Affiliation:
1Department of Internal Medicine, Cleveland Clinic, Cleveland, OH 44195, USA
ORCID: https://orcid.org/0000-0002-4087-3901
Affiliation:
2Section of Cardiovascular Imaging, Robert and Suzanne Tomsich Department of Cardiovascular Medicine, Sydell and Arnold Miller Family Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Cleveland, OH 44195, USA
ORCID: https://orcid.org/0009-0005-8594-3407
Affiliation:
2Section of Cardiovascular Imaging, Robert and Suzanne Tomsich Department of Cardiovascular Medicine, Sydell and Arnold Miller Family Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Cleveland, OH 44195, USA
ORCID: https://orcid.org/0000-0003-2915-2510
Affiliation:
2Section of Cardiovascular Imaging, Robert and Suzanne Tomsich Department of Cardiovascular Medicine, Sydell and Arnold Miller Family Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Cleveland, OH 44195, USA
Email: wangt2@ccf.org
ORCID: https://orcid.org/0000-0001-5570-9402
Explor Cardiol. 2025;3:101282 DOI: https://doi.org/10.37349/ec.2025.101282
Received: August 25, 2025 Accepted: October 10, 2025 Published: November 19, 2025
Academic Editor: Gaetano Nucifora, University Hospital of South Manchester NHS Foundation Trust, United Kingdom
Infective endocarditis (IE) remains a challenging diagnosis, particularly in patients with prosthetic valves, cardiac implantable electronic devices (CIEDs), or nonspecific presentations. With rising rates of healthcare-associated and device-related infections, the need for earlier and more reliable diagnosis has become increasingly important. Multimodality imaging now plays a central role in confirming IE, identifying complications, and guiding management. Echocardiography is the initial test of choice, with transesophageal echocardiography (TEE) offering better sensitivity for vegetations, leaflet perforation, and periannular extension, though its limitations in prosthetic valve endocarditis (PVE) have led to greater reliance on other modalities. Cardiac computed tomography (CT) provides detailed anatomical information that can reveal abscesses, pseudoaneurysms, and prosthetic dehiscence, and is frequently used for surgical planning. Functional imaging with 18F-fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT) and white blood cell single-photon emission CT/CT (WBC-SPECT/CT) improves diagnostic accuracy in PVE and CIED infections while also detecting systemic embolic events. Brain magnetic resonance imaging (MRI) has become an important tool to uncover neurologic complications, including silent emboli and mycotic aneurysms. This review summarizes the strengths and limitations of each modality, outlines a stepwise approach to imaging decisions, and considers how findings should be incorporated into overall clinical care. This review also highlights surgical indications, evolving antimicrobial strategies, and the future role of standardized imaging protocols. Taken together, thoughtful use of multimodality imaging is critical to improving outcomes in patients with suspected or confirmed IE.
Infective endocarditis (IE) is defined as an infection of the endocardial surface of the heart, most commonly affecting heart valves. Though uncommon, with an annual incidence of 3–7 per 100,000 person-years, IE is associated with substantial morbidity and mortality, ranking among the top life-threatening infections alongside sepsis, pneumonia, and intra-abdominal abscess. Over time, IE has evolved to involve an older, more comorbid population, with increasing cases tied to prosthetic valves and intracardiac devices. Staphylococcus aureus has become the predominant causative organism [1]. Both U.S. and international guidelines emphasize the ongoing threat of IE. The 2021 American College of Cardiology/American Heart Association (ACC/AHA) guideline on valvular heart disease emphasizes that IE is universally fatal without treatment. It stratifies IE based on native versus prosthetic valve involvement and timing post-intervention. Mortality remains high, with in-hospital rates of 15–20% and one-year mortality approaching 40%. Heart failure, embolic phenomena, intracardiac abscesses, and need for surgery are common, underscoring the importance of early detection and coordinated care [2]. The 2023 European Society of Cardiology (ESC) guideline echoes these concerns, highlighting that IE continues to carry a high in-hospital mortality rate (~17%)—even higher in prosthetic valve endocarditis (PVE)—and is associated with serious complications such as neurologic sequelae and embolization [3]. Against this backdrop, the role of advanced diagnostic strategies, particularly multimodality imaging, has become increasingly important to improve early detection, guide management, and optimize outcomes. This review will summarize current evidence, highlight gaps in knowledge, and discuss how imaging can be applied in clinical practice to address these challenges.
According to the Global Burden of Disease (GBD) analysis, there were approximately 1.09 million new IE cases and 66,322 deaths globally in 2019. Between 1990 and 2019, the age-standardized incidence rate rose from 9.91 to 13.80 and the mortality rate from 0.73 to 0.87 per 100,000 person-years, especially among older adults in high-income regions [4–6].
Over time, the average age of affected patients has increased, with more cases linked to prosthetic valves, cardiac devices, and healthcare exposures. In high-income countries, S. aureus has surpassed streptococci as the leading cause, while rheumatic heart disease (RHD) remains the main driver in lower-income settings [1, 7, 8]. Despite ongoing advances in diagnostic tools and therapeutic options, IE remains associated with substantial morbidity and mortality, with 1-year mortality rates approximating 30% [7].
S. aureus is now the leading cause of both native and PVE in high-income countries, accounting for 31–40% of cases [8, 9]. In some studies, S. aureus ranks as the second most common pathogen, positioned between viridans group streptococci (VGS) and enterococci, especially in developing nations [10]. VGS and Streptococcus gallolyticus are responsible for 30–40% of cases. Enterococci, particularly Enterococcus faecalis, account for 8–19% of cases. These infections are closely associated with healthcare exposures, the presence of prosthetic valves, and advanced age [8, 9]. Coagulase-negative staphylococci (CoNS) are more commonly involved in prosthetic valve and device-related IE [8, 11, 12]. Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, Kingella (HACEK) organisms are rare causes of IE but remain clinically important, especially in culture-negative cases [8]. Fungal endocarditis, most commonly due to Candida and Aspergillus species, is an uncommon but serious condition. It is most often seen in immunocompromised individuals and those with prosthetic material. Culture-negative endocarditis presents a significant diagnostic challenge and can account for up to 26% of all IE cases [12, 13].
