Affiliation:
1Laboratorio de Neuroinmunología, Centro Científico y Tecnológico de Excelencia Ciencia & Vida, Fundación Ciencia & Vida, Santiago 8580702, Chile
2Facultad de Medicina y Ciencia, Universidad San Sebastián, Santiago 7510156, Chile
ORCID: https://orcid.org/0000-0001-7015-9058
Affiliation:
1Laboratorio de Neuroinmunología, Centro Científico y Tecnológico de Excelencia Ciencia & Vida, Fundación Ciencia & Vida, Santiago 8580702, Chile
2Facultad de Medicina y Ciencia, Universidad San Sebastián, Santiago 7510156, Chile
Email: rpacheco@cienciavida.org; Rodrigo.pacheco@uss.cl
ORCID: https://orcid.org/0000-0001-8057-9806
Explor Neuroprot Ther. 2024;4:100–107 DOI: https://doi.org/10.37349/ent.2024.00073
Received: December 26, 2023 Accepted: February 02, 2024 Published: March 19, 2024
Academic Editor: Shile Huang, Louisiana State University Health Science Center, USA
Short-chain fatty acids (SCFAs) play a key role regulating immune and metabolic homeostasis. Consequently, dysregulation in SCFA levels is involved in the pathogenesis of autoimmune, inflammatory, metabolic, and neurodegenerative disorders. These metabolites are generated by gut microbiota, and their production is influenced mainly by diet. Here, an overview is provided of how SCFA production is associated with diet and with neurological disorders. The mechanisms by which SCFAs exert beneficial effects are analysed, along with how their production may be boosted by diet and how the use of specific dietary interventions might improve the outcome of neurological diseases.
Short-chain fatty acids (SCFAs) are metabolites generated by some bacteria of the microbiota through the anaerobic fermentation of dietary fibers. They are abundant in the colonic mucosa, reaching 50 to 200 mmol/L [1]. Acetate, propionate, and butyrate represent more than 95% of colonic SCFAs, where they are found in a molar ratio of approximately 60:25:15 under healthy conditions [2]. The exact levels of SCFAs depend on the dietary carbohydrate consumption rate and the intestinal microbiota composition. For instance, Akkermansia muciniphila and Bacteroides spp. may produce acetic acid through fermentation [3, 4]. Bacteroides and gram-negative bacteria, such as Roseburia inulinivorans and A. muciniphila, produce propionate [3, 4]. Faecalibacterium prausnitzii and Clostridium clusters IV and XIVa might synthesize butyrate [4]. This latter metabolite is then absorbed by intestinal epithelial cells, playing a critical role and contributing to the host’s energy.
SCFAs may exert their physiological effects on the host in three different ways. The first one is through the stimulation of the G-protein coupled receptors (GPCRs) GPR41, GPR43, and GPR109a, which are expressed on the cell surface of various cell types in the host (Figure 1A). The second one is through the regulation of gene transcription by a mechanism independent of the stimulation of surface GPCRs. SCFAs, particularly butyrate and propionate, might enter the cell through simple diffusion or using transporters such as the monocarboxylate transporter (MCT) and sodium-coupled MCT (SMCT) and act as histone deacetylase (HDAC) inhibitors, promoting gene expression and regulating cell metabolism, differentiation, and proliferation (Figure 1B). In the third way, SCFAs enter cells and provide energy for the colon (Figure 1C). The remaining SCFAs are primarily utilised by the hepatocytes, and only a tiny fraction reaches the blood circulation (Figure 1D). Besides their effects on intestinal epithelium and immune cells, SCFAs can also modulate the activity of the enteric nervous system (ENS). For example, butyrate-mediated HDAC inhibition in neurons of the ENS triggers increased firing of cholinergic neurons, leading to a rise in the motility of the colon [5] (Figure 1E). Since the latter mechanism is based on the ability of butyrate to increase the activity of cholinergic neurons in the ENS, other compounds that facilitate the activity of these neurons, such as nicotine [6], might also be used to treat gastrointestinal motility disorders associated with inhibition of colonic transit.
