Affiliation:
1Department of Neurology, University Hospital Essen, University of Duisburg-Essen, D-45122 Essen, Germany
2Center for Translational and Behavioral Neurosciences, University Hospital Essen, University of Duisburg-Essen, D-45122 Essen, Germany
ORCID: https://orcid.org/0000-0002-0213-9642
Affiliation:
3Institute for Transfusion Medicine, University Hospital Essen, University of Duisburg-Essen, D-45122 Essen, Germany
ORCID: https://orcid.org/0000-0003-2446-948X
Affiliation:
1Department of Neurology, University Hospital Essen, University of Duisburg-Essen, D-45122 Essen, Germany
2Center for Translational and Behavioral Neurosciences, University Hospital Essen, University of Duisburg-Essen, D-45122 Essen, Germany
Email: dirk.hermann@uk-essen.de
ORCID: https://orcid.org/0000-0003-0198-3152
Explor Neurosci. 2022;1:61–74 DOI: https://doi.org/10.37349/en.2022.00005
Received: May 25, 2022 Accepted: August 09, 2022 Published: October 09, 2022
Academic Editor: Aurel Popa-Wagner, University of Medicine and Pharmacy Craiova, Romania
Ischemic stroke is a highly prevalent condition that frequently results in life-long disability and death. Considerable efforts have been made to establish treatments that prevent secondary ischemic damage and promote stroke recovery. Until now, the recanalization of occluded blood vessels via thrombolysis and thrombectomy, although highly potent, remains the only treatment in humans that enhances stroke outcome. Small extracellular vesicles are non-replicating, nano-sized (70–150 nm) lipid bilayer-enclosed vesicles, which have shown remarkable biological activities in various physiological and pathophysiological contexts. When administered post-stroke, mesenchymal stromal cell-derived extracellular vesicles (MSC-EVs) induce neuroprotection, promote brain remodeling and plasticity, and enhance neurological recovery in rodents and non-human primates via mechanisms that involve immunomodulation and anti-inflammation. In this review, experimental studies on the therapeutic actions of MSC-EVs in animal stroke models are summarized and perspectives for clinical translation are outlined.
Stroke is one of the most common causes of death and long-term disability. Each year, stroke causes approximately 101.5 million cases, 143 million disability-adjusted life years (DALYs), and 6.6 million deaths worldwide. These cases and disability numbers are still increasing annually, imposing great economic burdens on societies [1, 2]. The most common type of stroke is ischemic stroke, which results from the occlusion of a cerebral vessel by a clot. Despite the extensive attempts in developing ischemic stroke treatments, only intravenous thrombolysis and endovascular thrombectomy have hitherto been shown to be effective in clinical settings. Both aim at removing the clot and restoring blood flow to the brain. Due to their narrow applicable time windows, only a small proportion of patients with ischemic benefit from these treatments.
Ischemic stroke activates components of innate and/or adaptive immunity and induces brain inflammatory responses, which besides removing damaged tissue exacerbate ischemic brain injury [3, 4]. Following a stroke, immune responses and neuroinflammation persist throughout all phases of injury, from early tissue damage to late brain tissue remodeling [3, 4]. Specifically, polymorphonuclear neutrophils (PMNs) have been shown to play a critical role in the exacerbation of ischemic brain injury in the acute stroke phase and in the postischemic brain remodeling and angiogenesis in the post-acute phase [5, 6]. Although animal studies have yielded promising results on immunomodulatory therapies targeting poststroke leukocyte brain entry, these approaches have without exception failed in clinical trials [7]. Among others, this study’s failure may be attributed to the wide utilization of animal models that lack prevalent vascular risk factors (e.g., age, hyperlipidemia, diabetes) and/or the use of treatments that act too unspecifically to achieve clinically sustainable improvements in stroke patients.
Recently, mesenchymal stromal cell (MSC)-derived small extracellular vesicles (EVs) have emerged as a potential new therapeutic agent for stroke treatment. EVs are nano-sized lipid bilayer-enclosed vesicles (70–150 nm) that are released by virtually all cell types. Apparently, EVs are essential components of an ancient cargo and communication system, we are just getting aware of. A proportion of EVs transmit complex signals from sending cells to selected target cells within the close and distant environment. Applying EVs from the right cell types, we gain new vehicles to interfere with biological processes and use them as novel therapeutic agents [8, 9]. In contrast to their parental MSCs, MSC-derived EVs are easier to handle and can be sterilized by filtration. Since EVs are non-replicating, they lack the risk of endogenous malignant transformation. Therefore, MSC-EVs became a promising tool for the development of novel stroke therapies.
This review summarizes existing evidence on the neuroprotective and neurorestorative effects of MSC-EVs in ischemic stroke, defining the role of immunomodulatory and anti-inflammatory actions in their recovery-promoting responses. Clinical perspectives of MSC-EVs are discussed and challenges for the translation from bench to bedside are outlined.