Native valve endocarditis (NVE) is the most common subtype, with an estimated incidence of 2 to 10 cases per 100,000 person-years. Endothelial leads to the formation of vegetations that are difficult to eradicate, even with targeted antimicrobial therapy [9, 14]. In low-resource settings, RHD is the dominant predisposing factor to NVE, accounting for up to 45% of NVE cases and associated with high mortality [15]. In contrast, mitral valve prolapse (MVP), bicuspid aortic valve, and congenital defects are more commonly implicated in high-income regions [14]. MVP, in particular, carries a 3.5- to 8.2-fold increased risk, with a mean prevalence of 8.5% among patients with NVE.
PVE accounts for 20–30% of all IE cases. Outcomes are generally worse than in NVE due to delayed diagnosis, higher complication rates, and more complex microbiology. Mechanical valves are more commonly infected with S. aureus (36% vs. 17% in bioprosthetic valves), whereas bioprosthetic valves tend to show broader microbiologic diversity. Although bioprosthetic valves carry a higher cumulative risk of infection over 10 years (3.4% vs. 1.9%), adjusted survival following PVE appears comparable between valve types [16–18]. Timing also matters: early-onset PVE (within one year of implantation) is more often associated with CoNS and Candida species, and carries a higher risk of perivalvular complications [19].
A growing subset of patients develops endocarditis following transcatheter aortic valve replacement (TAVR). Although the overall incidence of TAVR-associated IE (TAVR-IE) is similar to that of surgical aortic valve replacement (SAVR) (1.7–2.0% vs. 1.9–2.5% per patient-years), it typically occurs earlier and is more often caused by Enterococcus, likely due to femoral access and peri-procedural exposure. By comparison, surgical valve infections more frequently involve staphylococci. TAVR-IE is also more difficult to diagnose; classic echocardiographic findings may be absent in up to 15% of cases, and transesophageal echocardiography (TEE) sensitivity is reduced. In these cases, cardiac computed tomography (CCT) has become increasingly valuable in identifying complications such as abscesses or pseudoaneurysms [8, 20–23]. Despite guideline recommendations, many patients with TAVR-IE are not offered surgery due to comorbidities, contributing to high mortality—32–36% in-hospital and 37–45% at one year [24]. Risk factors include male sex, diabetes, younger age, and procedural complications [23].
Beyond structurally related risks, procedural and nosocomial exposures also contribute significantly to the burden of IE. Between 2000 and 2013, the proportion of IE hospitalizations attributed to intravenous drug use (IDU) increased from 7% to 12%, with some centers now reporting rates exceeding 50%. IDU often involves the tricuspid valve and is commonly due to S. aureus [25, 26]. Hemodialysis represents another high-risk setting. Patients on hemodialysis have an IE incidence over 1,000 per 100,000 person-years—markedly higher than those receiving peritoneal dialysis or kidney transplantation. Central venous catheters more than double the risk of IE compared to arteriovenous fistulas and are the leading cause of healthcare-associated IE in this population [27, 28]. More broadly, central venous catheters are implicated in 33–66% of nosocomial IE cases. Common pathogens include Staphylococcus, Enterococcus, and Candida. Patients with prosthetic valves, cancer, cardiac implantable electronic devices (CIEDs), or dialysis dependence are particularly vulnerable [29]. Invasive procedures—including vascular interventions, wound care, and transfusions—have also been associated with increased risk in case-crossover studies [30]. Across all these groups, S. aureus remains the predominant pathogen and is strongly associated with worse outcomes [30]. A comprehensive list of structural, device-related, and procedural risk factors for IE is summarized in Table 1.
Risk factors for IE.
| Category | Risk factor | Details/Examples |
|---|---|---|
| Patient-related | Previous history of IE | Strongest risk factor |
| Structural heart disease | Rheumatic heart disease, bicuspid aortic valve, mitral valve prolapse with regurgitation | |
| CHD | Cyanotic CHD, unrepaired defects, or repaired with prosthetic material | |
| Intravenous drug use | Strong association with right-sided IE | |
| Immunosuppression | Due to chemotherapy, HIV, immunosuppressive drugs | |
| Diabetes mellitus | Increased susceptibility for IE | |
| Chronic hemodialysis | Due to frequent vascular access | |
| Indwelling vascular catheters | Central lines, PICC lines | |
| Poor dentition or recent dental infection | Risk via oral flora | |
| Advanced age | Age-related degeneration of valves, comorbidities | |
| Liver disease | Especially cirrhosis | |
| Malignancy | Especially GI or genitourinary cancers | |
| Nosocomial exposure | Hospital-acquired bacteremia | |
| Valve/Device-related | Prosthetic heart valve (mechanical or bioprosthetic) | Risk highest in first year post-implantation (< 60 days) |
| Transcatheter valve | Similar early risk to surgical valves, unique microbiology | |
| Native valve disease | Mitral and aortic valves most affected | |
| Valve dysfunction | Regurgitation and/or stenosis | |
| Presence of intracardiac devices | Pacemakers, ICDs, CRT devices | |
| Annular calcification | Associated with increased risk | |
| Previous valve surgery | Residual suture lines or patches may harbor infection | |
| Procedure-related | Dental procedures | Manipulation of gingiva or perforation of oral mucosa |
| Respiratory tract procedures | Bronchoscopy with biopsy, tonsillectomy | |
| GI or genitourinary procedures | Especially in patients with active infection | |
| Invasive cardiac procedures | TAVR, pacemaker/ICD implantation, ablation | |
| Vascular catheter manipulation | Hemodialysis access, central line placement | |
| Non-sterile injection practices | Common in IV drug use | |
| Inadequate perioperative prophylaxis | Especially in high-risk individuals |
IE: infective endocarditis; CHD: congenital heart disease; HIV: human immunodeficiency virus; PICC: peripherally inserted central catheter; GI: gastrointestinal; ICDs: implantable cardioverter defibrillators; CRT: cardiac resynchronization therapy; TAVR: transcatheter aortic valve replacement; IV: intravenous.