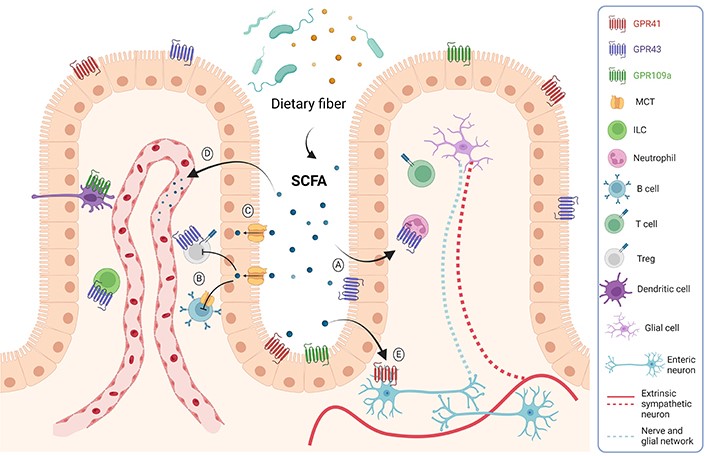
Mechanisms of SCFAs action. SCFAs are mainly produced by bacterial fermentation. These metabolites can act through GPCR expressed on epithelial and immune cells, reducing inflammation (A). They can also enter the cells and act as HDAC inhibitors (B). SCFAs might also reach the distal gut by simple diffusion or through the MCT transporter to be used as an energy source (C). SCFAs can also reach the bloodstream and affect other tissues, such as the brain, inducing beneficial metabolic effects (D). These metabolites might also act on the ENS, regulating gut motility and secretory activity (E). ILC: innate lymphoid cell; Treg: regulatory T cell. Created with BioRender.com
In addition to local protective effects in the gut, including those associated with intestinal motility and gut barrier integrity, SCFAs can act directly on immune cells regulating their differentiation, acquisition of tolerogenic/inflammatory behaviour, tissue tropism, and activation. SCFAs impact the differentiation of T helper cell 1 (Th1) and Th17 cells and Tregs. For example, propionate and butyrate enhance the degree of acetylation of the forkhead box protein P3 (Foxp3) locus, inducing higher Foxp3 expression and increasing Treg differentiation and their suppressive activity. In the same direction, through inhibiting HDAC activity, SCFAs increase interleukin-10 expression by lymphocytes (T and B cells) and macrophages [7]. Moreover, SCFAs regulate the activity of ILCs; they increase the number and activity of group 3 ILC (ILC3s) by stimulating surface SCFA receptors [8]. SCFAs induce tolerogenic behaviour in macrophages and dendritic cells through GPCR activation and HDAC inhibition [7]. Accordingly, recent studies have demonstrated the therapeutic effects of drugs targeting SCFA receptors in neuroinflammatory disorders. For instance, the stimulation of GPR43 by SCFA might limit the acquisition of central nervous system (CNS) tropism by autoreactive T cells in the intestinal mucosa [9], thereby affecting the development of CNS autoimmunity in a model of multiple sclerosis (MS). Also, acetate has been shown to reduce neuroinflammation and cognitive decline through a GPR41-dependent mechanism in a mouse model of Alzheimer’s disease (AD) [10]. In summary, SCFAs might mediate microbiota effects by inducing tolerance directly in the gut mucosa and distal organs, such as the CNS.
SCFAs that reach the bloodstream might promote CNS homeostasis by reinforcing blood-brain barrier (BBB) integrity, avoiding neuroinflammation, affecting levels of neurotrophic factors, promoting serotonin biosynthesis, or directly activating the vagal afferent. Of note, in addition to diet and dysbiosis, the plasma concentration of SCFAs might be affected by the renin-angiotensin system [11], and by the energy requirements and lipids metabolism [12]. In this regard, acetate and butyrate might be converted into acetyl coenzyme A and subsequently used as substrate in the tricarboxylic acid cycle or beta-oxidation, while propionate might act as a precursor for gluconeogenesis in the liver [12]. According to the crucial role of SCFAs in CNS homeostasis, altered levels of these metabolites have been associated with many neurological diseases. These alterations are associated with the infiltration of peripheral immune cells into the CNS, which favours neuroinflammation and neurodegeneration [13]. Considering the high GPR41 expression in the brain and the BBB, gut microbiota might impact vascular permeability, influencing the entrance of immune cells into the CNS and, consequently, neuroinflammation. In this regard, compared with healthy controls, patients with neuroinflammatory disorders, such as MS, AD, and Parkinson’s disease (PD), harbour dysbiosis in the gut microbiota involving changes in the levels of SCFA (Table 1). For instance, MS, a T cell-mediated autoimmune disease of the CNS that involves demyelination, axonal damage, and chronic neuroinflammation in the brain and spinal cord, has been associated with a significant reduction of SCFAs levels in the faeces and the blood [14]. Moreover, a high-fiber diet or oral SCFA treatment ameliorates the disease manifestation in an MS mouse model [9, 15]. Of note, the mechanistic analysis indicated that these beneficial effects of SCFAs were mediated by promoting an anti-inflammatory phenotype in mucosal colonic T cells. Another example is AD, the most common neurodegenerative disorder