Affiliation:
1Medical Faculty University of Belgrade, 11000 Belgrade, Serbia
Email: skali.ana7@gmail.com
ORCID: https://orcid.org/0009-0006-2359-5192
Affiliation:
2Cardiology Clinic, University Clinical Center Serbia, 11000 Belgrade, Serbia
ORCID: https://orcid.org/0000-0001-5610-7083
Affiliation:
2Cardiology Clinic, University Clinical Center Serbia, 11000 Belgrade, Serbia
ORCID: https://orcid.org/0000-0001-8987-4190
Affiliation:
1Medical Faculty University of Belgrade, 11000 Belgrade, Serbia
ORCID: https://orcid.org/0000-0003-1049-6321
Explor Cardiol. 2024;2:1–8 DOI: https://doi.org/10.37349/ec.2024.00016
Received: November 21, 2023 Accepted: December 09, 2023 Published: February 04, 2024
Academic Editor: Maria Grazia Andreassi, CNR Institute of Clinical Physiology, Italy
Coronary vasospasm stands as a widely acknowledged and frequent culprit behind chest pain, acute coronary syndrome, and sudden cardiac death, yet it remains a challenging diagnosis. Current guidelines recommend invasive coronary function testing to assess pathophysiology and mechanisms and to define treatment. In reality, this protocol is rarely applied, because it necessitates extended occupation of the cath lab, repetitive administration of nephrotoxic iodine contrast agents, the need for repeated testing on both coronary arteries leading to considerable radiation exposure, and significant direct expenses. The promising perspective for vasospasm testing is a noninvasive approach with advanced echocardiographic techniques, such as transthoracic Doppler echocardiography, with more sensitive indicators of ischemia. Hyperventilation and exercise tests are used for vasospasm directed testing, with assessment of the new parameters: coronary flow velocities and reserve, allowing to see deeper into macro and microvascular pathophysiology. Association between coronary flow, global longitudinal strain and microvascular dysfunction (MVD) and impaired values at hyperemia was previously demonstrated. Reduction in coronary flow velocity (CFV) despite heightened myocardial oxygen consumption and double product during hyperventilation are indicative of coronary vasospasm. Normal coronary angiography finding in patients with documented evidence of ischemia should initiate additional diagnostic testing in order to increase the yield of specific diagnosis in patients with suspected vasospasm, which could help to personalize treatment and prognosis. In order to achieve this, non-invasive provocative stress echocardiography tests should be included in the diagnostic workup. This approach, characterized by its simplicity, feasibility, safety, and efficacy, is currently undergoing extensive testing on a large scale.
A significant number of patients (up to 70%) who undergo coronary angiography due to angina and/or transient ischemic electrocardiographic (ECG) changes, have angiogram with no obstructive coronary artery disease. According to the latest guidelines, they are classified as having a specific endotype of chronic coronary artery disease syndrome, known as ischemia with non-obstructive coronary arteries (INOCA) [1, 2]. These patients often find themselves moving between cardiologists and psychiatrists, undergoing numerous diagnostic procedures without receiving a proper diagnosis and therapy, all while experiencing a poor quality of life and an underestimated risk of adverse cardiac events. This represents a significant financial burden for the health care system, considering high estimated lifetime costs.
Angina in INOCA patients occurs due to existence of epicardial spasm and/or microvascular dysfunction (MVD, known as microvascular angina). MVD is a consequence of: (1) structural remodeling, implying an increase in wall-to-lumen ratio and/or reduced capillary density (rarefaction) [3]. The hemodynamic correlates of these changes are reflected in an impaired response to an endothelium-independent vasodilator (e.g., adenosine), and increased microvascular resistance; and (2) functional arteriolar dysregulation implies the existence of endothelial dysfunction, which is associated with decreased vasodilation and also paradoxical vasoconstriction [4]. All of these mechanisms of vascular dysfunction may coexist and contribute to INOCA.
