Affiliation:
1Department of Neurology, Firoozgar Hospital, School of Medicine, Iran University of Medical Sciences, Tehran 1911883611, Iran
2Rasoul Akram Clinical Research Development Unit (RACRD), Iran University of Medical Sciences, Tehran 1981685113, Iran
Affiliation:
1Department of Neurology, Firoozgar Hospital, School of Medicine, Iran University of Medical Sciences, Tehran 1911883611, Iran
Email: nrgstaba@gmail.com
ORCID: https://orcid.org/0000-0001-8717-8757
Affiliation:
2Rasoul Akram Clinical Research Development Unit (RACRD), Iran University of Medical Sciences, Tehran 1981685113, Iran
3Department of Neurology, Rasoul Akram Hospital, School of Medicine, Iran University of Medical Sciences, Tehran 1981685113, Iran
Affiliation:
1Department of Neurology, Firoozgar Hospital, School of Medicine, Iran University of Medical Sciences, Tehran 1911883611, Iran
Affiliation:
2Rasoul Akram Clinical Research Development Unit (RACRD), Iran University of Medical Sciences, Tehran 1981685113, Iran
4Clinical Research Development Center of Shahid Rajaei, Alborz University of Medical Sciences, Karaj 3154686695, Iran
Affiliation:
2Rasoul Akram Clinical Research Development Unit (RACRD), Iran University of Medical Sciences, Tehran 1981685113, Iran
3Department of Neurology, Rasoul Akram Hospital, School of Medicine, Iran University of Medical Sciences, Tehran 1981685113, Iran
Affiliation:
1Department of Neurology, Firoozgar Hospital, School of Medicine, Iran University of Medical Sciences, Tehran 1911883611, Iran
Affiliation:
1Department of Neurology, Firoozgar Hospital, School of Medicine, Iran University of Medical Sciences, Tehran 1911883611, Iran
Affiliation:
1Department of Neurology, Firoozgar Hospital, School of Medicine, Iran University of Medical Sciences, Tehran 1911883611, Iran
Affiliation:
1Department of Neurology, Firoozgar Hospital, School of Medicine, Iran University of Medical Sciences, Tehran 1911883611, Iran
2Rasoul Akram Clinical Research Development Unit (RACRD), Iran University of Medical Sciences, Tehran 1981685113, Iran
Affiliation:
5Department of Electrical and Computer Engineering, University of Tehran, Tehran 1914395515, Iran
Affiliation:
3Department of Neurology, Rasoul Akram Hospital, School of Medicine, Iran University of Medical Sciences, Tehran 1981685113, Iran
Affiliation:
6Cognitive Systems Laboratory, Control and Intelligent Processing Center of Excellence (CIPCE), School of Electrical and Computer Engineering, College of Engineering, University of Tehran, Tehran 1914395515, Iran
7Department of Psychology, Faculty of Psychology and Education, University of Tehran, Tehran 1941556456, Iran
8School of Cognitive Sciences, Institute for Research in Fundamental Sciences, Tehran 1953833511, Iran
Explor Neuroprot Ther. 2025;5:1004109 DOI: https://doi.org/10.37349/ent.2025.1004109
Received: July 18, 2024 Accepted: April 30, 2025 Published: June 22, 2025
Academic Editor: Yujie Chen, Third Military Medical University, China
Aim: Our previous research (Abstract, J Vessels Circ. 2021;2) suggested an increased risk of thrombotic events, including ischemic strokes, in patients with COVID-19. This study aims to determine the mortality rate and its predictors in patients with stroke and concurrent COVID-19 infection.
Methods: A retrospective analysis was conducted on stroke patients admitted to three Iranian referral hospitals within a 3-month period during the COVID-19 pandemic (COV-pos and COV-neg groups). The mortality rate was compared to a similar period one year before the pandemic (non-COV group). The Cox proportional hazards model was used to assess the independent and interactive effects of various variables on mortality.
Results: Among 124 stroke admissions, 59 (47.6%) had confirmed COVID-19 infection. The COV-pos group had a significantly higher initial NIHSS score (P = 0.001) compared to other groups. Mortality rates were 49.2%, 24.2%, and 17.3% in the COV-pos, COV-neg, and non-COV groups, respectively (P < 0.001). Posterior cerebral artery (PCA) stroke (HR = 65.099), internal carotid artery (ICA) stroke (HR = 19.102), and a history of diabetes mellitus (HR = 3.824) were identified as the most significant predictors of mortality in patients with stroke and COVID-19 infection.