worldwide, which involves the accumulation of protein aggregates called amyloid-β, autoreactive lymphocytes, and neuroinflammation. Recent evidence has shown that AD involves a dysbiosis that leads to decreased butyrate levels in human and animal models (Table 1). Importantly, the administration of the butyrate-producing bacteria Clostridium butyricum significantly diminished the amyloid-β accumulation and levels of pro-inflammatory cytokines produced by microglial cells in the amyloid precursor protein/presenilin 1 (APP/PS1) mouse model of AD, dampening the disease manifestation [16]. PD is another neurodegenerative disorder that involves the accumulation of protein aggregates (containing α-synuclein), an autoimmune T cell-mediated response, and neuroinflammation, where SCFA levels have been found altered. Although SCFAs exert protective effects in many inflammatory disorders, the role of these metabolites in PD seems more complex. A transgenic mouse model study involving the overexpression of human α-synuclein demonstrated that a germ-free environment abrogates the disease development, and oral feeding with SCFAs rescued the Parkinsonian phenotype, including neuroinflammation [17]. These findings suggest that SCFAs are required to develop PD pathology. In contrast, recent studies show that PD patients display lower faecal but higher plasma concentrations of SCFA compared to healthy controls [18]. A plausible explanation for this discrepancy may come from increased intestinal permeability observed in patients, which might result in higher SCFAs entering the bloodstream. These elevated SCFAs in systemic circulation may cross the BBB and affect the physiology of neurons and glia, impacting the development of neuroinflammation. It is also essential to consider that transgenic mice involving the overexpression of human α-synuclein transplanted with faecal microbiota from PD patients displayed decreased acetate but increased propionate and butyrate in the faeces [17]. Thus, another possibility is that changes in the ratio of concentrations of different SCFAs play an important role in PD pathogenesis. These findings strongly suggest that SCFAs are essential microbiota-derived mediators on the gut-brain axis involved in neuroinflammatory disorders. However, further studies are required to address the exact mechanisms by which different SCFAs affect neurons, microglia, and immune cells in the context of neuroinflammatory disorders.
Alterations of SCFA levels in neuroinflammatory disorders
| Pathology | SCFA | Effect | Reference |
|---|---|---|---|
| MS | ↓ Propionate in stool and serum | Reduced Treg number, metabolism, and function. Increased Th17 number. | [14, 19] |
| ↓ Propionate in faeces and acetate in plasma | Reduced SCFA promotes an inflammatory phenotype in gut mucosal T cells. | [9, 15] | |
| AD | ↓ Butyrate | AD patients with brain amyloid accumulation and endothelial dysfunction had decreased blood butyrate. | [20, 21] |
| ↓ Butyrate | In faeces and brain of APP/PS1 AD mice model. Butyrate can hinder the oligomerization of amyloid-β 1-40. | [22, 23] | |
| PD | ↓ SCFA in faeces | Significant decline of butyrate, acetate, and propionate in faecal of PD individuals compared to controls. | [18, 24] |
↓ Acetate ↑ Butyrate and propionate in faeces | Microbiota-derived SCFA regulates motor deficits and neuroinflammation in mouse models. | [17] |
The relationship between diet, microbiota-associated metabolic machinery, and the immune system’s responsiveness to bacterial products implicates the usage of dietary substrates as potent sources of immunomodulators for therapeutic purposes. These strategies vary in complexity and could range from the restructuring of the whole bacterial consortium to the supplementation of specific metabolites that directly affect the host. (i) Faecal microbiota transplant (FMT) is the administration of a complex bacterial community preparation obtained from the stool of a healthy donor to a patient undergoing gut dysbiosis to treat the disease (Figure 2). The FMT success depends on the microbiome of the donor, and the improved success rate has been associated with higher richness of specific taxa, such as Ruminococcus spp. and A. muciniphila in the case of refractory ulcerative colitis [25]. Another interesting example is the success of FMT inhibiting neuroinflammation associated with traumatic brain injury [26]. (ii) A second approach is the modification of food components in the