Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
ORCID: https://orcid.org/0000-0003-4805-1758
Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
ORCID: https://orcid.org/0000-0003-1619-7592
Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
ORCID: https://orcid.org/0000-0002-6906-7444
Affiliation:
Department of Food Science and Nutrition, Nara Women’s University, Kita-Uoya Nishimachi, Nara 630-8506, Japan
Email: smatsuda@cc.nara-wu.ac.jp
ORCID: https://orcid.org/0000-0003-4274-5345
Explor Neuroprot Ther. 2022;2:74–86 DOI: https://doi.org/10.37349/ent.2022.00019
Received: February 24, 2022 Accepted: April 04, 2022 Published: April 24, 2022
Academic Editor: Raymond Chuen-Chung Chang, The University of Hong Kong, China
The article belongs to the special issue Intervention of Neuroimmune Responses
Altered immunity may have destructive consequences for the integrated central nervous system. This immune response often affects progressive neurodegenerative diseases such as Parkinson’s disease and/or psychiatric disorders such as schizophrenia. In particular, schizophrenia pathogenesis may be mediated by multiple neuro-immune interaction pathways. Gut microbiota might affect the brain and/or immune function. Significant machineries of immunity are commonly affected by the commensal gut microbiota. Therefore, schizophrenia may be connected with the gut-immune system. In addition, the brain and immune systems cooperate on multiple levels. The brain could save several pieces of information about specific inflammation in a body. This immunological memory named “engrams”, also called memory traces, could restore the initial disease state, which may help to explain key features of schizophrenia. Based on this concept, therapeutic strategies for schizophrenia could be the modification of the gut microbiota. Probiotics and/or fecal microbiota transplantation are now emerging as the most promising treatments for the modification. More consideration of the roles of gut microbiota will conduct the further development of immune-based therapeutics for the prevention and/or treatments of psychiatric disorders.
Schizophrenia is a psychiatric disorder described by intellectual impairment and emotional fragility with dysfunction of the central nervous system (CNS) [1, 2]. Impairment of attention, anxiety, depression, and suicidal ideation are also signs of schizophrenia. Subsequently, abnormalities in the brain have been observed in postmortem examinations of individuals with schizophrenia [3]. Evidence of high lipid peroxidation and alteration in antioxidant activity has also been detected in patients with schizophrenia [4]. The pathophysiology of schizophrenia patients might be partially attributed to increased oxidative stresses [5]. In fact, the reactive oxygen species (ROS) generation could increase with schizophrenia [6]. Particularly, mitochondrial dysfunction has been linked to neurodegeneration in schizophrenia [7]. Consequently, schizophrenia might belong to a neuron-degeneration disease. Neurons are usually susceptible to excess ROS because of relatively reduced antioxidant capabilities [8]. Therefore, oxidative stress and its metabolites could work as a biomarker for schizophrenia patients suggesting the progression of symptoms towards psychosis [9].
Gut microbiota is constituted of an assessed 100 trillion bacteria as well as viruses [10]. These microorganisms have evolved to be alive in symbiosis with their host. The body’s oxidative stresses are linked to changes in gut microbiota [11]. For example, Lactobacillus plantarum could improve inflammation and oxidative stresses by modulating gut microbiota [12]. Therefore, the conformation of the microbiota has the great potential to impact the pathogenesis of many disorders including neurodegenerative diseases [13]. Increasing evidence is demonstrating the intricate interactions between gut microbiota, immunity and CNS [14]. Whereas normal gut microbiota may protect the CNS, the dysbiosis of microbiota could accelerate to exacerbate psychiatric disorders [15]. Several studies have observed the possible link between gut microbiota and schizophrenia [16]. In particular, alterations of the gut microbiota may indicate to lead to schizophrenia [16]. Altered proportions of certain bacteria have been identified in the gut of patients with schizophrenia compared to healthy persons [17]. This review would highlight the roles of oxidative damage in the pathophysiology of schizophrenia. In addition, recent knowledge on treating schizophrenia with improved gut microbiota is presented along with information on how oxidant levels and/or inflammation in patients with schizophrenia might be controlled by the gut microbiota. Schizophrenia typically emerges in late adolescence or early adulthood. The alteration of gut microbiota might help to regulate schizophrenia development and provide preventive strategies.