Conclusions: Stroke patients with COVID-19 infection exhibited a significantly higher mortality rate compared to patients without COVID-19. The type of stroke involving the PCA or ICA and a history of diabetes emerged as the strongest predictors of mortality in the studied population.
The emergence of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the COVID-19 pandemic, has been associated with a wide range of health complications beyond respiratory illness [1, 2]. Neurologic manifestations, including acute cerebrovascular disease (CVD), have been increasingly recognized as significant sequelae of COVID-19 infection [3–5].
Studies have reported a notable increase in the incidence of acute ischemic stroke and intracerebral hemorrhage among COVID-19 patients [6–8]. Given the growing body of evidence suggesting a strong association between COVID-19 and stroke [6], this study aims to investigate the clinical characteristics, neuroimaging findings, and mortality of stroke patients during the pandemic period. By comparing stroke patients with and without COVID-19 infection, as well as a pre-pandemic control group, we aim to answer the following research questions: 1. What proportion of stroke patients had COVID-19 infection? 2. What are the clinical characteristics of stroke in COVID-19 patients? 3. What is the outcome of stroke in COVID-19 patients? 4. What factors may influence mortality in stroke patients with COVID-19 infection?
By addressing these questions, this study will contribute to a better understanding of the impact of COVID-19 on cerebrovascular health and inform clinical practice and public health strategies.
This retrospective cohort study included all stroke patients admitted to three tertiary care neurology centers in Tehran, Iran (Rasoul-Akram and Firoozgar Hospitals, affiliated with Iran University of Medical Sciences) and Karaj, Iran (Shahid Rejaee Hospital, affiliated with Alborz University of Medical Sciences). Patients were enrolled in two distinct periods: the pre-COVID-19 period which includes patients who came to the hospital one-year period prior to the COVID-19 pandemic and the post-COVID-19 period that is a three-month period following the onset of the COVID-19 pandemic in Iran.
Patients were categorized into three groups: the first was COVID-19 positive (COV-pos) that included patients who tested positive for SARS-CoV-2, the second was COVID-19 negative (COV-neg) composed of patients who tested negative for SARS-CoV-2, and the third was non-COVID-19 (non-COV) with patients from the pre-COVID-19 period.
To select eligible stroke patients, we initially performed a brain CT scan. For cases where the CT scan was inconclusive, we further utilized MRI to definitively diagnose the stroke subtype. Patients were then categorized into two groups: ischemic stroke and hemorrhagic stroke. Ischemic stroke was confirmed by a CT scan without evidence of intracranial hemorrhage, while hemorrhagic stroke was diagnosed by a CT scan demonstrating intracranial hemorrhage.
For all of the individuals, we collected demographic features, past medical history, the characteristics of stroke, laboratory findings, involvement of lungs and the need for mechanical ventilation, and the final outcome of death. The characteristics of stroke included the type of stroke, the circulation and territory involved, the initial National Institutes of Health Stroke Scale (NIHSS), and Modified Rankin Scale (MRS). Mortality rate, need for mechanical ventilation, and the days of hospitalization were also measured.
All data were gathered, coded, and analyzed using SPSS statistical software, version 26. The Cox proportional hazards ratio test was implemented using coxphfit in Matlab 2018b, considering covariates as: main variable, main variable × COVID-19. The frequency, mean, and standard deviation were utilized to compare the three groups of patients: COV-pos, COV-neg, and non-COV. The Kruskal-Wallis test was used for the comparison of means. To assess the relationship between nominal variables, the chi-square test was applied. For nominal variables such as smoking and alcohol use, we also checked the Fisher exact test and the results were the same (P < 0.001). A P value of less than 0.05 was considered statistically significant.
Next, the Cox proportional hazards model was applied separately for each specified variable and its interaction with COVID-19. The interaction term indicates how COVID-19 affects the impact of the main variable on the hazard ratio (HR). The HR for a binary variable is interpreted as the hazard rate when the variable is present compared to when it is absent. For continuous variables, the HR shows how much an increase of one unit in the variable affects mortality.
It is important to note that in the Cox proportional hazards model, the duration of survival of patients is considered, and the hazard rate determines the survival duration for deceased patients. Additionally, due to the limited number of patients, the effects of each variable were considered separately in independent models, and interactions or collinearity with other variables apart from COVID-19 were not considered in this study.
Overall, 124 individuals with stroke were admitted to three centers within three months of COVID-19 pandemic (COV-pos and COV-neg groups). None of the patients had been previously vaccinated against COVID-19, and no vaccination occurred during the study. In addition, 202 individuals that were admitted one year before COVID-19 and within the similar period, were considered as non-COV group. Of the 124 stroke patients hospitalized during COVID-19 pandemic, 59 (47.6%) were diagnosed with having COVID-19, including 8 subjects with positive polymerase chain reaction test and 51 subjects with typical clinical features conjunctive with typical appearance of lung involvement in chest CT scan. The demographic features of the recruited patients are presented in Table 1.