Stroke is the second leading cause of death and the third leading cause of death and disability worldwide [2]. Generally, stroke can be classified into two major subtypes, namely ischemic stroke and hemorrhagic stroke, caused by brain vessel blockage or rupture, respectively. Of all strokes, 87% are ischemic [1]. Globally, in 2019 approximately 77.2 million people suffered from ischemic stroke and a total of 3.3 million people died [1, 10]. Stroke statistics exhibit sex-related differences. Age-specific stroke incidence is similar between males and females at ages up to 55 years, but greater in males than females at ages 55–75 years [11, 12]. Age-standardized rates of deaths and DALYs from ischemic stroke are substantially greater in males than females, but prevalence rates are greater in females than males [13]. Due to population growth and aging, stroke incidence and prevalence rates are annually raising, imposing further economic burdens on societies [1, 11, 13].
So far, only two treatments have succeeded in clinical trials and become standard treatments for acute ischemic stroke. Both of them aim at the restoration of cerebral blood flow for the salvageable tissues in the penumbra. The first one is intravenous thrombolysis with recombinant tissue-plasminogen activator (tPA) and the second is endovascular thrombectomy. In 1995 tPA was proven to significantly improve stroke patients’ neurological outcome within 3 h of symptom onset, regardless of age, severity, and stroke subtype [14]. Thereafter, in 1996 tPA was approved by the United States Food and Drug Administration as a thrombolytic drug. A later clinical trial expanded the time window for administration of intravenous tPA from 3 h to 4.5 h of symptom onset [15], and using mismatch concepts inclusion criteria have even been expanded recently under well-defined clinical conditions [16]. Although tPA represents great progress in the development of stroke treatments, it has many drawbacks, such as narrow time windows, strict contraindications, and potential life-threatening complications. In fact, only up to 15% of patients with acute ischemic stroke are eligible for tPA in the United States [17, 18]. On the contrary, endovascular thrombectomy with a stent retriever device was proven to improve stroke outcomes up to 24 h after symptom onset [19, 20] and also as an add-on treatment to thrombolysis [21–23]. Although the number of patients eligible for thrombolysis and thrombectomy is on the rise [23–25] and the patients treated with one of the two treatment options is currently between 20–30% in well-established stroke centers, additional neurorestorative treatments are urgently needed, which allow improving stroke recovery once an ischemic injury has occurred.
Ischemic stroke activates brain-resident microglia and recruits blood-derived leukocytes, causing secondary brain injury (reviewed in [3, 4]). Microglia are the resident macrophages in the central nervous system that act as first-line innate immune defense. Microglia are activated when they sense danger signals, namely danger-associated molecular patterns released from the ischemic tissue, such as high-mobility group box 1, histones, and heat shock proteins [26–28]. Activated microglia display distinct phenotypical characteristics and polarize between a pro-inflammatory (M1-like) phenotype and an anti-inflammatory (M2-like) phenotype [29–31]. Activated M1-like microglia further recruit peripheral leukocytes into the brain by endothelial adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) [32–34]. Consequently, brain-invading leukocytes aggravate brain damage through production of reactive oxygen species (ROS), matrix metalloproteinases (MMPs), and cytokines/chemokines, further leading to blood-brain barrier (BBB) disruption, brain edema, hemorrhagic transformation, and neuronal death [35–40]. To limit excessive inflammation, the peripheral immune system may transiently adopt an immunosuppressive lymphopenic state. This poststroke immunosuppression facilitates infections, such as pneumonia, which worsen stroke outcome and increase mortality [41]. Interestingly, microglia depletion increases neuroinflammation and brain injury in acute ischemic stroke [42]. This observation emphasizes that microglia have important brain protective roles in ischemic stroke pathology, perhaps via their M2-like phenotype.
PMNs are the most abundant type of leukocytes in humans and act as first-line immune response to ischemic stroke [6]. It has been suggested that PMNs play a decisive role in poststroke brain injury and neurological recovery (reviewed in [5]). PMNs already appear in brain parenchyma at 12 h and peak within 24–72 h after ischemic stroke [43, 44]. The number of brain-infiltrating PMNs is positively correlated with the severity of ischemic injury and neurological outcome [32, 45]. Specifically, the brain accumulation of PMNs due to impaired microglial phagocytosis worsens stroke outcomes [46], and high neutrophil-lymphocyte ratios in poststroke peripheral blood indicate poor neurological recovery [47]. Therefore, targeting PMNs might be a strategy for stroke therapy. Indeed, preclinical studies have shown that depletion of PMNs or prevention of PMN brain entry by anti-lymphocyte antigen 6 complex locus G (Ly6G) antibody or by C-X-C motif chemokine receptor 2 or very-late-antigen-4 (VLA-4) blockade, respectively, reduces neurological deficits and infarct volume [5, 32, 45, 48]. Yet, PMN depletion has recently been shown to compromize brain microvascular remodeling and angiogenesis in the post-acute stroke phase [49], presumably by preventing the release of proteases and ROS that facilitate extracellular matrix remodeling [6]. Hence, the role of PMNs in ischemic stroke pathology differs between the acute and post-acute stroke phase. Treatments targeting PMNs appropriately need to consider treatment timing in order to ensure that beneficial actions are not put at risk by interventions administered in the wrong stroke phase.