The landscape of IE is shifting, marked by a growing incidence of PVE, particularly following TAVR. As TAVR expands into younger and lower-risk populations, the total number of TAVR-IE cases is rising [31–33]. Enterococcus species have become prominent in TAVR-IE, especially in early post-implantation cases. This shift is likely attributable to procedural factors (e.g., transfemoral access) and the advanced age and comorbidities of the TAVR population. In contrast, staphylococcal species remain more prevalent in SAVR-IE. The highest risk period is within one-year post-procedure, and many cases are nosocomial or healthcare-associated, reflecting the procedural environment and recovery settings [34, 35].
The clinical presentation of IE is often nonspecific. Classic findings such as fever, new murmur, or peripheral stigmata are often absent, particularly in early stages or in cases involving the right heart. Delayed recognition can result in life-threatening complications, including heart failure, systemic embolism, and death [1].
The diagnosis of IE relies on an integration of clinical, microbiological, and imaging findings. The modified Duke criteria, first published in 1994 and subsequently updated, are the foundation for diagnostic classification, categorizing cases as definite, possible, or rejected IE [2]. Minor criteria include predisposing heart conditions or intravenous (IV) drug use, fever ≥ 38°C, vascular phenomena (such as emboli or Janeway lesions), immunologic phenomena (such as glomerulonephritis or Osler nodes), and positive blood cultures not meeting major criteria. Major criteria are based on blood culture and imaging findings:
Microbiologic evidence: (a) two separate blood cultures positive for typical IE organisms (viridans streptococci, Streptococcus gallolyticus, HACEK group, S. aureus, or community-acquired enterococci without a primary focus); (b) persistently positive cultures (two drawn 12 h apart, or three of four positive cultures drawn at least one hour apart); or (c) a single positive culture for Coxiella burnetii or antiphase I IgG titer > 1:800.
Imaging evidence of endocardial involvement, including vegetation, abscess, new partial dehiscence of a prosthetic valve, or new valvular regurgitation.
Electrocardiogram (ECG) findings are not formally part of the Duke criteria but may help detect conduction abnormalities suggestive of perivalvular extension, such as new atrioventricular block [1].
The 2023 ESC guidelines reaffirm the Duke framework but highlight its limitations in prosthetic valve and device-related IE, where echocardiography alone may be inconclusive [3]. Key imaging features that support the diagnosis of endocarditis include vegetations, periannular abscesses or pseudoaneurysms, fistula formation, leaflet perforation, and prosthetic valve dehiscence, all of which carry major diagnostic and prognostic significance.
Echocardiography remains first-line, but sensitivity is reduced in PVE, small vegetations, or periannular extension [9]. Echo can also miss small vegetations, struggle to differentiate thrombus from vegetation, and may be insufficient for detecting periannular involvement [36]. As a result, the updated ESC criteria now incorporate multimodality imaging into the diagnostic pathway, elevating certain advanced modalities to major criteria. Specifically, 18F-fluorodeoxyglucose positron emission tomography/CT (FDG-PET/CT) and white blood cell single-photon emission CT/CT (WBC-SPECT/CT) are recognized as major Duke criteria in prosthetic valve and CIED infection [37]. In response to these evolving diagnostic needs, current ACC/AHA guidelines recommend a multimodality imaging approach, particularly in patients with prosthetic valves, CIEDs, or inconclusive echocardiography [2, 38]. Both the AHA and ISCVID now formally recommend CCT, 18F-FDG-PET/CT, and WBC-SPECT/CT for evaluating complex cases of PVE and CIED-associated IE, particularly when standard imaging is inconclusive [8, 39–41].
This review will examine how the strategic use of multimodality imaging enhances diagnostic precision, facilitates risk stratification, and guides treatment planning in patients with suspected or confirmed IE. The indications, advantages, and limitations of each imaging modality are outlined in Table 2. A stepwise approach to multimodal imaging in suspected IE is illustrated in Figure 1.
The role of multimodality imaging in the evaluation of IE.
| Imaging modality | Indications | Advantages | Limitations | Special considerations | Key role in IE evaluation |
|---|---|---|---|---|---|
| Transthoracic echocardiography (TTE) | Initial imaging for suspected endocarditis; screening tool | Widely available, non-invasive, bedside use, good for large vegetations or valve dysfunction | Limited sensitivity (especially in prosthetic valves or obese patients), operator dependent | Should be done in all suspected cases; may need to be followed by transesophageal echocardiography (TEE) | Initial assessment of valve structure, function, and large vegetations |
| TEE | High-risk patients, prosthetic valves, inconclusive TTE, suspected complications | High resolution; superior for detecting abscess, perforation, prosthetic valve endocarditis | Semi-invasive, sedation required, less accessible | Essential in suspected prosthetic valve endocarditis or device-related infections | Gold standard for valve and periannular complication assessment |
| Cardiac CT (CTA/CT angiography) | Assessment of periannular complications, prosthetic valves, coronary evaluation pre-surgery | High spatial resolution, visualizes abscesses, pseudoaneurysms, and fistulas | Radiation, contrast use, less sensitive for vegetations | Valuable in surgical planning and in prosthetic valve endocarditis | Complement to TEE for structural complications, especially in prosthetic valves |
| 18F-FDG-PET/CT (nuclear imaging) | Prosthetic valve endocarditis, device infections, fever of unknown origin with suspicion for IE | Functional imaging, detects inflammatory activity, whole-body evaluation | False positives (post-op), poor spatial resolution, not ideal for native valves | Requires strict preparation; best > 3 months post-surgery to reduce false positives | Detection of prosthetic valve and device-related infections; extracardiac emboli |
| Radiolabeled leukocyte scan | Suspected IE when other imaging is inconclusive, prosthetic material infections | Specific for active infection, especially useful when 18F-FDG-PET/CT is equivocal | Limited availability, time-consuming, less anatomical detail | May complement PET/CT in complex or inconclusive cases | Alternative nuclear method for infection localization |
IE: infective endocarditis; CT: computed tomography; FDG: fluorodeoxyglucose; PET: positron emission tomography.