Demographic features of three groups including COV-pos and COV-neg groups within the period of COVID-19 pandemic, and non-COV group within the same period one year before emerging of COVID-19 pandemic
| Variables | Subgroups | Within the COVID-19 pandemics | Non-COV | P value | |
|---|---|---|---|---|---|
| COV-pos | COV-neg | ||||
| Total number of individuals | 59 | 65 | 202 | - | |
| Age (mean ± SD) | 70.90 ± 14.3 | 69.03 ± 16.0 | 64.02 ± 14.3 | Kruscal-Wallis test: < 0.001 | |
| Gender (male/female) | Male | 25 (42.4%) | 40 (61.5%) | 120 (59.4%) | Chi2 test: 0.046Fisher exact test: 0.049 |
| Female | 34 (57.6%) | 25 (38.5%) | 82 (40.6%) | ||
| Hx of hypertension | Yes | 38 (64.4%) | 46 (70.8%) | 119 (59.5%) | Chi2 test: 0.406Fisher exact test: 0.438 |
| No | 21 (35.6%) | 19 (29.2%) | 81 (40.5%) | ||
| Hx of atrial fibrillation | Yes | 4 (6.8%) | 9 (13.8%) | 18 (9.1%) | Chi2 test: 0.280Fisher exact test: 0.409 |
| No | 55 (93.2%) | 56 (86.2%) | 179 (90.9%) | ||
| Hx of cardiac valvular disease | Yes | 9 (15.3%) | 9 (13.8%) | 13 (6.4%) | Chi2 test: 0.168Fisher exact test: 0.092 |
| No | 50 (74.7%) | 56 (86.2%) | 188 (93.6%) | ||
| Hx of ischemic heart disease | Yes | 8 (13.6%) | 6 (9.2%) | 57 (28.6%) | Chi2 test: 0.004Fisher exact test: 0.002 |
| No | 51 (76.4%) | 59 (90.8%) | 142 (71.4%) | ||
| Hx of diabetes melitus | Yes | 19 (32.2%) | 25 (38.5%) | 54 (27.3%) | Chi2 test: 0.239Fisher exact test: 0.308 |
| No | 40 (67.8%) | 40 (61.5%) | 144 (72.7%) | ||
| Hx of any comorbidity | Yes | 47 (79.7%) | 54 (83.1%) | 162 (80.2%) | Chi2 test: 0.857Fisher exact test: 0.902 |
| No | 12 (20.3%) | 11 (16.9%) | 40 (19.8%) | ||
| Number of previous comorbidities (mean ± SD) | 1.41 ± 1.2 | 1.55 ± 1.0 | 1.72 ± 1.5 | Kruscal-Wallis test: 0.376 | |
| Hx of smoking | Yes | 6 (10.2%) | 13 (20.0%) | 30 (19.5%) | For both Chi2 test and Fisher exact test: < 0.001 |
| No | 53 (89.8%) | 52 (80.0%) | 124 (80.5%) | ||
| Hx of alcohol consumption | Yes | 1 (1.7%) | 1 (1.5%) | 4 (2.5%) | For both Chi2 test and Fisher exact test: < 0.001 |
Hx: history; COV-pos: COVID-19 positive; COV-neg: COVID-19 negative; non-COV: non-COVID-19
The initial NIHSS was 14.59 ± 6.9, 10.85 ± 7.9, and 8.80 ± 6.4 in COV-pos, COV-neg, and non-COV patients, respectively (P = 0.001). The most common type of stroke in all the three groups was the ischemic stroke. There was no difference in type of ischemic stroke, the involved circulation, and the vascular territory between the three groups (P > 0.05). The location of hemorrhagic stroke was statistically different between groups (P = 0.024). Subarachnoid hemorrhage was more prevalent in COV-pos patients compared with the other groups. Despite higher initial MRS of COV-pos patients, this difference was not statistically significant (P = 0.761). Indeed, MRS at hospital was significantly higher in COV-pos patients (P < 0.001) (see Table 2).