This entity is recognized for quite some time, but often neglected, despite the fact that attacks of angina could be fatal. Prinzmetal and his colleagues [5] were the first to propose the concept of coronary artery spasm, suggesting that the angina they observed was due to vasospasm, leading to reduced blood supply to the heart muscle and causing distinctive electrical changes on the ECG, such as transient ST segment elevations or depressions. Starting from 1970’s, Maseri et al. [6], Maseri and Chierchia [7] performed a series of studies demonstrating that coronary vasospasm is a frequent cause of chest pain, acute ischemic syndromes and sudden cardiac death [6, 7]. Angina like chest pain, ECG changes during exercise, normal angiogram are also known as syndrome X (CSX). CSX is pathophysiologically linked to MVD which is the result of anatomic and functional abnormalities of coronary microcirculation [8].
Currently, for the comprehensive diagnostic assessment of patients with INOCA, the European Society of Cardiology (ESC) guidelines endorse invasive coronary functional testing. The gold-standard technique utilizes invasive coronary angiography to directly visualize coronary spasm, utilizing both pharmacological (acetylcholine, ergonovine, methylergonovine) and non-pharmacological (hyperventilation, cold pressor) triggers for provocative spasm testing. Confirmation of coronary artery spasm via the provocative test necessitates: (1) reproducing characteristic chest pain; (2) manifesting ischemic ECG changes; and (3) observing ≥ 90% vasoconstriction on angiography [1]. Currently approved invasive diagnostic protocols and hemodynamic correlates are presented in Figure 1.
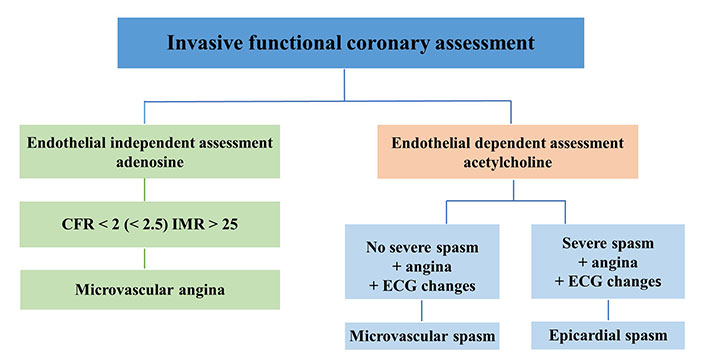
Invasive functional coronary assessment. CFR: coronary flow reserve; IMR: index of microvascular resistance
Normal coronary angiography finding in patients with documented angina symptoms and evidence of ischemia should initiate additional diagnostic testing immediately in the catheterization laboratory. Regrettably, this scenario is infrequent due to overwhelming scheduling demands, high occupancy rates, and a lack of specific reimbursement. Alongside these logistical and administrative challenges, the invasive approach presents additional conceptual and pragmatic limitations. This includes the necessity for sequential coronary angiography tests utilizing nephrotoxic iodine contrast material, employment of a coronary catheter carrying inherent potential to perturb vasomotor function, exposure to substantial cumulative radiation doses associated with oncogenic risks, potential disruption of vasomotor function due to the known toxic effects of ionizing radiation on the endothelium, a notable incidence (around 2%) of major side effects, and the requirement to install a temporary pacemaker as a precaution against potential arrhythmic complications.
This procedure is protracted, expensive, hazardous, and intricate. It is not commonly performed in the majority of invasive cardiology laboratories, despite the acknowledged significance of coronary vasospasm as both a pathogenic mechanism and a target for therapeutic intervention in these patient populations [9–11].
In order to increase the yield of specific diagnosis in patients with INOCA, non-invasive provocative stress echocardiography tests should be included in the diagnostic workup.
Noninvasive testing for vasospasm, whether epicardial or microvascular, poses different challenges from the proper patient selection (identifying angina like chest pain occurring during rest, early in the morning or during the night, on cold exposure or on effort and hyperventilation, circadian variability in exercise tolerance), the preparation (test in the morning hours, withdrawal of the therapy) as well as stressor selection. Regional wall motion abnormality (RWMA) as the echocardiographic hallmark of ischemia due to a transient severe spasm of the epicardial artery, followed by ECG ischaemic changes (usually ST elevation or severe arrhythmias), could also occasionally occurs with MVD, followed with ECG changes (ST depression) and anginal pain [12]. RWMA was occasionally observed during aminophylline administration after provocative test for ischemia with dipyridamole [13], or after metoprolol administration at the end of the dobutamine test [14]. One should have in mind that these drugs and drugs used for lactation cessation and exaggerate uterine bleeding, or migraine attacks, such as ergotamine preparation, bromocriptine, and beta blockers could provoke and aggravate spasm in susceptible patient.
