Affiliation:
1Allergy Department, Complejo Hospitalario de León, 24071 León, Spain
Email: fsantos@saludcastillayleon.es
ORCID: https://orcid.org/0000-0003-1493-6152
Affiliation:
1Allergy Department, Complejo Hospitalario de León, 24071 León, Spain
ORCID: https://orcid.org/0000-0003-1220-0993
Affiliation:
3Research & Development Department, Roxall, 48170 Zamudio, Spain
ORCID: https://orcid.org/0000-0001-7645-2773
Explor Asthma Allergy. 2023;1:55–59 DOI: https://doi.org/10.37349/eaa.2023.00008
Received: February 17, 2023 Accepted: April 27, 2023 Published: June 30, 2023
Academic Editor: Ingrid Terreehorst, University of Amsterdam, The Netherlands
A case report of fish allergy is exposed. The responsible allergen was fish collagen, and there was no sensitization to parvalbumin (main fish allergen). The patient acquired collagen sensitization by occupational exposition, not by ingestion.
Fish allergy is a common food allergy all over the world. The main allergen to which allergic people react is generally parvalbumin (prevalence rates 70–95%). However, other allergens, such as enolase, aldolase, vitellogenin, tropomyosin, and collagen, have been described [1]. Proteins are present in fish muscle, roe, skin, or blood [2].
Collagen was identified as a new fish allergen by Hamada et al. [3].
Fish gelatin (hydrolyzed collagen) is made from fish skin and bones and has been described as a potentially dangerous sensitizer, mainly in Japan [4]. Isinglass largely contains collagen. Some foods (beverages, candy) and also some pharmaceutical gel capsules and coatings can contain this allergen [5, 6].
An atopic 46-year-old man (allergic rhinoconjunctivitis and asthma due to grass pollen and dander sensitization) began to work by cutting and packaging fish collagen and shark cartilage seven years ago in a factory. The powder obtained from this manufacturing was packed in capsules. At the beginning of his work there was a direct exposition of the powder, but later the factory installed isolation measures.
He had been working there for about ten months when he began to notice nasal and ocular symptoms, as well as pruritus and contact urticaria, while he manipulated those products. Later, he also experienced dyspnea, chest tightness, and cough. The symptoms began a few minutes after the work began and gradually worsened along the day. He felt better on weekends and holidays. He managed the situation with inhalers [a combination of long-acting bronchodilators (formoterol) and inhaled corticosteroids (budesonide)] twice a day and antihistamines (cetirizine, 10 mg), on demand.
Two years later, he suddenly began to have anaphylaxis when eating fish, with general urticaria, bronchospasm, and rhinoconjunctivitis. He had previously eaten fish without any problem throughout his life.
From then on, he left this job. He cannot eat fish from then on as he experiences the same anaphylactic symptoms.
To perform the prick-to-prick test, the patient provided fish collagen and shark cartilage powder. The powder was obtained after drying and micropulverizing the animal’s own cartilage tissue. Control test histamine dichlorhydrate: 10 mg/mL.
Total and specific immunoglobulin E (IgE) were determined using the ImmunoCAPTM system (Thermo Fisher Scientific) according to the manufacturer’s instructions. Specific IgE greater than 0.15 kU/L was considered positive.
Enzyme allergo-sorbent test (EAST): solid phase was obtained by coupling panga collagen and shark cartilage powder (1 mg/mL) to 6 mm diameter cyanogen bromide-activated paper discs. EAST was performed in accordance with the manufacturer’s instructions [specific IgE enzyme immunoassay (EIA) kit, HYTEC, HYCOR Biomedical Ltd, UK].
The assay was carried out as described by Laemmli [7]. A pool of sera from non-atopic subjects was used as negative control serum in the assay.
The method of this technique is described by Van Hengel et al. [8].
The patient refused any bronchial provocation test due to the potential risk. A pool of sera from non-atopic subjects was used as negative control serum in the assay.
Prick control test histamine dichlorhydrate (10 mg/mL): 6 mm. Prick-to-prick test with collagen fish powder (from panga): 10 mm papule with surrounding erythema. Prick-to-prick test with shark cartilage powder: 9 mm papule with surrounding erythema.
Specific IgE (ImmunoCapTM system) to cod, sole, hake, and carp parvalbumin were negatives.
EAST: specific IgE to panga collagen (the same powder of skin prick test): 1.2 kU/L (class 2) and to shark cartilage powder: 0.5 kU/L (class 1).
The assay showed a clear IgE-reactive band higher than 97 kDa in the sample of shark cartilage as well as in panga and shark extracts. In the shark cartilage sample, an IgE-reactive area between 6–30 kDa was also detected, probably originating from the same degradation of the protein > 97 kDa. The panga collagen sample showed various IgE-reactive bands between 20–48 kDa surely originating from collagen degradation (Figure 1).