Features related to the stroke comparing three groups including COV-pos and COV-neg groups (within the period of COVID-19 pandemic), and non-COV group (within the same period one year before emerging of COVID-19 pandemic)
| Variables | Within the COVID-19 pandemics | Non-COV | P value | |
|---|---|---|---|---|
| COV-pos | COV-neg | |||
| Stroke types (all) | 59 | 65 | 202 | 0.867 |
| Ischemic stroke (arterial) | 42 (71.2%) | 49 (75.4%) | 148 (73.3%) | |
| Venous stroke | 0% | 0% | 2 (1%) | |
| Intracerebral hemorrhage | 14 (23.7%) | 15 (23.1%) | 45 (22.3%) | |
| Subarachnoid hemorrhage | 3 (5.1%) | 1 (1.5%) | 7 (3.5%) | |
| Circulation involved (all) | 59 | 65 | 202 | 0.922 |
| Anterior | 45 (76.3%) | 47 (72.3%) | 148 (73.27%) | |
| Posterior | 11 (18.6%) | 15 (23.1%) | 47 (23.27%) | |
| Mixed | 3 (5.1%) | 3 (4.6%) | 7 (3.46%) | |
| Type of ischemic stroke (all) | 42 | 49 | 150 | 0.433 |
| Territorial | 37 (88.1%) | 39 (79.6%) | 122 (81.3%) | |
| Lacunar | 2 (4.8%) | 7 (14.3%) | 19 (12.7%) | |
| Hemodynamic | 3 (7.1%) | 2 (4.1%) | 7 (4.7%) | |
| Arterial dissection | 0% | 0% | 0% | |
| Others | 0% | 1 (2.0%) | 2 (1.3%) | |
| Ischemic stroke: vascular territory (all) | 42 | 49 | 148 | 0.350 |
| ACA | 0% | 2 (4.1%) | 8 (5.4%) | |
| MCA | 28 (66.66%) | 27 (55.1%) | 81 (54.72%) | |
| PCA | 2 (4.76%) | 3 (6.12%) | 21 (14.19%) | |
| Vertebrobasilar | 6 (14.3%) | 9 (18.36%) | 18 (12.16%) | |
| ICA | 4 (9.52%) | 3 (6.12%) | 11 (7.43%) | |
| Others | 2 (4.76%) | 5 (10.2%) | 9 (6.1%) | |
| Hemorrhagic stroke: locations (all) | 17 | 16 | 52 | 0.024 |
| Basal ganglia and internal capsule ICH | 10 (58.82%) | 9 (56.25%) | 13 (25.0%) | |
| Lobar ICH | 3 (17.64%) | 3 (18.75%) | 26 (50.0%) | |
| Posterior fossa ICH | 1 (5.9%) | 3 (18.75%) | 3 (5.8%) | |
| SAH | 3 (17.64%) | 1 (6.25%) | 8 (15.4%) | |
| Isolated IVH | 0% | 0% | 0% | |
| Others | 0% | 0% | 2 (3.8%) | |
| Initial NIHSS (mean ± SD) | 14.59 ± 6.9 | 10.85 ± 7.9 | 8.80 ± 6.4 | 0.001 |
| MRS before stroke (mean ± SD) | 0.78 ± 1.5 | 0.34 ± 0.8 | 0.38 ± 0.8 | 0.761 |
| MRS during admission (mean ± SD) | 3.81 ± 1.5 | 2.98 ± 1.7 | 2.55 ± 1.5 | < 0.001 |
COV-pos: COVID-19 positive; COV-neg: COVID-19 negative; non-COV: non-COVID-19; ACA: anterior cerebral artery; MCA: middle cerebral artery; PCA: posterior cerebral artery; ICA: internal carotid artery; ICH: intracerebral hemorrhage; SAH: subarachnoid hemorrhage; IVH: intraventricular hemorrhage; NIHSS: National Institutes of Health Stroke Scale; MRS: Modified Rankin Scale
Overall, 93 individuals (28.5%) of all patients needed mechanical ventilation during the admission period. Mechanical ventilation was needed in 59.3% of COV-pos patients which was significantly higher than other groups (P < 0.001). Eighty (24.5%) of all individuals died. Death was occurred in 49.2% and 24.2% of COV-pos and COV-neg patients, respectively, with marked statistical significance (P < 0.001). However, in non-COV group, the death was observed in 35/202 (17.3%) of stroke patients. The involvement of lungs was observed in 16/24 (66.7%) patients who died, while only 26/101 (25.7%) of patients without final outcome of death had involvement of lung (P < 0.001) (Table 3).