Lymphocytes, especially T lymphocytes, are likewise associated with deleterious effects in acute ischemic stroke [48]. However, their early detrimental effects in experimental ischemic stroke are not related to adaptive immunity [50]. T cells accumulate in the brain lesion and mediate postischemic damage as early as 24 h after reperfusion [51, 52]. It has been reported that recombination activating gene 1 (RAG1)-deficient mice (which are T cell and B cell-deficient) are protected from focal cerebral ischemia and this protection is attributed to T cells because RAG1–/– mice reconstituted with T cells are no longer protected from the injury [50, 53]. More specifically, among T cell subpopulations, CD4+, CD8+, and γδ T cells have been shown to have detrimental roles in acute ischemic brain injury through mechanisms involving release of cytotoxic cytokines [51, 54], while regulatory T cells (Tregs) were reported to have neuroprotective effects by secreting the protective cytokine interleukin (IL)-10 [55]. Thus, the role of T cells in ischemic stroke pathology is cell subset-dependent. A comprehensive understanding of T cell biology is key for the development of T cell-targeting stroke treatments.
Although preclinical studies have demonstrated the detrimental role of leukocyte brain infiltration in ischemic stroke, immunomodulatory strategies targeting leukocyte brain entry have repeatedly failed in clinical trials (reviewed in [7]). For example, natalizumab, a humanized monoclonal antibody against VLA-4, did not influence infarct growth [56] and enlimomab, a murine monoclonal antibody against ICAM-1, failed to show benefits and even increased mortality in patients with acute ischemic stroke [57]. The failures can be attributed, at least partially, to the scarce utilization of animals with different sex, age, and comorbidity in stroke research. In line with previous reports, our group observed that aged mice exhibited worse cerebral blood flow recovery following postischemic reperfusion, greater neurological deficits, larger infarct volume and brain edema, and higher BBB permeability than young mice after intraluminal middle cerebral artery occlusion (MCAO) [58], similar to aged rats exhibiting more severe motor-coordination deficits and larger brain infarcts than young rats after permanent distal MCAO [59]. Moreover, the aggravated stroke outcome is associated with the exacerbated age-related immune alteration, that is, more severe peripheral immune responses and brain infiltration of leukocytes in aged ischemic brains. Specifically, PMN frequency and activation and activated CD8+ T cell counts are significantly increased in the peripheral blood, and the number of PMNs, T cells, and ED1+ macrophages is elevated in the ischemic brain of aged rodents [45, 58, 59]. Comorbidities, such as hyperlipidemia, substantially influence ischemic injury and stroke outcome (reviewed in [60]). We have shown that hyperlipidemic mice, fed with a cholesterol-rich Western diet, have increased BBB permeability and brain edema, impaired cerebral blood flow, attenuated cerebral angiogenesis and vascular remodeling, and disturbed stroke recovery [61–63]. This hyperlipidemia-exacerbated ischemic brain injury is associated with increased blood neutrophilia and brain PMN infiltration [32, 63]. Since comorbid conditions alter poststroke immune responses and have a huge impact on stroke outcome, immunotherapies targeting leukocytes should be thoroughly evaluated in preclinical studies under conditions of risk factors. Besides, leukocytes have both detrimental and beneficial roles in ischemic stroke pathology, and unfortunately, the immunomodulatory therapies that failed invariably also blocked the beneficial actions. For instance, PMNs besides their injury-promoting actions have recently been reported to have a pro-angiogenic role in the post- acute stroke phase [6, 49], and natalizumab, which inhibits T cell brain invasion, similarly prevents the brain entry of cytotoxic CD8+ effector cells and of neuroprotective Tregs. There is a need for more targeted therapies in the stroke field that selectively influence the detrimental immune cell actions without influencing the beneficial ones.