When it comes to selecting a specific test, it’s worth noting that ergonovine, although highly effective, is not commercially available in most countries. In the large study, Om et al. [15] demonstrated the safety and prognostic value of ergonovine echocardiography in the large group of 14,012 patients. The overall frequency of positive results was 15.3% (n = 2,144) and most of these patients (99.6%) showed RWMAs [15]. According to the Japanese Circulation Society, the diagnosis of definitive or suspected vasospastic angina can be established even through a non-drug-induced coronary spasm provocation test [16]. Hyperventilation and cold pressor test are usually used with stress echocardiography, sometimes accelerated with exercise test. The effectiveness of accelerated exercise following mild hyperventilation test has been shown to be equivalent to the intracoronary acetylcholine test during coronary angiography, a current gold standard [17].
Variable sensitivity of ECG and RWMA (8–61.7%) in vasospasm testing has limit the use of stress echo (SE) as a potent, available and promising diagnostic method, directing this huge group of the patients directly to invasive coronary angiography, where in the limited number of cases physiologic testing is performed. Recently, advanced echocardiographic techniques, drow in a focus vasospasm directed testing, allowing us to see deeper into macro and microvascular pathophysiology. In order to introduce a noninvasive method to test coronary reactivity, more sensitive indicators of ischemia should be used.
Subtle impairments in left ventricular (LV) contractility, impairment in LV longitudinal function caused by MVD, are not detectable with conventional echocardiography based on RWMA. This early marker of subclinical systolic dysfunction can be assessed by speckle-tracking derived LV global longitudinal strain (LVGLS) [18], which has high sensitivity but low specificity. To overcome this limitation, SE assessment of myocardial deformation capacity (LVGLS reserve) in patients with MVD should be iationused to provide greater insight into the LV systolic function [19, 20]. In the publication from the iPOWER cohort, authors reported that women with MVD had significantly lower LVGLS at hyperemia as well as an impaired delta LVGLS [19, 20]. Transthoracic Doppler echocardiography (TDE), has been proven to be an efficacious, versatile, and reproducible non-invasive method to assess functional state of microcirculation by measuring coronary flow velocity reserve (CFVR) [21]. Impairment of CFVR is the first sign of ischemic cascade and in the absence of epicardial disease is sensitive marker of MVD [22, 23]. In the new Guidelines of the American College of Cardiology/American Heart Association for Evaluation and Diagnosis of chest pain, this method gain recommendation IIb [24] in evaluation of patients with chest pain and INOCA during provocative tests. Markedly lower CFVR values in left anterior descending artery (LAD) have been reported in women with CSX [25], and moreover, impairment of CFVR correlated with impaired global longitudinal strain values in women with cardiac CSX and slow coronary flow [26]. Reduction of CFVR, reflecting dynamic microvascular changes was observed during spasm provocation, with ergonovine and hyperventilation-cold pressor test in the cath lab [27, 28].
With noninvasive trans thoracic echocardiography (TTE)-Doppler coronary flow velocity (CFV) measurement methods, microvascular functional testing could be entirely conducted noninvasively. We recently presented protocol based on stress echocardiography and CFV-LAD evaluation after vasoconstrictive and vasodilator stimuli in patient with INOCA and suspected vasospasm [29]. Typical example of spectral Doppler flow in LAD during provocation is presented in a Figure 2.