Features related to the mortality and morbidity of stroke comparing three groups including COV-pos, COV-neg, and non-COV groups
| Variables | Subgroup | Within the COVID-19 pandemics | Non-COV(n = 202) | P value | |
|---|---|---|---|---|---|
| COV-pos(n = 59) | COV-neg(n = 65) | ||||
| Need for mechanical ventilation (%) | Yes | 35 (59.3%) | 19 (29.2%) | 39 (19.3%) | For both Chi2 test and Fisher exact test: < 0.001 |
| No | 24 (40.7%) | 46 (70.8%) | 163 (80.7%) | ||
| Death rate | Dead | 29 (49.2%) | 16 (24.2%) | 35 (17.3%) | For both Chi2 test and Fisher exact test: < 0.001 |
| Alive | 30 (50.8%) | 49 (75.8%) | 167 (82.7%) | ||
| Duration of hospitalization (days) | 12.61 ± 11.3 | 8.53 ± 8.6 | 10.3 ± 9.2 | Kruskal wallis test: 0.008 | |
COV-pos: COVID-19 positive; COV-neg: COVID-19 negative; non-COV: non-COVID-19
Table 4 denotes the calculation of HR for each variable and its interaction with COVID-19 infection in stroke patients. After considering the interaction with COVID-19 infection, the greatest HR was observed for posterior cerebral artery (PCA) stroke (HR = 65.099), internal carotid artery stroke (ICA) (HR = 19.102), history of diabetes mellitus (HR = 3.824), arterial ischemic stroke (HR = 2.947), and history of hypertension (HR = 2.194), respectively (P < 0.05) (Figure 1).
The Cox proportional hazards model ratio (HR) for each variable and its interaction with having COVID-19 infection in stroke patients
| Variables | Main variable | Interaction with COVID-19 infection | ||||
|---|---|---|---|---|---|---|
| Beta | HR | P value | Beta | HR | P value | |
| Age | 0.012 | 1.012 | 0.115 | 0.009 | 1.009 | 0.006 |
| Gender | –0.070 | 0.932 | 0.780 | 0.407 | 1.502 | 0.006 |
| Hx of hypertension | –0.007 | 0.993 | 0.978 | 0.786 | 2.194 | 0.006 |
| Hx of atrial fibrillation | –0.042 | 0.959 | 0.916 | 0.649 | 1.913 | 0.357 |
| Hx of cardiac valvular disease | 0.988 | 2.687 | 0.006 | –0.652 | 0.521 | 0.233 |
| Hx of ischemic heart disease | –0.251 | 0.778 | 0.486 | 1.080 | 2.945 | 0.055 |
| Hx of diabetes melitus | –0.714 | 0.490 | 0.037 | 1.341 | 3.824 | 0.004 |
| Hx of any comorbidity | –0.039 | 0.962 | 0.892 | 0.672 | 1.958 | 0.010 |
| Number of previous comorbidities | 0.006 | 1.006 | 0.946 | 0.281 | 1.324 | 0.012 |
| Hx of smoking | –0.842 | 0.431 | 0.069 | 0.446 | 1.563 | 0.685 |
| Hx of alcohol consumption | –1.374 | 0.253 | 0.185 | –12.484 | 0.000004 | 0.990 |
| Ischemic stroke (arterial) | –0.567 | 0.567 | 0.031 | 1.081 | 2.947 | < 0.001 |
| Venous stroke | –1.346 | 0.260 | > 0.999 | 0 | 1 | > 0.999 |
| Intracerebral hemorrhage | 0.294 | 1.342 | 0.258 | –0.017 | 0.983 | 0.965 |
| Subarachnoid hemorrhage | 0.257 | 1.293 | 0.618 | 0.138 | 1.147 | 0.903 |
| Anterior circulation | –0.100 | 0.904 | 0.724 | 0.445 | 1.560 | 0.090 |
| Posterior circulation | –0.293 | 0.746 | 0.442 | 1.220 | 3.388 | 0.033 |
| Mixed circulation | –0.739 | 0.478 | 0.463 | 2.087 | 8.064 | 0.071 |
| ACA stroke | –13.999 | 8e–07 | > 0.999 | 0 | 1 | > 0.999 |
| MCA stroke | –0.321 | 0.725 | 0.231 | 0.600 | 1.822 | 0.117 |
| PCA stroke | –1.142 | 0.319 | 0.258 | 4.176 | 65.099 | 0.004 |
| ICA stroke | –0.920 | 0.399 | 0.362 | 2.950 | 19.102 | 0.008 |
| Initial NIHSS | 0.107 | 1.113 | < 0.001 | 0.009 | 1.009 | 0.575 |
| MRS before stroke | 0.234 | 1.264 | 0.162 | 0.148 | 1.16 | 0.457 |
