scale studies on HRT have been conducted in predominantly Caucasian populations [38, 42], which limits the generalizability of findings to other ethnic groups. Genetic polymorphisms in estrogen metabolism and receptor activity may alter HRT safety and efficacy, requiring more diverse cohort studies and pharmacogenomics research.
The review highlights the emerging role of GLP-1 receptor agonists [39] and SGLT2 inhibitors [40] in diabetes care, but limited research exists on their interactions with HRT. Given that HRT and modern diabetes medications influence cardiovascular health, weight, and glucose metabolism, future studies should evaluate synergistic or antagonistic effects.
While HRT’s benefits on glycemic control, lipid profiles, and cardiovascular health are well-documented, its long-term risks—especially regarding breast and endometrial cancer [34–36]—remain debated. Most cancer risk assessments are derived from general postmenopausal populations, but additional studies are needed to assess HRT’s cancer risk profile, specifically in T2DM patients, who may have altered estrogen metabolism.
The review primarily focuses on clinical and metabolic outcomes, but the impact of HRT on QoL, mood, sleep, and cognitive function in women with T2DM requires further investigation [12, 34, 41]. Longitudinal studies assessing patient-reported outcomes would strengthen the understanding of HRT’s overall benefits beyond metabolic markers.
Comprehensive cardiovascular risk assessment (e.g., history of thromboembolism, hypertension, dyslipidemia) before HRT initiation [27].
Diabetes and metabolic risk evaluation (HbA1c, fasting glucose, insulin resistance markers) to assess glycemic control [32].
Breast and endometrial cancer screening for patients with risk factors for hormone-sensitive malignancies [31].
Liver and renal function tests to rule out contraindications for oral estrogen therapy [13].
Route of administration: Transdermal estrogen is preferred in women with cardiovascular risk, while oral formulations may be used cautiously in low-risk individuals [14].
Hormonal composition: Estradiol-based therapies are favored over CEE due to a more favorable metabolic profile [15].
Adjunctive therapies: Combining metformin, SGLT2 inhibitors, or GLP-1 receptor agonists may provide additional metabolic benefits [29].
Age and menopausal duration: HRT should be initiated within 10 years of menopause to optimize cardiovascular and metabolic benefits [4].
Tailored dosing strategy: The lowest effective dose should be used, with a duration typically not exceeding 5 years [30].
Continuous reassessment: Monitoring lipid levels, glycemic parameters, and cardiovascular function is critical for ongoing safety [31].
HRT offers significant benefits for postmenopausal women with T2DM, including improved glycemic control, lipid metabolism, and renal function. Early initiation within 10 years of menopause has been associated with reduced insulin resistance and cardiovascular protection, while transdermal estrogen appears to be the safest option for women at moderate cardiovascular risk.
Despite these advantages, HRT use must be carefully personalized due to risks such as thromboembolic events, breast and endometrial cancers, and potential cognitive effects. Individualized risk assessments, multidisciplinary evaluations, and adherence to guidelines—such as using the lowest effective dose for no more than five years—are essential for optimizing safety and efficacy.
Future research should focus on long-term safety, the impact of HRT in diverse populations, and its interactions with modern diabetes therapies like GLP-1 receptor agonists and SGLT2 inhibitors. Additionally, more studies are needed to explore HRT’s role in enhancing QoL and reducing diabetes-related complications in high-risk women.
While HRT remains a valuable therapeutic tool, clinical decision-making should be patient-centered, balancing metabolic benefits with potential risks. Tailored treatment strategies and ongoing monitoring will help maximize its benefits while minimizing adverse effects in postmenopausal women with T2DM.
Personalized care: HRT should be prescribed after a comprehensive risk-benefit assessment, particularly in women with CVD or advanced diabetes.
Multidisciplinary evaluation: A team including endocrinologists, cardiologists, gynecologists, and primary care physicians should guide HRT decisions. This ensures proper risk stratification, appropriate therapy selection (transdermal vs. oral estrogen), and continuous monitoring. To prevent complications, standardized screening should assess cardiovascular status, metabolic profile, and cancer risk.
Early initiation: Starting HRT within 10 years of menopause maximizes metabolic and cognitive benefits while minimizing long-term risks.
Preferred route: Transdermal estrogen is recommended for women with moderate-to-high cardiovascular risk due to its lower thromboembolic risk.
Minimizing risks: HRT is linked to thromboembolic events and hormone-sensitive cancers, necessitating individualized evaluation. Guidelines recommend the lowest effective dose for no more than five years to reduce risks. Long-term HRT use (> 5 years) is associated with increased risks of malignancies and clotting events.
Transition planning: A meta-analysis confirms that short-term HRT improves metabolic and cardiovascular health while avoiding long-term complications. After five years, women at higher cardiovascular risk should discontinue or transition to non-hormonal therapies.
Long-term safety and efficacy: Further studies are needed to assess the prolonged impact of HRT in postmenopausal women with T2DM, focusing on cardiovascular risks and cancer outcomes.
Comparative studies: Clinical trials should evaluate the effectiveness and safety of different HRT delivery routes (oral, transdermal, vaginal) across varied risk profiles.
HRT and modern diabetes therapies: Investigate the interactions between HRT and advanced diabetes treatments, including GLP-1 receptor agonists and SGLT2 inhibitors, to determine optimal therapeutic combinations.
Diversity in treatment outcomes: Explore racial and ethnic disparities in HRT response to ensure equitable, personalized care strategies for women with diverse genetic and metabolic backgrounds.
QoL assessment: Evaluate HRT’s influence on physical, emotional, and social well-being, particularly in postmenopausal women with multiple diabetes-related complications.
CEE: conjugated equine estrogen
CKD: chronic kidney disease
CVD: cardiovascular diseases
ERT: estrogen replacement therapy
ET: estrogen-only therapy
GLP-1: glucagon-like peptide 1
HbA1c: glycated hemoglobin
HDL: high-density lipoprotein
HOMA-IR: homeostatic model assessment for insulin resistance
HRT: hormone replacement therapy
LDL: low-density lipoprotein
QoL: quality of life
RCTs: randomized controlled trials
SGLT2: sodium-glucose co-transporter 2
T2DM: type 2 diabetes mellitus
VTE: venous thromboembolism
WHI: Women’s Health Initiative
The authors thank Fatima M.A. Odhaib for her artistic contribution of Figure 1.
BAAS and SAO: Conceptualization, Investigation, Validation, Writing—original draft, and Writing—review & editing. Both authors read and approved the submitted version.
The authors declare that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 12279
Download: 266
Times Cited: 0
Nagamani Gumpeny ... Sridhar R. Gumpeny
Bruno Vecchiatto ... Fabiana S. Evangelista