Schizophrenia is considered by several fundamental behavioral features, in which gastrointestinal symptoms are often reported [18]. Furthermore, activation of gastrointestinal inflammation may contribute to the neuronal damage and degeneration found in patients with schizophrenia [18, 19]. Several infections during pregnancy, who later develop schizophrenia, have been repeatedly reported [20]. In addition, signs of inflammation have been detected in brains with schizophrenia [21]. In these ways, an inflammatory reaction has been suggested for schizophrenia [22]. Consistently, a chronic inflammatory encephalitic process in the pathogenesis of schizophrenia has been offered [23]. An array of neuro-immune aberrations have also been identified in patients with schizophrenia [24], suggesting that oxidative stresses and/or systemic inflammation may be an invariant feature of schizophrenia [25] (Figure 1). Commonly, an increased level of inflammatory marker in blood and/or in brain tissues have been detected in patients with schizophrenia [26]. Therefore, it has been suggested the prospective usage of anti-inflammatory medications as secondary treatments in the patients [27]. Furthermore, levels of oxidative stress may associate with the severity of several symptoms of schizophrenia [28]. It is now accepted that levels of inflammation and/or oxidative stresses may connect with the level of mental impairment in patients with the schizophrenia-episode [29]. In addition, psychosocial stresses are a strong risk factor for the development of several forms of psychiatric disorders including schizophrenia [30].
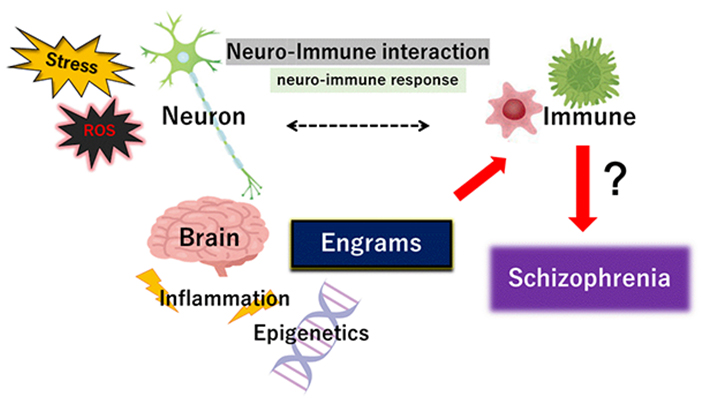
Schematic representation of the pivotal role of neuro-immune interaction including neuro-immune response during the pathogenesis of schizophrenia. The immune system is not self-regulated but interconnects in close relationship with the nervous system. Illustration of the pathogenic role of stress, ROS, inflammation, and engrams has also been shown. Note that some important events have been excluded for clarity
Inflammatory oxidative stress may generate ROS, which are oxygen-containing molecules competent to react with various biological molecules through an oxidation-reduction redox machinery [31]. Intracellular sources of ROS may often include mitochondria during their ATP synthesis. Significant biological response affected by excessive levels of ROS is autophagy that delivers a cellular recycling system which may provide defense mechanisms to preserve cellular homeostasis [32]. ROS induction could initiate the activation of autophagy in cells [33]. Autophagy usually organizes for a protective role in cells, on the other hand, autophagy is also faithfully associated with necrosis and apoptotic cell death. Autophagy may be involved in the pathogenesis of schizophrenia [34]. For example, olanzapine is an anti-psychotic drug frequently prescribed for the treatment of schizophrenia, which has been known to bring about brain volume-loss [35]. Unusual oxidative stresses and/or autophagy have been associated with the role in this olanzapine-induced neurodegeneration [35]. In addition, it has been reported that different levels of autophagy-related mRNA are expressed in schizophrenia patients treated with olanzapine [36]. Olanzapine may ameliorate cellular damage by activating autophagy in neuronal cells. As an indispensable molecular step for autophagy initiation, the signaling activity of phosphoinositide 3-kinase (PI3K)/AKT and/or downstream effector mammalian target of rapamycin (mTOR) might be repressed under oxygen deprivation or nutrient starvation [37]. In particular, the mTOR is a key exploitive component of the autophagy-signaling [38]. Hence, mTOR inhibition has been revealed during the autophagy initiation followed by a ROS upsurge in cells [39]. In addition, altered mTOR signaling implicated in synaptic plasticity is associated with neurological disorders including schizophrenia [40], in which autophagy has been described to follow in combination with mTOR inactivation due to huge intracellular ROS [41]. Autophagy could also be induced by activated AMP-activated protein kinase (AMPK) during energy deficit in cells. AMPK is imperative in cells of CNS for maintaining neuronal integrity and for anti-stress survival [42].