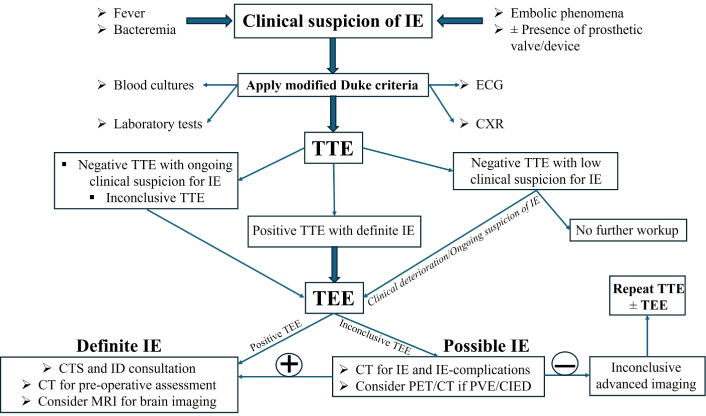
Cardiac multimodality imaging algorithm for the diagnosis and management of infective endocarditis (IE). ECG: electrocardiogram; CXR: chest X-ray; TTE: transthoracic echocardiography; TEE: transesophageal echocardiography; CTS: cardiothoracic surgery; ID: infectious disease; CT: computed tomography; MRI: magnetic resonance imaging; PET: positron emission tomography; PVE: prosthetic valve endocarditis; CIED: cardiac implantable electronic device.
Echocardiography is the first tool used for diagnosis and management in IE, with both transthoracic echocardiography (TTE) and TEE playing critical roles. The AHA and Infectious Diseases Society of America recommend TTE as the initial imaging modality in all suspected cases of IE. TTE is noninvasive, widely available, and valuable for evaluating hemodynamics, ventricular function, and right-sided involvement. Its sensitivity for detecting vegetations in NVE is moderate (50–70%), and lower in PVE (36–69%), though specificity remains high (> 90%) [1, 2, 9]. Typical echocardiographic findings include oscillating masses (vegetations), annular abscesses, new valvular regurgitation, and prosthetic valve dehiscence—each of which constitutes a major Duke criterion for diagnosis [9]. Prognostically, large vegetations (> 10 mm) are associated with increased embolic risk [42]. Embolic events, severe regurgitation, abscesses, and signs of heart failure may indicate a need for early surgical intervention.
In general, TEE offers superior sensitivity (up to 95%) and comparable specificity (approximately 90%) compared to TTE, particularly for detecting vegetations, abscesses, and prosthetic valve complications. This is especially critical in the perioperative period, where rapid changes in anatomy or new complications may arise and directly impact surgical planning and intraoperative management [1].
The evaluation and interpretation of valvular regurgitation should follow the most recent guidelines from the American Society of Echocardiography (ASE) and the ACC/AHA. Both societies recommend a comprehensive, integrative approach using multiple echocardiographic parameters—qualitative, semi-quantitative, and quantitative—to assess regurgitation severity. Severity should be graded as mild, moderate, or severe, with “trace” used for physiologic, clinically insignificant regurgitation, particularly in right-sided valves or the mitral valve [43, 44].
In NVE, TTE performance is highly dependent on image quality and pretest probability. It provides a high negative predictive value when technically adequate in low-risk patients. However, in patients with intermediate or high clinical suspicion, TEE is usually required to confirm or exclude IE. TEE demonstrates excellent sensitivity (up to 95%) and specificity (~90%) for vegetations, abscesses, and leaflet perforation. Both TTE and TEE can produce false negatives if vegetations have embolized or are very small. When clinical suspicion remains high after negative TTE, or when surgical intervention is anticipated, TEE is strongly indicated [1, 8]. Native mitral valve IE with perforation and severe regurgitation is depicted in Figure 2.
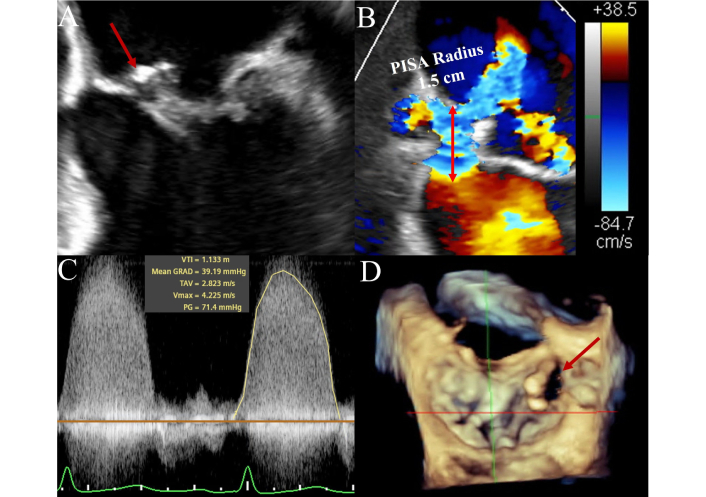
Mitral valve native infective endocarditis on transesophageal echocardiography. Figure 2 illustrates a case of a native mitral valve infective endocarditis in a 56-year-old female manifesting as a large vegetation on transesophageal echocardiography attached to the anterior mitral leaflet (AML) (Panel A: four-chamber view, see arrow) with a suspicious echo free space in the body of the AML for leaflet destruction and perforation, confirmed with color Doppler in the same view. Red arrow pointing to Doppler flow across the valve (Panel B). Large proximal isovelocity surface area (PISA) radius (1.5 cm) with Nyquist limit lowered to 38.5 cm/s. Panel C represents continuous wave Doppler through the leaflet perforation, with findings confirming severe regurgitation through this orifice. Panel D represents three-dimensional multiplanar reconstruction of the mitral valve (3-D MPR, surgeon’s view), with the red arrow highlighting the perforation of the AML medially.
Diagnosing PVE poses additional challenges due to acoustic shadowing and reverberation artifacts from the prosthetic material. These artifacts are particularly problematic in prosthetic valves in the aortic position, whereas in the mitral position, vegetations are often on the atrial side and may be less affected. They can nonetheless obscure vegetations and perivalvular pathology, warranting careful evaluation with adjunctive imaging. Recent data support the utility of three-dimensional TEE, which improves spatial resolution and anatomical clarity, enhancing detection of vegetations and complications [45]. TAVR-IE presents unique imaging difficulties, particularly in early post-implantation periods. The stent frame and associated shadowing complicate visualization, and distinguishing vegetation from pannus or sterile inflammation is often difficult. An illustrative example of TAVR-IE with pseudoaneurysm and leaflet thickening is shown in Figure 3. In these scenarios, multimodal imaging, including PET/CT, is increasingly used to support diagnosis when echocardiography is inconclusive [46, 47].
