Affiliation:
1Independent Researcher, E-1-8, Girija Shankar Vihar, Pune 411052, Maharashtra, India
Email: milind.watve@gmail.com
ORCID: https://orcid.org/0000-0003-0730-8393
Affiliation:
2Sustainability & Environment, Pune Knowledge Cluster, Savitribai Phule Pune University, Pune 411007, Maharashtra, India
ORCID: https://orcid.org/0000-0003-2958-1366
Explor Neuroprot Ther. 2024;4:108–118 DOI: https://doi.org/10.37349/ent.2024.00074
Received: January 22, 2024 Accepted: March 07, 2024 Published: April 07, 2024
Academic Editor: Rafael Franco, Universidad de Barcelona, Spain
The article belongs to the special issue The Urgent Need for New Hypotheses to Develop Effective Therapeutic Tools Against Alzheimer's Disease
Behavioural environment and behavioural responses of an individual are known to affect multiple aspects of physiology including neuroendocrine and growth factor signalling, angiogenesis, stem cell dynamics, tissue homeostasis, and maintenance. Despite substantial evidence, the role of behaviour-physiology interface in human health and disease remains underappreciated. The hypothesis proposed here suggests that deficiencies of certain behaviours that have evolved to become essential or “vitactions” can potentially trigger multiple health problems. Altered growth factor expression because of vitaction deficiencies affects angiogenesis and vascular function, neuronal maintenance, transport of glucose and other nutrients to the brain, mitochondrial function, oxidative stress, inflammation, and protein aggregation dynamics all implicated in Alzheimer’s disease (AD). Exercise is already known to be effective in prevention of AD. The hypothesis suggests that it is the behavioural component of exercise over mechanical activity and calorie burning that has crucial effects on brain health through multiple signalling pathways. Similar to vitamin deficiencies, where supplying the deficient vitamin is the only effective solution, for vitaction deficiencies supplying the deficient behavioural stimuli through behaviourally enriched exercise can be the most effective remedy.
Over half a century, a cluster of non-communicable diseases and conditions are becoming increasingly common in the modern urban industrialized societies including obesity, type 2 diabetes, essential hypertension, cardiovascular disease, stroke, Alzheimer’s disease (AD), certain types of sexual and reproductive disorders including erectile dysfunction and polycystic ovary syndrome (PCOS), osteoporosis, depression, certain types of cancers, chronic liver, and kidney diseases. They are largely believed to be lifestyle disorders. Serious mismatch between the lifestyle for which the human body has evolved versus the sedentary urban lifestyle today is thought to be responsible for the set of pathophysiological mechanisms responsible for these disorders.
Lifestyle is an inclusive term potentially referring to a wide diversity of factors but only a few factors have been largely focused on, namely diet, lack of physical activity, and substance use such as tobacco and alcohol [1–4]. There are other elements in lifestyle that can potentially affect physiology and pathophysiology but they are rarely addressed. We suggest here that there is a major mismatch between human ancestral and current lifestyles with respect to the behavioural environment and the corresponding behavioural responses. The behavioural mismatch can potentially be responsible for multiple elements of the pathophysiology of lifestyle disorders [5].
We state below with available evidence that the behavioural environment and the need for giving appropriate coping responses substantially influences short-term and long-term physiologic states. The behavioural environment includes food related cues, presence or absence of predator, aggressive competitors, and other rewarding as well as risk factors. Appropriate responses to these challenges include physical aggression, defense, agility, quick strategic moves, complex motor coordination, strategic optimization, and the like. Mechanisms behind the behavioural responses commonly involve catecholamines, neurotransmitters, neuropeptides, building and remodeling neuronal pathways, growth factor expression, hormonal responses, angiogenesis, wound healing cascades, immune responses, stem cell dynamics, and tissue microenvironment. The same set of factors are implicated in different ways in different lifestyle associated disorders and therefore it is worth exploring the possibility of behavioural origins of lifestyle disorders.