| MRS during admission | 0.795 | 2.214 | < 0.001 | 0.009 | 1.009 | 0.884 |
| Need for mechanical ventilation | 3.653 | 38.585 | < 0.001 | 0 | 1 | 0.999 |
The first column presents the HR for the variable alone, and the second column displays the interaction of that variable with COVID-19, indicating how much COVID-19 has increased the hazard rate due to that variable. Hx: history; ACA: anterior cerebral artery; MCA: middle cerebral artery; PCA: posterior cerebral artery; ICA: internal carotid artery; NIHSS: National Institutes of Health Stroke Scale; MRS: Modified Rankin Scale
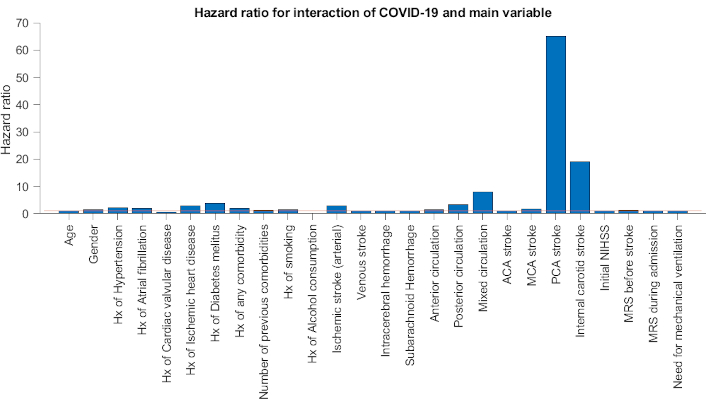
This hazard ratio chart shows the interaction between COVID and main characteristics of stroke factors. The red line represents a hazard ratio of 1. Hx: history; ACA: anterior cerebral artery; MCA: middle cerebral artery; PCA: posterior cerebral artery; NIHSS: National Institutes of Health Stroke Scale; MRS: Modified Rankin Scale
In the present study, we retrospectively investigate the characteristics and outcomes of patients infected with SARS-CoV-2 and stroke and compare them with stroke patients in non-COVID era, to provide novel insights into the spectrum of this disease. Of 124 individuals with stroke who were admitted to three centers, 59 patients had laboratory and/or radiographically-confirmed SARS-CoV-2.
The pathophysiological mechanisms associated with the risk of acute ischemic stroke (AIS) appear to be a complex interplay of multiple factors. A primary consideration is the presence of underlying conventional risk factors. These factors may predispose individuals to either incidental stroke or increase their susceptibility to stroke triggered by the infectious process.
Several potential mechanisms have been proposed. The inflammatory and cytokine response associated with COVID-19 may destabilize atherosclerotic plaques, leading to thromboembolism. Additionally, matrix metalloproteinases (MMPs) are enzymes involved in various physiological and pathological processes, making them fewer specific biomarkers for COVID-19. Other factors, such as chronic diseases or comorbidities, can influence MMP levels in the context of SARS-CoV-2 infection and accordingly increase stroke risk. Moreover, impaired cardiac function, resulting from myocardial dysfunction or arrhythmias, can increase the risk of cardioembolic stroke. The downregulation of ACE-2 receptors, which may reduce the activity of the renin-angiotensin system, could contribute to vascular dysfunction and increased thrombotic risk. Furthermore, emerging evidence suggests a prothrombotic state in COVID-19 patients, which may further elevate stroke risk [1, 9–16].
Although there have been many reports of young strokes in COVID-19 patients [11, 17, 18], in our study, the mean age of patients with concomitant stroke and COVID-19 was 70.90 ± 14.3, which is significantly higher than mean age of stroke patient in pre-COVID period (P value < 0.001). Moreover, as opposed to a systematic review by Stefania Nannoni et al. [19], comparison with non-infected patients with stroke showed no special differences in mean age (70.90 ± 14.3 vs. 69.03 ± 16.0) [19]. We observed a female predominance in patients with COVID-19 infection suffering a stroke which is unlike previous studies that showed equivalent results [20] or a higher number of male involvements [18, 21, 22].