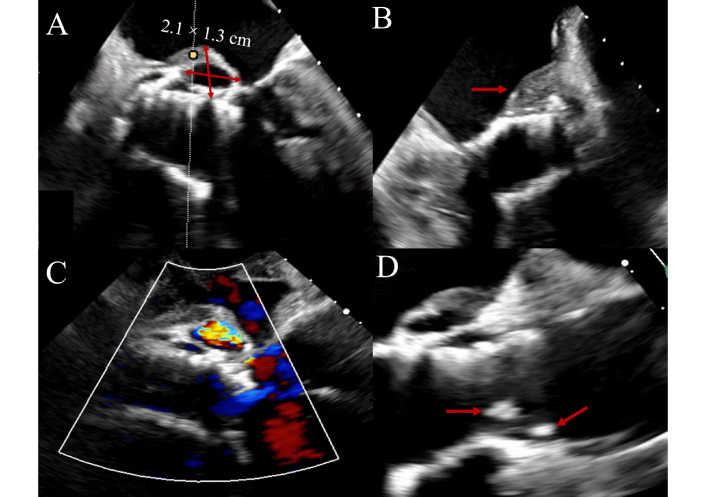
Transcatheter aortic valve replacement-associated infective endocarditis (TAVR-IE) on transesophageal echocardiography. Figure 3 shows transesophageal echocardiogram imaging of a 77-year-old patient with late-onset TAVR-IE manifesting with vegetations, leaflet thickening and interval development of an aortic root pseudoaneurysm. Panel A: 2.1 cm × 1.3 cm pseudoaneurysm (pulsatile echo-free space with systolic expansion) arising on the posterior aspect of the aortic root with a thickened surface (arrow, Panel B) and with abnormal color Doppler. Panel C: Thickened leaflets with obvious attached vegetations. Panel D: Long-axis view demonstrating communication between the pseudoaneurysm cavity and the left ventricular outflow tract (red arrows) consistent with a contained rupture along the posterior aortic root. The arrows highlight the margins of the pseudoaneurysm neck and adjacent thickened endocardial surface.
The Heart Rhythm Society and the Infectious Diseases Society of America recommend TEE as the preferred imaging modality for diagnosing CIED IE. TEE is superior to TTE in sensitivity for detecting endocarditis and perivalvular extension of infection, with TTE having a sensitivity of only 32% compared to TEE’s much higher sensitivity. TEE is particularly important in patients with S. aureus bacteremia or suspected CIED infection, as the rate of lead-associated endocarditis is substantial in these populations. TEE should be considered in all patients with documented or suspected bloodstream infection or CIED pocket infection, as device pocket infection often involves intravascular lead infection even in the absence of systemic symptoms [48, 49].
CCT is an increasingly valuable adjunct in IE diagnosis, particularly in the evaluation of PVE and perivalvular extension. It is recommended by the ACC and AHA when echocardiography is limited or equivocal and is especially helpful for assessing aortic valve IE and preoperative planning in prosthetic valve cases [2]. CCT surpasses echocardiography in identifying perivalvular complications such as abscesses, pseudoaneurysms, and prosthetic valve dehiscence. It is less hindered by artifacts from prosthetic material [50–52]. Indications for CCT include: suspected abscess or pseudoaneurysm, PVE, equivocal TEE results, and preoperative coronary artery assessment [53, 54]. CCT can identify vegetations (especially large), abscesses, leaflet perforations, pseudoaneurysms, and valve dehiscence [50, 53, 55]. Sensitivity of CCT for vegetations ranges from 72–97%, with specificity from 84–97%, though it is less effective for small vegetations and leaflet perforation. However, CCT excels in identifying perivalvular complications, with sensitivity for abscess detection approaching 100% [51, 55]. Advantages include high spatial resolution, multiplanar visualization, and comprehensive anatomical assessment, but limitations include radiation exposure, contrast use, and lower sensitivity for small vegetations [51, 52, 55]. CCT aids diagnosis, risk stratification, and surgical planning—especially when high-risk features like large vegetations or abscesses are present, or when coronary artery disease must be excluded prior to surgery [53, 54]. The Society of Cardiovascular Computed Tomography, the ACC, and other societies also highlight the role of CT in delineating extracardiac manifestations of endocarditis, including septic emboli and mycotic aneurysms [56].
Multimodal imaging in PVE, including TEE and CCT, is shown in Figure 4. A comparative summary of key imaging features and diagnostic performance between echocardiography and CT is provided in Table 3.
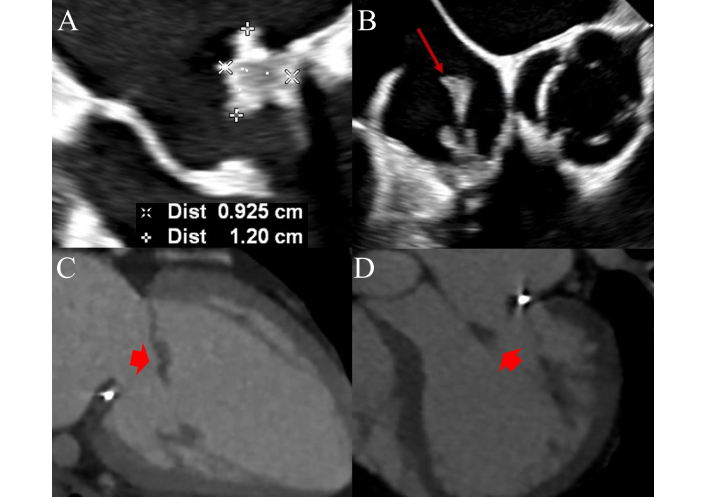
Mitral prosthetic valve endocarditis on transesophageal echocardiography (TEE) and cardiac computed tomography (CT). Figure 4 shows a case of prosthetic valve endocarditis in a 45-year-old male with prior history of mitral annuloplasty repair. Panel A: TEE showing a four-chamber view showing a large vegetation attached to the posterior mitral leaflet. Panel B: Large mobile vegetation attached to the tricuspid valve noted as well, indicating poly-valvular involvement; aortic and pulmonary valves were not affected (not shown). Arrow pointing to the vegetation. Panels C and D represent cardiac CT imaging performed in preparation for mitral and tricuspid valve replacement. Arrowheads show the anterior mitral leaflet (AML) vegetation appearing as a low-attenuation, soft-tissue mass.