In invertebrates, olfactory cues of food, predator, or infection trigger a multitude of responses involving neuropeptides, calcium channels, fat tissue dynamics, Toll-Imd-JNK-JAK/STAT pathways, cyclic nucleotide-gated or transient receptor potential channels, stem cell dynamics, ovulation, and reproductive behaviour [6–11]. In a social wasp the levels of aggression of a potential new queen appears to trigger her ovarian development along with correlated physiological and behavioural phenotypic traits [12]. Individuals or genotypes vary in their responses to a given environmental or a social behavioural challenge and the responses are also highly flexible and context specific [12, 13]. Many of the signaling pathways linking behaviour to physiology are conserved across animal taxa [7, 11, 14].
Mammals also show evidence that the behavioural environment and behavioural responses of individuals shape their physiological states and thereby disease risk substantially. One of the best demonstrations of the effect of behavioural environment on pathophysiology is the experiment in which an enriched behavioural environment led to shrinkage of implanted tumors whereas in the control group housed in traditional cages the tumors grew [15]. This experiment and its variations have been reproduced independently by many groups [16–20]. Although the mechanisms are not yet completely clear, behavioural environment appears to modulate growth factor expression and tumor immunity in these experiments. Most genes and molecules associated positively with aggression have a negative association with metabolic syndrome, and the ones negatively associated with aggression have a pro-obesity or pro-insulin-resistance action [5, 21]. Social aggression and the dominance subordinate axis in social animals are also linked to physiology through multiple signaling pathways involving serotonin, dopamine, oxytocin, cholesterol, corticosteroids, sex hormones, insulin, insulin-like growth factor 1 (IGF1), and many other growth factors [5]. Subordinate females are known to suppress ovulation in many species involving mechanisms similar to human PCOS [5, 22–26]. Aggression, risk taking, and anticipation of injuries stimulate salivary expression of growth factors [27, 28]. Breast feeding and maternal aggression are behaviourally linked through hormones and neuropeptides and together appear to reduce anxiety and blood pressure [29, 30]. Angiogenesis is a complex process affected by a diversity of hormones, growth factors, neuropeptides, and neuroamines that are behaviourally regulated [31–39].
There have been some attempts to study the evolutionary origins of the behaviour-physiology links and its role in lifestyle disorders. Multiple pathways of food intake regulation in the human body operate through foraging optimization [40]. Predator presence or other foraging risks increase cocaine-amphetamine regulated transcript (CART) expression and the interaction of CART with leptin and glucagon-like peptide 1 (GLP-1) regulate food intake [40, 41]. As a result, body weight regulation is significantly different with and without predators in many animals [42–45]. In modern lifestyle, since feeding is detached from foraging, the mechanisms of energy regulation through foraging optimization fail to work. Apart from energy regulation CART, which is responsive to foraging risk, is important in mitochondrial function, and neuronal health and thereby has a protective role in neurodegenerative disorders [46–53].
It is unlikely to be a coincidence that many of the behaviourally regulated growth factors and neuropeptides-neuroamines are involved in wound healing and angiogenesis. These links have adaptively evolved since social conflicts, aggression, adventure, and risk prone behaviours anticipate injuries. Since animals’ lick wounds, salivary glands are the best places to secrete some critical growth factors on receiving a behavioural stimulus [5, 27, 28]. It can be expected therefore that chronic deficiency of these behaviours can lead to impaired regulation of growth factor signaling, wound healing, and angiogenic pathways. Growth factors also influence mitochondrial working and thereby normal function of cells including neurons [54–57].
Although there is ample evidence that the behavioural environment and behavioural responses affect several aspects of physiology, the behavioural mismatches remain largely underappreciated in the pathophysiology of lifestyle disorders. Although the links are demonstrated reproducibly across multiple animal species, the recognition of behaviour as a possible causal factor in non-communicable diseases is largely missing. This hypothesis is based on the exploration of the behaviour-physiology interface.