There was a decrease in stroke admissions after the onset of COVID-19 pandemic. The same decrease was reported in other studies conducted in other countries all over the world [23–25]. In addition, detecting signs of ischemic stroke in critically-ill COVID-19 patients who are often intubated and strongly sedated, is especially difficult, masking their clinical features [26]. It also could be due to the fear of becoming infected, that patients with asymptomatic and mild to moderate strokes may be reluctant to seek hospital care [23, 27]. Reduction in cardiology services for coronary artery disease admissions during the COVID-19 pandemic was also noticed [28]. Furthermore, a decrease was observed in the utilization of acute stroke treatments, such as mechanical thrombectomy and recombinant tissue plasminogen activator (rtPA) [29]. Accordingly, this finding is probably not limited to stroke, nor to the geographic region where the present study conducted. Moreover, we observed that mean NIHSS score in COVID-19 period was higher than the same period a year before. Thus, the findings of our study about the age and NIHSS of stroke patients signifies the hypothesis that in the COVID era, all of the patients with any cause including stroke, did not come to hospital because of fear of infecting to COVID virus [30]. Albeit, when the symptoms of stroke were markedly disabling, they came to the hospital for treatment. A recent review by Ménard et al. [31] highlighted the importance of early intervention, patient education, and timely medical care for stroke patients, even during the COVID-19 pandemic. However, interestingly, we observed that the NIHSS was higher in COV-pos patients compared with COV-neg patients. In a study that compared the stroke characteristics of COV-pos patients with COV-neg patients and historical controls, consistent findings were reported. They have demonstrated that patients with concomitant COVID-19 suffered more severe strokes, with a higher NIHSS score [26]. This finding may signify the effect of concomitant COVID-infection on the signs of stroke. The study of Shahjouei et al. [8] revealed that 24.4% of patients with stroke and COVID-19 infection had no identifiable risk factor for vascular events. In addition, they found that 37.8% of patients were initially admitted with COVID-19 infection in the hospital and were asymptomatic for stroke at the onset of admission.
Observational studies support the role of modifying lifestyle-related risk factors (e.g., smoking, alcohol use, physical activity, diet) in stroke prevention [32]. The observation of lower rate of smoking and alcohol consumption among COV-pos patients in our study (P value < 0.001) signifies the role of COVID-19 as the cause of stroke. Furthermore, the number of previous comorbidities was the same in both COVID-era and a year before. In both periods, the most prevalent comorbidities were hypertension and diabetes mellitus, followed by cardiovascular diseases. The same prevalence of risk factors for stroke was reported in a meta-analysis of 8 studies from China [33, 34].
Similar to what previous studies have shown, the most common type of stroke in our study was ischemic stroke as well [19, 35]. We did not observe any differences in types of ischemic stroke, whether it is territorial, lacunar, hemodynamic, or dissection in comparison to the data from a year before COVID-19 pandemic. Although many studies have categorized ischemic stroke subtypes to cryptogenic, cardioembolic, large vessel occlusion, and small vessel occlusion showing the higher prevalence of cryptogenic causes [26, 36], we have used another system. In our study, the most common type of ischemic stroke in COV-pos patients was territorial one, including 88.1% of cases, followed by hemodynamic strokes accounting for 7.1% of cases, and lacunar strokes in 4.8% of cases. We observed that posterior circulation strokes were as common as anterior and mixed circulation strokes between three groups. This is in contrast with the recently described phenotype in two other case series, which shows a high incidence of posterior territory predisposition, and multi-territory involvement [6, 37].
Our study also included patients with CVD caused by hemorrhagic stroke. It was the second most prevalent type of stroke. We have not observed any differences in its prevalence in COVID-era and non-COVID-era (P value = 0.867). Interestingly, there was a substantially higher proportion of lobar intracerebral hemorrhage (ICH) in a year before COVID-19 pandemic, probably highlighting greater referral approaches before this pandemic (18.75% vs. 50%). Besides, there was a significant difference in the incidence of subarachnoid hemorrhage (SAH) between COV-pos and COV-neg patients (17.6% vs. 6.3%), but this difference was not apparent in non-COV patients (15.4%).
Furthermore, we observed a high rate of functional dependency during the hospitalization period for COV-pos patients (MRS = 3.81 ± 1.5), compared to MRS of our COV-neg patients and prior to the pandemic (P value < 0.001). This could be alluded to extreme illness and general weakness secondary to COVID-19 disease. Moreover, mortality and morbidity rates both were higher in COV-pos patients. In line with our results, a study by Hernández-Fernández et al. [37] illustrated that functional prognosis during the hospital period was unfavorable in 73.9% of their patients (17/23 MRS 4–6). Our findings revealed that, more than half of COV-pos patients needed mechanical ventilation, but it was 29.2% in COV-neg patients and 17.4% a year before pandemic, highlighting the prominent role of COVID infection in respiratory failure and related complications.