Characteristic imaging features of infective endocarditis on multimodality imaging.
| Finding | Echocardiography (TTE/TEE) | Cardiac PET | Cardiac CT | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Vegetation | Mobile echodense mass attached to valve or device; best seen on TEE | Focal increased 18F-FDG uptake on valve leaflets or prosthetic components | May be seen as low-attenuation mass on valve, but limited sensitivity | TTE: 50–70%TEE: 90–100%PET: 30–40% (native), 73–100% (PVE)CT: 60–90% | TTE: 90–95%TEE: 90–100%PET: 80–100%CT: 88–95% |
| Abscess | Echolucent or echodense periannular area; better seen on TEE | Perivalvular fluid collection with intense 18F-FDG uptake | Periannular low-attenuation area; ring enhancement | TTE: 25–40%TEE: ~80–90%PET: 80–87%CT: 90–95% | TTE: 90–95%TEE: 90%PET: 83–95%CT: 95% |
| Pseudoaneurysm | Pulsatile echo-free space adjacent to annulus, with systolic expansion | Outpouching adjacent to valve annulus on that may or may not exhibit 18F-FDG uptake | Contrast-filled outpouching with communication with cardiac chamber | TTE: 20–40%TEE: 85–98%PET: 60–80%CT: 95% | TTE: 90–95%TEE: 90–100%PET: 90–100%CT: 90–100% |
| Leaflet perforation | Color Doppler shows flow through perforation; direct visualization on TEE | Not directly seen on PETMay show secondary signs like vegetation or uptake in perforated area | May see small discontinuity; less sensitive | TTE: 25–50%TEE: 80–90%PET: poor detectionCT: 30–60% | TTE: 90–95%TEE: 90–100%PET: poor detectionCT: 100% |
| Fistula | Abnormal color Doppler jet between chambers (e.g., LV-RA); best on TEE | Linear 18F-FDG uptake along tract if inflammation present | Direct tract visualization with contrast; better delineation of anatomy | TTE: 30–50%TEE: 85–95%PET: limitedCT: ~90% | TTE: 90–95%TEE: 95%PET: limitedCT: ~95% |
| Paravalvular leak | Color Doppler shows turbulent flow around sewing ring; TEE is key | Low or no 18F-FDG uptake unless associated with infection/inflammation | Seen as contrast jet extending outside valve annulus | TTE: 30–60%TEE: 90%PET: poor detectionCT: 80–95% | TTE: 90–95%TEE: 95–100%PET: poor detectionCT: 90–100% |
| Prosthetic valve dehiscence | Rocking motion of prosthesis, paravalvular regurgitation | Increased 18F-FDG uptake at the annular interface | Visualized as separation of prosthesis with abnormal angulation or movement | TTE: 30–50%TEE: 90–95%PET: 65–85%CT: ~85% | TTE: 90–95%TEE: 95%PET: 80–90%CT: 90% |
TTE: transthoracic echocardiography; TEE: transesophageal echocardiography; PET: positron emission tomography; CT: computed tomography; FDG: fluorodeoxyglucose; PVE: prosthetic valve endocarditis; LV: left ventricle; RA: right atrium.
For NVE, CCT is not a first-line modality and is primarily used when echocardiographic data are inconclusive or to assess suspected perivalvular complications. Sensitivity is inferior to TEE for small vegetations and leaflet perforations. CCT has limited utility in detecting cerebral or visceral emboli, where other imaging like magnetic resonance imaging (MRI) or abdominal CT/MRI is preferred. It may be included in a comprehensive preoperative screen to detect silent embolic complications [1, 2, 57].
In PVE, CCT offers excellent visualization of perivalvular structures, particularly when TEE is inconclusive. It reliably detects abscesses, pseudoaneurysms, and inflammatory extension, with high sensitivity (89–100%) and specificity when compared to surgical findings [58, 59]. CT is less sensitive for small vegetations and leaflet perforation, and echocardiography remains superior for identifying paravalvular leaks. Nonetheless, combining CT and TEE improves overall diagnostic accuracy. Disadvantages include radiation, contrast need, and lower sensitivity for mobile vegetations [58, 60]. CCT is especially beneficial in TAVR-IE due to its ability to overcome limitations posed by the valve stent frame and prosthetic artifacts. It effectively delineates abscesses, pseudoaneurysms, fistulae, and dehiscence and facilitates detailed preoperative planning [51, 60].
Building on the adjunctive role of CCT, recent expert consensus and clinical data emphasize that CCT is particularly valuable for delineating the extent of infection and identifying structural complications in CIED infection when echocardiography is inconclusive or limited by device-related artifacts. CCT offers high spatial resolution for detecting perivalvular abscesses, pseudoaneurysms, fistulae, and other anatomic sequelae that may not be well visualized with ultrasound-based modalities, especially in the presence of prosthetic material or lead-associated shadowing [49].
18F-FDG-PET/CT and WBC-SPECT/CT are endorsed by the Infectious Diseases Society of America, ACC, AHA, and partner societies as valuable tools in diagnostically complex cases of IE—particularly PVE, cardiac device infections, and cases where echocardiography or blood cultures are inconclusive [61].
These modalities are also instrumental in identifying extracardiac complications and metastatic infection, which support both diagnosis and risk stratification. On 18F-FDG-PET/CT, key findings include focal or perivalvular increased 18F-FDG uptake, while WBC-SPECT/CT reveals localized radiolabeled leukocyte accumulation. Reported sensitivity for 18F-FDG-PET/CT in PVE ranges from 74–93%, with specificity between 84–95%. For NVE, sensitivity is much lower (22–31%), although specificity remains high (98–100%). WBC-SPECT/CT shows pooled sensitivity and specificity of 86% and 97%, respectively, with higher accuracy in PVE than in NVE [52, 62–64]. These techniques enhance diagnostic confidence in PVE and device infection, facilitate detection of systemic emboli, and may reclassify “possible” IE to “definite” per the modified Duke criteria. However, they have limited use in NVE due to low sensitivity, and false positives can occur in the context of post-surgical inflammation. Furthermore, specialized imaging protocols and technical expertise are required. 18F-FDG-PET/CT is now recognized as a major diagnostic Duke criterion for PVE, with moderate to intense uptake associated with adverse event risk [52, 62–64].