The mismatch between the ancestral set of behaviours for which the human physiology has evolved and the behavioural repertoire of today’s urban lifestyle is an important causative factor in lifestyle disorders. Many behaviours that are essential for shaping normal physiology are deficient in today’s lifestyle. Since every behaviour is linked to a network of neuronal, endocrine, immunological, and metabolic cascades, chronic deficiency of a behaviour can exert multiple effects on the network (Figure 1). These behavioural deficiencies are comparable to vitamin deficiencies. Similar to vitamins we would like to coin the word “vitactions” for the set of behaviours whose deficiency can potentially cause any kind of physiological problems.
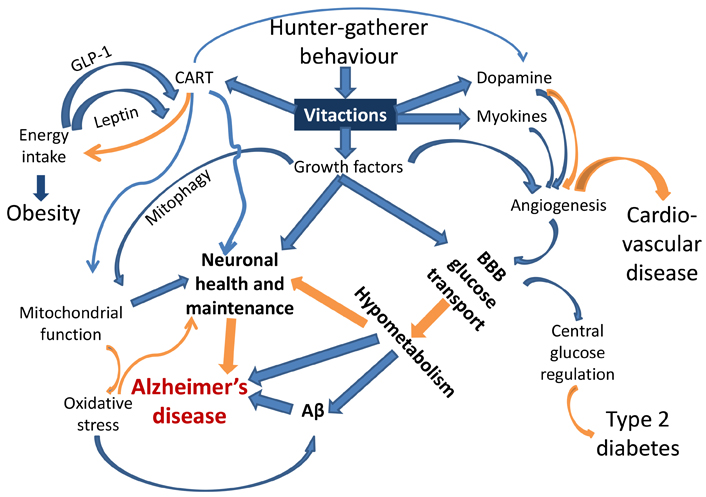
A schematic representation of the links joining behaviour responsive signaling pathways and the pathophysiology of AD and other lifestyle related disorders. Blue arrows indicate up-regulation, and orange arrows indicate down-regulation or prevention. Each of the links is experimentally demonstrated (references in the text). This is the first attempt to put them together to visualize causal pathways to AD. BBB: blood brain barrier; Aβ: amyloid β
Of specific relevance to AD are impaired angiogenesis and growth factors required for neuronal health. Neuronal mitochondrial function is evidently impaired in AD [58] and mitochondrial function is dependent on CART and growth factor signaling [45, 54–57]. Mitophagy, altered in AD [59–61], is also under growth factor regulation [62, 63]. Oxidative stress, also implicated in AD [64–66], is generated by defective mitochondria or impaired fission-fusion dynamics [67, 68]. Stimuli from behavioural environments are also suspected to alter the dynamics and distribution of immune cells and thereby systemic inflammatory responses [69] which are also associated with AD [70, 71].
Subnormal or defective angiogenesis impairs supply of glucose and other nutrients across the blood brain barrier (BBB) [72, 73]. The rate of glucose transport across the BBB is known to be reduced in obesity and type 2 diabetes, which are known risk factors for AD [74, 75]. Subnormal vasculature, reduced transport of glucose through BBB, and lower glucose metabolism in the brain are also known to be associated with AD [76–80]. CART has a neuroprotective role in dementia [48, 50]. Dopamine expression is behaviour-responsive and also associated with CART [51], and has complex roles in angiogenesis and neurogenerative conditions [81, 82]. Myokines generated from skeletal muscle activity also promote angiogenesis along with neuroprotection [38, 83].
With inadequate or defective brain vasculature, the nutrient supply to neurons is expected to be subnormal leading to neuronal death and degenerative changes. However, all parts of the brain need not be equally affected. The brain is expected to have evolved mechanisms by which regions being used more frequently would be selectively spared. Mechanisms by which vasculature in different regions of the brain can be differentially affected have been demonstrated [84]. For a sedentary lifestyle motor coordination functions are underutilized and therefore they would degenerate preferentially sparing the cognitive functions. Therefore, in sedentary lifestyle-related neurogenerative disease, the motor coordination is likely to degenerate first. This may not be noticed in a lifestyle where complex coordination is hardly challenged. Specifically designed tests can detect this degeneration very early which may serve as an early warning or timely diagnosis marker.