The brain and immune system cooperate on various levels, but how the brain remembers immune-challenge has remained ambiguous until now. Amazingly, it has been recently revealed that the brain retrieves information about specific inflammation in the body [43]. The immunological memory could restore the initial disease state, which may be immunological memory called “engrams” [44]. In this Perspective-manuscript, we would like to define the word “engrams” as the meaning of “ensembles of neurons” including the phrase “memory traces”. The engrams have been fairly theoretical concepts of the most basic units of memory, which is described as the remaining though hidden modification in the moody substances produced by environmental stimuli. The synergistic arrangement of engrams might bring in several disease progress and/or refreshed mental health, which involves the concept that any of complex psychological outcomes could result from the interplay of many engrams associated memory traces. Assemblies of these engrams may be thought to synergize in the improvement of conditions both of health and disease by arrangements that are reliant on environmental situations [45]. Frequently originated from stressful events, the engrams are made that might devote rise to a delicate progression of psychiatric disorders including schizophrenia [46]. In particular, epigenetic changes are integral components of the neuronal response to stimulation for continuing changes in behavior including learning and/or memory [47], which is emerging as a key contributor to the adjustment of brain function and the creation of neuronal engrams in brain health or disease [48]. Inflammatory ROS may also facilitate various changes to cellular actions by modifying signaling pathways or epigenetic alterations [49]. The engrams could be related to immune consequences [50]. In addition, a stable engram could be also formed under the blockade of inflammation signaling during prior engrams consolidation [51]. Again, engrams may help to explain key features of psychiatric conditions such as schizophrenia [52] Memories are encoded by engrams represented within subsets of neurons that are synchronously activated during learning, and epigenetics such as DNA methylation within the neuronal assemblies may stabilize engrams for successful memory retrieval [53]. Synaptic modifications may permit the specific formation of engrams during learning for sustaining memory, which may be encouraged by epigenetic regulators such as histone deacetylases (HDACs) [54]. Dysregulated HDACs-related epigenetic pathways have been systematically linked to modifications in various genes expression [55]. Consequently, external inflammation-stimuli could result in epigenetic changes of DNA with the following alterations of some transcriptions. Epigenetics affected by environmental factors have also been proposed to play a key role in schizophrenia [56]. These epigenetic mechanisms might be one method which CNS utilizes to translate environmental stimuli into strong memory engrams. In fact, engrams formation could begin early on in brain development [57]. Subtle structural modifications and/or inflammation would seem to be best matched as physical individuals for engrams development. The immune challenge might also result in the formation of multiple engrams [44, 51, 58]. Thus, the brain could stock specific immune and developmental responses to neuronal representations of inflammatory information. This concept might explain the pathogenesis of several psychiatric disorders including schizophrenia (Figure 1). Epigenetic changes specifically to the neurons in schizophrenia might be helpful in understanding the role of the epigenetic process [59], which might constitute a new model for therapeutic interventions against schizophrenia. However, several possibilities may exist indicating that engrams formation may be influenced by the malfunctioning of various peripheral systems [60].