diet to impact the composition of the intestinal microbiota, finally affecting a particular immune modulatory effect (Figure 2). Fruits, nuts, legumes, vegetables, and whole grains are the natural sources of dietary fibers converted into SCFAs, which impact the balance between pro- and anti-inflammatory mechanisms on mucosal immune cells. In this regard, a recent pilot study with AD patients revealed that the modified Mediterranean-ketogenic diet alleviates AD symptoms and improves AD biomarkers in the cerebral spinal fluid by affecting SCFA production [27]. (iii) A more specific therapeutic tool is prebiotics, which is currently defined as substrates that are selectively utilised by host microorganisms, conferring health benefits. The administration of fiber supplements results in specific changes in the gut microbiome enhancing the selective growth or function of a subset of bacteria (Figure 2). These changes are fiber-type specific, affecting the production of specific metabolites by particular bacteria. For instance, the use of galacto-oligosaccharides has been shown to alleviate neuroinflammation and cognitive impairment in a mouse model of AD [28]. (iv) The most utilised strategy today is probiotics, a single strain or mixture of live microorganisms added to the diversity of the native gut ecosystem. An interesting example in this category is the probiotic formulation called SLAB51, made of nine bacterial strains [including Streptococcus thermophilus, bifidobacteria (Bifidobacterium longum, B. breve, B. infantis), lactobacilli (Lactobacillus acidophilus, L. plantarum, L. paracasei, L. delbrueckii subsp. Bulgaricus, L. brevis)], which has been tested as a therapeutic approach in an AD mouse model. This study demonstrates that SLAB51 significantly limited disease progression by reducing oxidative stress in AD mice [29], thus representing a promising therapeutic adjuvant in AD treatment. In the same line, another study showed that a mixture of L. fermentum, B. bifidum, L. casei, and L. acidophilus ameliorates cognitive decline in AD patients [30]. (v) Finally, the most specific strategy is postbiotics, bioactive molecules produced by bacteria. Postbiotics might contain dead microorganisms or their components that confer benefits to the host (Figure 2). For instance, the direct supplementation of MS patients with SCFAs as postbiotics has recently been investigated. This research demonstrates that propionate treatment might rectify altered gut microbiome composition and SCFA levels in MS patients and thus ameliorate the disease course, probably re-establishing the induction of Treg suppressive function [19]. Altogether, these studies suggest that the rational manipulation of gut microbiota represents an attractive and novel therapeutic strategy for treating neuroinflammatory disorders. Nevertheless, further work is required to understand the underlying mechanisms and to use this information to design better disease-specific intervention strategies.
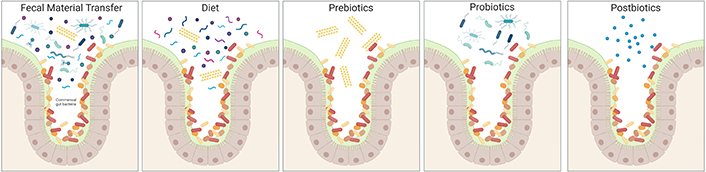
Constituents of microbiome-based interventions. Common constituents of microbiome-based intervention methods are illustrated, showing their different degree of complexity. The native microbiome members are on colours ranging from red to orange, while introduced species are on blue tones, and prebiotic fibers are yellow. Created with BioRender.com
AD: Alzheimer’s disease
BBB: blood-brain barrier
CNS: central nervous system
ENS: enteric nervous system
FMT: faecal microbiota transplant
GPCRs: G-protein coupled receptors
HDAC: histone deacetylase
ILC: innate lymphoid cell
MCT: monocarboxylate transporter
MS: multiple sclerosis
PD: Parkinson’s disease
SCFAs: short-chain fatty acids
Th1: T helper cell 1
Treg: regulatory T cell
CP: Conceptualization, Writing—original draft, Writing—review & editing. RP: Conceptualization, Writing—review & editing. All authors contributed to manuscript revision, read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work was supported by “Financiamiento Basal para Centros Científicos y Tecnológicos de Excelencia de ANID” Centro Ciencia & Vida [FB210008] (to Fundación Ciencia & Vida) from “Agencia Nacional de Investigación y Desarrollo de Chile (ANID)”. This work was also supported by the grant [MJFF-021112] from the Michael J. Fox Foundation for Parkinson’s research, and by [FONDECYT-1210013] (to RP) and [FONDECYT-11190251] (to CP) from ANID. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