The amyloid plaques, characteristic of AD may be a consequence of reduced glucose metabolism in the brain [85] or triggered by oxidative damage [66]. Amyloid proteins have a tendency to polymerize spontaneously with a small probability. However, if the rate of turnover of proteins is sufficiently large, the polymerization remains limited. If the rate of turnover is reduced below a threshold, proteins can aggregate rapidly [86]. If the reduced rate of metabolism reduces the protein turnover rate, aggregated proteins will be found, whether or not they have any further role in the pathology [87].
In short, alteration in many pathways radiating from vitaction deficiencies can potentially explain the multiple factors implicated in AD including amyloid plaques, altered vasculature, reduced glucose metabolism, subnormal mitochondrial function, and impaired neuronal maintenance. This could be the most parsimonious explanation for the multiple pathophysiological changes characterizing AD.
The deficiency of physical activity is well recognized as a risk factor in lifestyle diseases but in the classical view burning calories and achieving energy balance is the main relevance of physical activity. However, the benefits of physical activity are not the same and may even be contradictory under different behavioural contexts [88–90]. Therefore, the behavioural context of physical activity needs to be examined carefully. Available literature suggests that different types of exercises may have differential cardiovascular, emotional and metabolic effects [88] as well as growth factor expression [91–93]. Most studies appear to report only a limited classification of exercises in terms of low or high intensity and aerobic or resistance exercise types. Exercise can have different behavioural components which generally do not get recorded but they may be largely responsible for the beneficial effects of exercise [88]. Most sports activities mimic various components of a hunter-fighter’s behaviour such as chasing, hitting, aiming, dodging, escaping, risk taking, competing, attacking or defending, and therefore are expected to have different neuroendocrine effects than equal calorie mechanical and monotonous exercise. We hypothesize that the health benefits of exercise and sports are not obtained by burning calories alone but by partially supplementing the deficient vitactions. Apart from physical activity Watve [5] lists physical aggression, agility and rapid action, adventure and injury proneness, skin exposure to the sun, heat, cold, and minor injuries as some of the possible vitactions deficient in the modern lifestyle.
Exercises of different types are known to be effective in preventing and managing AD [92–98]. The beneficial effects of exercise are likely to be via its links to growth factor expression [99–103]. However, the differential effects of the behavioural components of exercise have not been tested in randomized control experiments (RCT). A typical design of RCT should involve comparison of behaviourally enriched exercise with a control group performing energetically comparable mechanical monotonous exercise. Using such a design, the effects of different behavioural components of exercise can be tested individually as well as collectively.
The prediction of the hypothesis that motor coordination is expected to degenerate first is also supported [104–105]. It needs to be explored how this can be systematically used for early warning and timely intervention.
Just as vitamin deficiencies can be prevented or treated by supplementing the deficient vitamin and no other drug works, for vitaction deficiencies supplementing the deficient behaviour in the form of appropriate sports, activities, and exercises is expected to be the only effective solution. Since behaviour-physiology interface has multiple links (Figure 1), all of which may not even be discovered, a single target molecule approach typical of pharma research is unlikely to work.
Behaviourally enriched exercises are therefore the most promising answer for effective prevention and possibly treatment of AD. The limitation of treatment might be that neurons already dead or irreversibly damaged may not be able to regenerate by behavioural exercises. Nevertheless, prevention of AD by behaviourally enriched exercise is likely to be highly effective.
AD: Alzheimer’s disease
BBB: blood brain barrier
CART: cocaine-amphetamine regulated transcript
MW: Conceptualization, Writing—original draft. AKS: Data curation, Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Rafael Franco, Joan Serrano-Marín
Karolina Armonaite ... Luigi Laura
Jesús Avila ... Félix Hernández
Glòria Salort ... Jesús A. García-Sevilla
Iyawnna Hazzard ... Forshing Lui