The neuro-immune response may be a component of the gut-brain-immune axis (Figure 2). Recent findings have highlighted the role of the axis with the gut microbiota playing an important role in several psychopathologies including schizophrenia [61]. Increased stresses could alter the composition of the gut microbiota [62]. Therefore, possible propagation of neuro-immune signaling cascades in response to various stresses could be indirectly organized by features of physiology in the gut-brain axis and/or gut-immune axis. In fact, several reports have shown that considerable dysbiosis of the gut microbiota could worsen many neuropsychiatric disorders [63], indicating that cross-talk of the gut-brain axis plays an imperative role in the pathogenesis of neuronal diseases [64]. In addition, accumulating evidence shows gut microbial dysbiosis represents a risk factor for schizophrenia [65]. Accordingly, the gut-brain axis might have crucial roles in physiology, certain metabolism, and homeostasis for maintaining good mental health [66]. There have been many studies into probable therapies intended at normalizing microbiota signaling to the CNS [67].
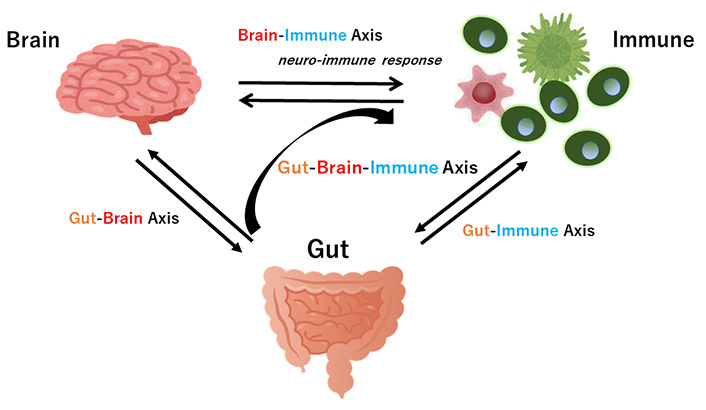
Hypothetical schematic overview and appearance of gut-brain axis cross-talk between the brain and the gut (intestine/colon) in addition to the brain-immune axis and the gut-immune axis have been shown. Arrowhead means stimulation. Note that some key pathways such as the liver-brain axis have been excluded for clarity
The gut-brain axis characterizes a bidirectional relationship between gut and brain, which is indeed an important assembly in the pathophysiology of neurodegenerative disorders [68] (Figure 2). For example, gut microbiota composition may be associated with narcolepsy type 1 [69]. This conception may include the interaction between gut microbiota and the neurological system. The gut microbiota is usually influenced by many factors including environmental stimulus, exercise, past illnesses, nutrition, use of antibiotics, and so on [70]. Recent studies have demonstrated that some species of bacteria can produce catecholamines, which also contributes to sympathetic nerve reactions [71]. Moreover, microbial specific metabolism may deliver some precursors involved in some neuromodulator synthetic pathways such as glutamic acid, noradrenaline, serotonin, dopamine, gamma amino butyric acid (GABA), and so on [72]. Several possible effectors in the gut could activate the sympathetic pathway through the gut-brain axis [73]. A subset of distal intestine-projecting vagal neurons in the sympathetic pathway may have an afferent role in the microbiota-mediated modulation [73]. In addition, microbes in the gut could also stimulate the sensory neurons [74]. Therefore, there might be a malicious or decent interaction between the gut and the nervous system [75]. The mechanism producing psychotic disorders appears to include brain inflammation and/or degeneration, which could be associated with the gut-brain axis [76].