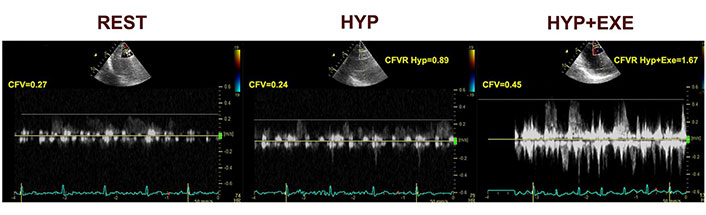
CFV at rest, during hyperventilation and at exercise [29]. HYP: hyperventilation; EXE: exercise
Note. Reprinted from “Noninvasive evaluation of dynamic microvascular dysfunction in ischemia and no obstructive coronary artery disease patients with suspected vasospasm,” by Dikic AD, Dedic S, Jovanovic I, Boskovic N, Giga V, Nedeljkovic I, et al. J Cardiovasc Med (Hagerstown). 2024;25:123–31 (https://journals.lww.com/jcardiovascularmedicine/fulltext/2024/02000/noninvasive_evaluation_of_dynamic_microvascular.4.aspx). CCBY-NC-ND.
CFV Doppler measurements were done at the baseline and at the end of the each step of the test protocol, with the dedicated probe settings for the coronary flow. As vasoconstrictive stimuli targeting the dysfunctional endothelium, we used hyperventilation test (deep and fast breathing during 5 min) followed by semi-supine exercise targeting endothelial dependent vasodilatation. Adenosine test was performed after resting period as the vasodilatory stimulus, acting through a mainly endothelium-independent mechanism and vascular smooth muscle cell relaxation, reflecting structural microvascular changes. We found that coronary vasoconstriction after hyperventilation is common (in half of the patients), in absence of structural coronary microvascular disese (CMD), as such is considered to be related to microvascular functional impairment. However, this approach does not separate an epicardial from a microvascular origin of coronary vasospasm in positive tests, but absence of ST segment elevation or multi segment or severe RWMA makes existence of transmural ischemia and epicardial artery vasospasm unlikely [29]. This is a pilot study, of the Stress Echo for coronary vasoSPASM detection (SESPASM) protocol, one of the 12 protocols on the large international, multicenter, prospective, SE 2030 platform that covers a broad spectrum of cardiovascular disease protocol, and needs a prospective confirmation with a large-scale study [30]. Suggested algorithm for noninvasive functional coronary assessment is presented in Figure 3.
Despite the clear advantages of a noninvasive approach, the clinical experience in the large scale studies with a noninvasive approach is missing to date. Comparison of this two approaches, invasive and noninvasive are presented in Table 1.
Detection of coronary vasospasm
| Test parameters | Invasive approach | Noninvasive approach |
|---|---|---|
| Operating theater | Catheterization laboratory | Echocardiography laboratory |
| Stress test | Intracoronary acetylcholine | Hyperventilation + exercise |
| Main biomarker | Coronary vasoconstriction | RWMA |
| Ancillary biomarker | IMR | CFV |
| Radiation exposure | 15 mSv (750 chest X-rays) | Absent |
| Iodine contrast material | 50 mL | Absent |
| Intracoronary catheter | Present, perturbs coronary tone | Absent |
| X-rays effect on endothelium | Present, may alter function | Absent |
| Large scale experience | Present | Missing |
mSv: milli Sievert (radiation dose)
Vasospasm is unfairly neglected as the cause of anginal pain. Microvascular vasospasm obviously exists more often than epicardial and contributes to the higher cardiovascular risk and poor life quality. The fact that we could not see it, does not absolve us from the fact that we should not look for it. Stress echocardiography and transthoracic Doppler measurements of coronary flow with dedicated protocols can be used to detect epicardial and coronary small vessel vasospasm. RWMA and CFVR combined with specific stressors targeted at eliciting coronary vasospasm such as hyperventilation + exercise, dobutamine + beta-blockers, and dipyridamole + aminophylline, and measured during the test are promising and available parameters. We believe that it is time to endorse more available, versatile, noninvasive methods to increase the yield of specific diagnosis in patients with suspected vasospasm, which could help us to personalize treatment and prognosis.
CFV: coronary flow velocity
CFVR: coronary flow velocity reserve
CSX: syndrome X
ECG: electrocardiographic
IMR: index of microvascular resistance
INOCA: ischemia with non-obstructive coronary arteries
LAD: left anterior descending artery
LV: left ventricular
LVGLS: left ventricular global longitudinal strain
MVD: microvascular dysfunction
RWMA: regional wall motion abnormality
SE: stress echo
ADD: Writing—original draft, Conceptualization. SD: Writing—original draft, Visualization. NB and VG: Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