In NVE, 18F-FDG-PET/CT has limited utility due to its low sensitivity (22–31%), although specificity remains high. It is best used as an adjunct in patients with persistent clinical suspicion and inconclusive TTE/TEE findings, or for identifying extracardiac complications such as septic emboli. Its role is not in primary diagnosis but in supplemental evaluation [3, 39, 65, 66].
In PVE, 18F-FDG-PET/CT significantly improves sensitivity and specificity, with pooled sensitivities of 85–93% and specificities of 85–95%. It is particularly valuable in reclassifying “possible” cases and is most effective when performed early, prior to significant antibiotic exposure. Representative patient cases are shown in Figure 5 to illustrate their clinical utility. Quantitative metrics such as standardized uptake value (SUV) ratios add to diagnostic precision. WBC-SPECT/CT is also effective in identifying inflammation and perivalvular abscesses, although its sensitivity is generally lower than PET/CT [65, 67]. In TAVR-IE, PET/CT is increasingly utilized to identify prosthetic infections and systemic septic emboli. However, interpretation is challenging early post-implantation due to inflammation-related false positives. Diagnostic accuracy is generally lower than for surgical valves, necessitating careful correlation with clinical and other imaging data [61, 68].
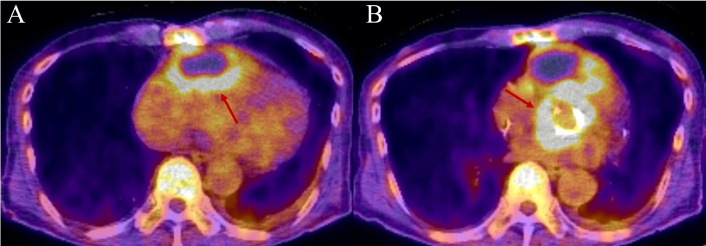
PET/CT imaging of prosthetic aortic valve endocarditis. This is the case of a 70-year-old male patient with a complex cardiac surgical history, initially undergoing aortic valve replacement with a #25 Inspiris valve, along with ascending aorta and hemiarch replacement, and saphenous vein graft (SVG) bypass of the ramus coronary artery due to aortic intramural hematoma. A few years after the initial surgery, the patient developed prosthetic aortic valve endocarditis with an aortic root and right ventricular free wall abscess. Panel A: PET/CT axial view highlighting a large cystic mass located in the anterior pericardial region. The mass exhibits increased peripheral 18F-FDG uptake with a maximum standardized uptake value (SUV) of 5.6 and central photopenia, which is suggestive of an abscess. Red arrow is pointing to the area of increased uptake. Panel B: PET/CT axial view at the level of the aortic valve demonstrating heterogeneous increased 18F-FDG uptake with a maximum SUV of 12.4. The uptake corresponds to soft tissue density surrounding the prosthetic valve, raising suspicion for active infection. Red arrow is pointing to the area of increased uptake. PET: positron emission tomography; CT: computed tomography; FDG: fluorodeoxyglucose.
The American Society of Nuclear Cardiology, Infectious Diseases Society of America, Heart Rhythm Society, and others state that 18F-FDG-PET/CT is useful for detecting both local device infection and systemic septic emboli and can improve diagnostic accuracy when integrated with clinical and microbiological data. WBC-SPECT/CT is also endorsed for its high specificity in identifying infection, especially in cases where 18F-FDG-PET/CT results are equivocal or when there is a need to distinguish infection from sterile inflammation [49].
MRI is useful in the detection of cerebral complications—with plain MRI detecting embolic phenomena and MRA angiography detecting mycotic aneurysms. The AHA and Infectious Diseases Society of America state that MRI has a major impact on the diagnosis and management of IE, particularly for detecting cerebral embolic events, many of which are clinically silent. They note that MRI may be considered in all patients with left-sided endocarditis, even in the absence of neurological symptoms, as MRI findings can influence subsequent medical and surgical management [1].
Management for endocarditis can be broken down into antimicrobial vs. antimicrobial + surgical. Once a diagnosis is suspected or confirmed, empiric antibiotics should be started.
Blood cultures should be obtained prior to the initiation of antimicrobial therapy. Early involvement of infectious disease specialists improves diagnostic accuracy and outcomes [69]. Empiric therapy should begin after blood cultures are drawn and should target the most likely pathogens based on patient characteristics and epidemiologic context. The WikiGuidelines Group recommends vancomycin or daptomycin to cover MRSA, enterococci, and CoNS in prosthetic valve cases, combined with a beta-lactam such as ceftriaxone for gastrointestinal or odontogenic sources, or cefazolin for suspected methicillin-susceptible S. aureus (MSSA) [69, 70]. For NVE with low risk for resistant organisms, monotherapy with cefazolin or ceftriaxone may be appropriate [9].
Once the causative organism and susceptibilities are known, therapy should be tailored accordingly. For MSSA, the preferred regimen is a beta-lactam such as nafcillin or oxacillin; cefazolin is an option for patients without severe beta-lactam allergy [70]. MRSA, vancomycin, or daptomycin is used. Streptococcal IE is treated with penicillin G or ceftriaxone, and gentamicin may be added briefly for synergy in select cases, though its use is typically limited to ≤ 2 weeks due to nephrotoxicity. Enterococcus faecalis IE is most effectively treated with ampicillin plus ceftriaxone, especially in older adults and those with renal dysfunction, as recommended by the ACC/AHA [2, 8, 9]. For HACEK organisms, ceftriaxone or a fluoroquinolone is appropriate [70].