A well-balanced connection between CNS and the immune system might verify good performance of the body in animals. Remarkably, raised expression of immune system-related proteins in the brain has been assumed as an indication of ongoing autoimmune progressions, which may be also correct for chronic brain inflammatory processes in schizophrenia [77]. Therefore, disorders of the immune system could precede impairments in the brain [78]. In addition, immunity-linked processes could be associated with neuronal memory responses [79]. But, there is no substantial evidence of an auto-immune origin in the case of schizophrenia. Alterations in genes that regulate the immune cells have been described in schizophrenia patients [80]. In addition, prenatal exposure to various inflammatory stimulants considerably raises the risk of the development of schizophrenia [81]. Gut and immune homeostasis are perturbed in psychiatric disorders [82]. The lymphoid tissue of the intestinal mucosa contains about 70% of all immune cells in the body [83]. Diet may influence the balance between regulatory T (Treg) and T helper 17 cells (Th17) [84]. Specifically, short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate made by commensal bacteria in gut could stimulate the development of Treg [85]. In contrast, long-chain fatty acids might be a key regulator of the proliferation [86] and differentiation [87] of Th17. Some clostridia strains in gut have been associated with increased levels of SCFAs in the cecum and Treg-promotion [88]. Likewise, Clostridium perfringens might work as adjuvants supporting Th17 differentiation [89]. There is a broad range of mutual interactions between the gut microbiota and immune-inflammatory responses which has crucial consequences on brain function and mental health [90] (Figure 2).
Synapse elimination may be accomplished by microglia as a mechanism underlying the forgetting of memories by engram cells [91]. In addition, there are significant correlations between microglial activation and/or demyelination in the brain and the certain type of gut microbiota, suggesting that gut-microbiota-microglia cross-talk may play a role in the facilitation of remyelination in the brain [92]. Diet is recognized to be an essential controller of gut microbiota in maintaining mental health. For example, caffeine consumption could have a favorable effect on schizophrenia [93]. Furthermore, exercise has a positive effect on microbiota associated with higher gut microbial diversity [94], which may benefit patients with schizophrenia as a complement to pharmacological treatment [95]. Alterations in the composition of gut microbiota with schizophrenia have been correlated with altered immunity and symptom-severity, which have also been identified as potential biomarkers in diagnosis [96].
The effects of some antidepressants might be partly achieved with the renewal of beneficial microbiota. For example, the cooperative effects of fluoxetine might be adopted by good modifications of the gut microbiota [97]. Besides, treatment with alternative antidepressant such as valproate or lithium also rises the quantity of favorable bacteria species [98]. Probably, the gut-brain-immune axis might be affected by the creation of inflammatory cytokines and by reduction in beneficial substances such as SCFAs [99]. SCFAs have been revealed as regulatory elements with the ability to affect emotional condition through the gut-brain axis [100]. In addition, SCFAs might upturn the permeability of blood brain barrier (BBB), which exhibits that SCFAs could manage the brain homeostasis [101]. Moreover, definite levels of SCFAs created by the gut microbiota have been revealed to possess some anti-inflammatory properties. Consistently, butyric acid, one of the major SCFAs, has an imperative role in the gut-microbiota-brain axis and/or for the brain function [102]. It is plausible that increasing the abundance of butyrate-producing bacteria may be a considerable potential therapeutic way to improve gut microbiota. For example, C. perfringens could perform this in concert with environmental and genetic factors [102, 103].
New therapies for schizophrenia are emerging. One method that may effectively impact gut microbiota composition is fecal microbiota-transplantation (Figure 3). By transplanting a healthy new gut microbiota, it may be probable to improve the efficacy of microbiota for the treatments in schizophrenia [103]. In particular, transplantation of Faecalibacterium prausnitzii could restore the intestinal structure, which might be used as a potential therapeutic approach against inflammation [104]. Additionally, F. prausnitzii might work as a diagnostic and therapeutic biomarker for the usage of fecal microbiota-transplantation [105]. However, the beneficial effects of the transplantation are dependent on the host’s responses [106]. Through the fecal microbiota-transplantation and its consequences, the character of certain gut microbiota and its SCFAs-production have been also verified in the treatment of renal dysfunction with diabetic nephropathy [107]. Consistently, transplantation of the fecal microbiota from patients with schizophrenic into antibiotic-treated mice has caused behavior anomalies such as severe hyperactivity, impaired learning/memory in the recipient mice [108]. By the way, it has been believed that novel interventions to develop stem-cell-based treatment could improve cognitive and social functions in schizophrenia [109]. In these meanings, several experimental studies have also pointed to an association between intestinal microbiota and neurogenesis [110]. Dietary approach for neural regeneration could be useful [111]. As ROS have been described to unfavorably affect stem cells properties, it is important to reduce the extent of ROS level with the stem-cell-oriented regeneration therapy [112, 113]. Gut microbiota could control the ROS level and/or D-amino acids level to keep the brain healthy [114]. Possibly, fecal microbiota-transplantation from a specific healthy donor with a certain gut microbiota could have considerably attenuated symptoms by engrams-improvement in the brain with schizophrenia (Figure 3).