We have observed that the duration of hospitalization was less in non-infected stroke patients (8.53 ± 8.6) compared to a year before (10.3 ± 9.2). Having in mind the higher mean NIHSS score of both COV-pos and COV-neg patients in comparison with a year before, this could be due to fear of infection and higher rate of self-discharges. It has been said that patients with stroke had a 65% proportion of critical rate of COVID-19 in mainland China, which is higher than the average 5% critical rate [38], which can explain longer length of hospital-stay in the patients with stroke and COVID-19 infection (12.61 ± 11.3) than the patients without COVID-19 [39]. The overall mortality rate among COV-pos patients was 49.2%, which was much higher than COV-neg patients (24.2%) and non-COV-era (17.3%). Although Shahjouei et al. [8] did not compare the mortality of stroke in COV-pos and COV-neg patients, their study reported an in-hospital mortality rate of 131 out of 432 patients (30.3%) with stroke and COVID-19; among these patients, 323 (74.8%) had acute ischemic stroke, 91 (21.1%) had intracranial hemorrhage, and 18 (4.2%) had cerebral venous or sinus thrombosis. The rate of mortality at ICH and SAH patients was significantly higher than AIS patients (62.5% and 55% vs. 27.6%, respectively) [8]. In this regard, our study revealed that HR of mortality for ICH and SAH was not statistically different, with and without the effect of COVID-19 infection (P value > 0.05). Interestingly, the HR for ischemic stroke is 0.567 which means that ischemic stroke had less mortality outcome than other types of strokes. But when we consider the effect of COVID-19 infection, the HR reaches 2.947 (P value < 0.001). In other words, these findings suggest that COVID-19 infection has a significant effect on mortality of patients with ischemic stroke.
Furthermore, the results of our study revealed that COVID-19 infection has the most effect on poor outcome of patients with PCA and ICA strokes, and those having the history of diabetes mellitus and of hypertension. The markedly significant HR of PCA infarction (HR = 65) and ICA infarction (HR = 19), draws our attention to the notion that these types of strokes should carefully be managed in the context of COVID-19 infection. In a large multicentric study [8] in 135 tertiary centers of stroke worldwide, large vessel occlusion was observed in 44.5% of AIS plus COVID-19 infection, however, mechanical thrombectomy was performed only in 7.4% of patients. Even, intravenous thrombolysis was administered in 13.6% of patients. These low rates may reflect the delay to reach hospital in COVID-era or delay in diagnosis of stroke in the context of COVID-19 symptoms.
One final point is the effect of comorbidities on the mortality of stroke in COVID-19 patients. In line with other studies, our study showed that having the history of hypertension, diabetes mellitus, and at least one comorbidity contributes to unfavorable outcomes and higher mortality rates.
Nevertheless, we need to highlight that, this study includes a short time interval which may not adequately reflect the impact of a pandemic on the healthcare stroke systems, and this can lead to underestimations due to insufficient observation time.
In this study, we conducted some interesting data by analyzing the effect of different variables when COVID-19 exists relative to when COVID-19 is absent. These data showed that COVID-19 infection changed markedly the characteristics and the outcome of stroke.
In conclusion, the findings of this study revealed that the number of stroke admissions during the COVID-19 period has fallen and showed an increase in the proportion of elder patients and more severe strokes. It demonstrated higher baseline NIHSS scores and MRS during admission. Patients with stroke admitted during the COVID-19 pandemic also had a higher risk of in-hospital mortality. The major HR for mortality was observed in patients with PCA and ICA stroke and those having cardiovascular risk factors. These observations require further confirmation by observation of the evolution of stroke epidemiology and care delivery over the total span and phases of the COVID-19 pandemic.
AIS: acute ischemic stroke
COV-neg: COVID-19 negative
COV-pos: COVID-19 positive
CVD: cerebrovascular diseases
HR: hazard ratio
ICA: internal carotid artery
ICH: intracerebral hemorrhage
MMPs: matrix metalloproteinases
MRS: Modified Rankin Scale
NIHSS: National Institutes of Health Stroke Scale
non-COV: non-COVID-19
PCA: posterior cerebral artery
SAH: subarachnoid hemorrhage
MAD: Conceptualization, Project administration, Supervision, Writing—review & editing, Visualization. ST: Conceptualization, Data curation, Project administration, Supervision, Writing—original draft, Writing—review & editing. FM, TK, MRG, TL, MH, ZS, and RY: Investigation. ZM: Supervision, Conceptualization, Software. SMT: Writing—review & editing. MM: Resources, Validation. AHV: Data curation, Formal analysis, Methodology, Software. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
This study was approved by the Ethics Committee of Iran University of Medical Sciences, Iran (approval number: IR.IUMS.FMD.REC.1400.431).
Written informed consent to participate in the study was obtained from the participants.
Written informed consent to publication of the study was obtained from the participants.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2961
Download: 12
Times Cited: 0