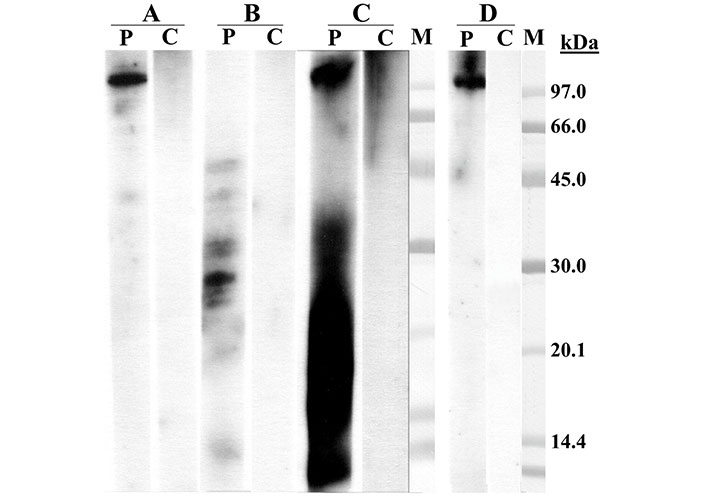
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) immunoblotting. (A) Panga extract; (B) panga collagen; (C) shark cartilage; (D) shark extract. Lane P: patient serum; lane C: control serum (pool of sera from non-atopic subject); lane M: molecular mass standard
Immunoblotting-inhibition assay was performed with panga extract in a solid phase and using two concentrations (1 mg/mL and 10 mg/mL) of panga collagen and shark cartilage samples as inhibitor phases. Both panga collagen and shark cartilage produced a total IgE-binding inhibition on the band > 97 kDa from the panga, identifying this band as panga collagen and showing that among all panga proteins, the patient is only sensitized to collagen (Figure 2).
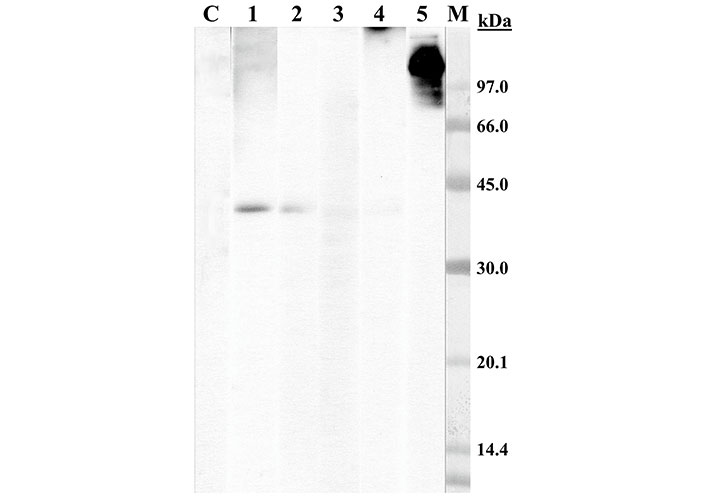
Immunoblotting-inhibition with panga extract in solid phase. Lane C: control serum (pool of sera from non-atopic subjects); lane 1–5: patient serum pre-incubated with panga collagen (1 mg/mL, lane 1), with panga collagen (10 mg/mL, lane 2), with shark cartilage (1 mg/mL, lane 3), with shark cartilage (10 mg/mL, lane 4), with lamb extract (10 mg/mL, lane 5); lane M: molecular mass standard
In conclusion, IgEs have been detected in patient serum, which recognizes proteins from the sample of panga collagen, shark cartilage, and panga and shark extracts which could explain the allergic reactions due to aerosolized exposition, contact, or fish ingestion. Also, according to the results, this patient is only sensitized to collagen. The exact and complete composition of the powder extracts could not be evaluated, but even if they could contain some other allergens such as parvalbumin, the truth is that the patient is not sensitized to it. No IgE to parvalbumin was detected either by skin prick test or specific IgE.
Collagen comes from the skin, scales, bones, and cartilage of different types of fish [9]. Denaturized collagen is exposed to hydrolysis and alkali and acid treatment to produce gelatin after a final process called gelation. Therefore, fish gelatin is fish collagen.
There are several types of collagen. Shark cartilage is made up of 40% collagen and the remaining 60% of several other components such as chondroitin sulfate, hyaluronic acid, phosphates, and calcium. Its collagen is two/thirds type II collagen, and the other third type I collagen, which is by far the most common type [10].
Talking about collagen, gelatin, or shark cartilage powder, used in the study and to which the patient is sensitized, is all similar, it is at the end, all about collagen sensitization, though going through different chemical or physical processes.
Nowadays, fish gelatin is used in the food industry (wine clearance, jellies, marshmallows), but also in the pharmaceutical industry (as capsule gels, or even vaccines or sublingual immunotherapy). In addition, its use is becoming more frequent in the cosmetic industry. So some occupational works are related to fish collagen exposition, and so, occupational asthma due to shark cartilage has been described, even with fatal consequences [11].
Therefore, this is a case of occupational collagen allergy where the route of sensitization could be a mix one: inhaled and cutaneous [12], and as a consequence of this sensitization, the patient developed fish ingestion allergy where parvalbumin is not involved and collagen is the unique fish allergen implicated.
EAST: enzyme allergo-sorbent test
IgE: immunoglobulin E
FSV: Conceptualization, Investigation. MLE: Writing—original draft. BSL: Writing—review & editing. BBZ: Investigation, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study was approved by the Hospital of León Ethics Committee and was carried out in compliance with ethical guidelines outlined in the Declaration of Helsinki and its later amendments. The researchers have conducted the study according to the approved protocol and acceptable research standards.
The informed consent to participate in the study was obtained from all participants.
The informed consent to publication was obtained from relevant participants.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