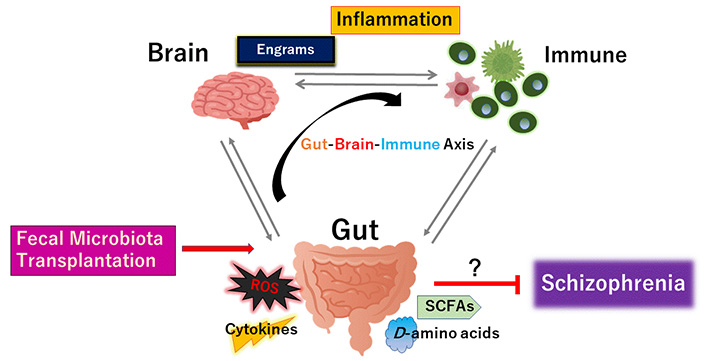
The gut microbiota could promote to the favorable creation of the cytokines, ROS, SCFAs, and certain D-amino acids against disease-progression of schizophrenia. Fecal microbiota transplantation is made up of fecal microbiota infusion from a healthy donor into a recipient subject, which has been likely more successful than just conventional pharmacotherapy for schizophrenia. Arrowhead suggests stimulation whereas hammerhead indicates inhibition. Note that some critical events such as cytokine-induction signaling have been omitted for clarity
As shown here, compositional changes of gut microbiota in schizophrenia have been correlated with altered brain function and/or altered immunity accompanying altered symptom severity of schizophrenia. In addition, the microbiota may influence the pathophysiology of schizophrenia through modulation of functional pathways related to neuro-immune signaling. Altered gut metabolic pathways have been associated with inflammatory cytokines in schizophrenia [115]. However, it is indefinite whether gut microbiota could reverse risks of neuropsychiatric disorder as a consequence of the key pathological processes. Understanding the functional alterations in microbial metabolic pathways as well as the effect on clinical outcomes may provide great contribution to the progression of microbiota-targeted interventions for schizophrenia. This approach might be appropriate for exploring the adjustments in good functional pathways of gut microbiota. In addition, the research and/or critical mission should be to explore the active formation of schizophrenia-specific engrams a long time before the disease could be diagnosed. Future investigation is required to understand the intricate and precise interactions between a host brain engram and its associated microbial communities with certain immunity. Consequently, future research should focus on the identification of disease-specific molecular engrams stored over time during the latent stage of psychiatric disorders. Voluminous researchers have to be united to comprehend the molecular activities and/or mechanisms in further clarity. Deep functional profiling of the gut microbiota may proffer great potential for discovery of therapeutic interventions in schizophrenia.
CNS: central nervous system
mTOR: mammalian target of rapamycin
ROS: reactive oxygen species
SCFAs: short-chain fatty acids
Th17: T helper 17 cells
Treg: regulatory T cells
HS and SM contributed conception of the study. Each author (HS, KT, YI, AT, YK, SM) has participated sufficiently in this work of drafting the article and/or revising the article for the important rational content. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Sarah Otaru, David A. Lawrence
Fábio José Coelho Souza-Junior ... Sabrina Francesca Lisboa
Niklas Frank ... Carola Y. Förster
Mydhili Radhakrishnan ... Sumana Chakravarty