Standard duration is 4 weeks for NVE and 6 weeks for PVE [70]. For select patients with left-sided IE caused by streptococci, enterococci, or staphylococci, the ACC/AHA guidelines support a switch to oral step-down therapy after initial IV treatment, provided that TEE excludes perivalvular complications and that close monitoring is feasible [2]. The POET trial further supports the safety and efficacy of partial oral therapy in these carefully selected cases [71].
Antimicrobial management alone is not enough for many patients, especially for patients with heart failure from valve damage, uncontrolled infection (e.g., abscess, persistent bacteremia), or embolic risk from large vegetations (> 10 mm). Early surgery—often within 48 h—can reduce embolic events, particularly in patients with large vegetations and severe regurgitation, though mortality benefit varies by patient selection. Surgical options include valve repair or replacement, with debridement as needed. Transcatheter therapies are not standard for left-sided IE and are reserved for select right-sided cases.
Referral to an expert surgical center is recommended for patients requiring intervention, as outcomes are best in high-volume centers with specialized experience in complex valve and prosthetic infections. Surgical decision-making and perioperative care should also be guided by a multidisciplinary endocarditis team, incorporating cardiology, cardiac surgery, infectious diseases, and imaging specialists to ensure optimal management of complicated cases. The ACC and AHA recommend early surgery is indicated in patients with IE who develop heart failure due to valvular dysfunction, have uncontrolled infection (e.g., persistent bacteremia > 5–7 days, annular or aortic abscess, heart block, or infection with fungi or highly resistant organisms), or are at high risk for embolic events, particularly with large (> 10 mm), mobile vegetations or recurrent emboli despite appropriate therapy. The ACC also highlights these indications and notes that early surgery (during the index hospitalization and before completion of antibiotics) is associated with a reduction in embolic complications, especially in patients with severe valve dysfunction and large vegetations [57].
Timing of surgery is stratified as emergency (within 24 h), urgent (within 7 days), or elective, based on the severity of complications and risk of further deterioration [72]. For example, surgery should not be delayed in patients with heart failure or uncontrolled infection, while in cases of recent major ischemic stroke with extensive neurological damage or intracranial hemorrhage, the ACC recommends delaying surgery for at least four weeks if the patient is hemodynamically stable. The presence of neurological complications without hemorrhage is not a contraindication to early surgery [2].
Surgical techniques are tailored to the extent of infection and valve involvement. Valve repair is preferred when feasible, particularly for the mitral valve, to preserve native tissue and reduce prosthesis-related complications [72]. Radical debridement of infected tissue and management of perivalvular abscesses are critical, and prosthetic valve replacement may be necessary in cases of extensive destruction or PVE [72]. The American Association for Thoracic Surgery notes that in right-sided IE, surgery is most often reserved for failure to control infection, persistent septic pulmonary emboli, or severe tricuspid regurgitation with right heart failure [73].
Transcatheter procedures, such as percutaneous aspiration of right-sided vegetations, have limited and highly selective utility, primarily in patients with isolated right heart involvement who are poor surgical candidates. These approaches are not standard of care for left-sided IE and are not recommended by major societies except in exceptional circumstances [73]. Overall, surgical intervention in IE should be guided by a multidisciplinary team with expertise in cardiology, infectious diseases, and cardiac surgery, with careful consideration of patient-specific risks and comorbidities [2].
Despite major advances in imaging technology and guideline development, significant challenges remain in the diagnosis and management of IE. Future efforts should focus on validating standardized imaging protocols across modalities, particularly for PVE and cardiac device infections, where diagnostic uncertainty remains high. The integration of artificial intelligence into imaging analysis may enhance diagnostic accuracy, especially in cases with subtle or equivocal findings. Multicenter prospective studies are needed to refine imaging-based risk stratification tools and to evaluate how multimodal imaging influences clinical decision-making, surgical timing, and long-term outcomes. Additionally, the role of emerging techniques—such as hybrid PET/MRI or targeted radiotracers—merits further exploration.
IE is a serious and complex disease with high morbidity and mortality, particularly in patients with prosthetic valves, cardiac devices, and healthcare-associated infections. Timely diagnosis and coordinated multidisciplinary management are essential. While echocardiography is the cornerstone of initial evaluation, advanced imaging modalities such as CCT, 18F-FDG-PET/CT, WBC-SPECT/CT, and brain MRI are increasingly important in diagnostically challenging cases. These tools offer complementary information that can improve diagnostic certainty, guide surgical planning, and detect extracardiac complications. Future work should focus on standardizing imaging protocols, improving access to advanced modalities, and generating prospective data to inform imaging-based clinical pathways. Optimizing the use of multimodality imaging is critical to improving outcomes in patients with suspected or confirmed endocarditis.
ACC: American College of Cardiology
AHA: American Heart Association
CCT: cardiac computed tomography
CIEDs: cardiac implantable electronic devices
CoNS: coagulase-negative staphylococci
CT: computed tomography
ESC: European Society of Cardiology
FDG: fluorodeoxyglucose
HACEK: Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, Kingella
IDU: intravenous drug use
IE: infective endocarditis
IV: intravenous
MRI: magnetic resonance imaging
MSSA: methicillin-susceptible Staphylococcus aureus
MVP: mitral valve prolapse
NVE: native valve endocarditis
PET: positron emission tomography
PVE: prosthetic valve endocarditis
RHD: rheumatic heart disease
SAVR: surgical aortic valve replacement
TAVR: transcatheter aortic valve replacement
TAVR-IE: transcatheter aortic valve replacement-associated infective endocarditis
TEE: transesophageal echocardiography
TTE: transthoracic echocardiography
VGS: viridans group streptococci
WBC-SPECT/CT: white blood cell single-photon emission computed tomography/computed tomography
TC: Investigation, Writing—original draft, Writing—review & editing. JER: Investigation, Writing—review & editing, Visualization. SLPV: Writing—review & editing, Validation. TKMW: Conceptualization, Supervision, Writing—review & editing, Project administration. All authors read and approved the final manuscript.
The authors declare that they have no conflicts of interest.
Ethical approval is not required for a review study according to the local Ethics Committee.
Informed consent to participate is not required for a review study according to the local Ethics Committee.
Not applicable.
All relevant data is contained within the manuscript.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2332
Download: 102
Times Cited: 0