MSCs and their secretome have meanwhile emerged as such therapies. MSCs are multipotent non-hematopoietic progenitor cells with the potential to develop along the osteogenic, chondrogenic, and adipogenic lineage [64]. Since their discovery in the bone marrow by Friedenstein et al. [65] in the 1960s, the therapeutic potential of MSCs has been explored in various disease models and more than 1,000 registered clinical trials (https://clinicaltrials.gov/) [66]. MSCs have attracted great attention because of their easy sampling access from a variety of tissues including bone marrow, adipose tissue, and umbilical cord, their low immunogenicity due to the low expression of major histocompatibility complex (MHC)-I and no expression of MHC-II, and their potent immunomodulatory capabilities that regulate wide-ranging immune cells such as monocytes/macrophages, PMNs, natural killer cells, T cells, and B cells [8, 64, 67, 68]. Over the past two decades, preclinical studies have demonstrated the therapeutic efficacy of MSCs in ischemic stroke, where MSC administration reduces ischemic brain damage as well as neurological deficits, and promotes neurological recovery, associated with peripheral blood immunomodulation, anti-inflammation, increased angiogenesis, and increased neurogenesis [69–73]. Based on these promising preclinical data, multiple clinical trials of MSCs as a therapy for ischemic stroke have been registered (Table 1). The mechanism of action (MoA) of MSC in ischemic stroke was—as in many other diseases—at first assumed to be cell replacement [74]. Due to the observation that infusion of MSC-derived conditioned medium likewise improved disease outcomes in various settings, a paracrine MoA was discussed quickly [75]. As a possible mediator, MSC-EVs have been discussed. Indeed, in a head-to-head study in mice, Doeppner et al. [69] have demonstrated that MSC-EV preparations are equally effective as their parental MSCs in reducing poststroke motor-coordination deficits and promoting long-term angiogenesis and neurogenesis.
Registered clinical trials of MSCs and MSC-EVs as therapeutics in ischemic stroke
| Identifier | Country | Intervention | Tissue source | Origin | Route | Phase | Status |
|---|---|---|---|---|---|---|---|
| NTC00875654 | France | MSCs | Bone marrow | Autologous | Intravenous | II | Completed |
| NTC00908856 | United States | MSCs | Bone marrow | Autologous | Intravenous | I | Withdrawn |
| NTC01091701 | Malaysia | MSCs | Not defined | Allogeneic | Intravenous | I/II | Withdrawn |
| NCT01297413 | United States | MSCs | Bone marrow | Allogeneic | Intravenous | I/II | Completed |
| NCT01461720 | Malaysia | MSCs | Bone marrow | Autologous | Intravenous | II | Unknown |
| NCT01468064 | China | MSCs | Bone marrow | Autologous | Intravenous | I/II | Completed |
| NCT01678534 | Spain | MSCS | Adipose tissue | Allogeneic | intravenous | II | Completed |
| NCT01714167 | China | MSCs | Bone marrow | Autologous | Intracerebral | I | Unknown |
| NTC01716481 | South Korea | MSCs | Bone marrow | Autologous | Intravenous | III | Unknown |
| NCT01849887 | United States | MSCs | Bone marrow | Allogeneic | Intravenous | I/II | Withdrawn |
| NCT01922908 | United States | MSCs | Bone marrow | Allogeneic | Intravenous | I/II | Withdrawn |
| NCT02378974 | South Korea | MSCs | Umbilical cord | Not defined | Intravenous | I/II | Completed |
| NCT02564328 | China | MSCs | Bone marrow | Autologous | Intravenous | I | Unknown |
| NTC02580019 | China | MSCs | Umbilical cord | Not defined | Intravenous | II | Unknown |
| NTC03176498 | China | MSCs | Umbilical cord | Allogeneic | Intravenous | I/II | Suspended |
| NTC03186456 | China | MSCs | Umbilical cord | Allogeneic | Intravenous | I | Suspended |
| NCT03356821 | Netherlands | MSCs | Bone marrow | Allogeneic | Intranasal | I/II | Completed |
| NCT03384433 | Iran | MSC-EVs | Not defined | Allogeneic | Intraparenchymal | I/II | Recruiting |
| NCT04093336 | China | MSCs | Umbilical cord | Allogeneic | Intravenous | I/II | Recruiting |
| NCT04097652 | China | MSCs | Umbilical cord | Not defined | Intravenous | I | Recruiting |
| NCT04280003 | Spain | MSCs | Adipose tissue | Allogeneic | Intravenous | II | Recruiting |
| NCT04434768 | China | MSCs | Umbilical cord | Allogeneic | Intravenous and intraarterial | I | Recruiting |
| NCT04590118 | China | MSCs | Not defined | Allogeneic | Intravenous | I/II | Recruiting |
| NCT04811651 | China | MSCs | Umbilical cord | Not defined | Intravenous | II | Recruiting |
| NCT04953663 | China | MSCs | Bone marrow | Allogeneic | Intravenous | I/II | Recruiting |
| NTC05008588 | Indonesia | MSCs | Umbilical cord | Not defined | Intraparenchymal | I/II | Not yet recruiting |
| NCT05158101 | Antigua and Barbuda | MSCs | Umbilical cord | Allogeneic | Intravenous | I | Recruiting |
| NCT05292625 | Vietnam | MSCs | Umbilical cord | Allogeneic | Intravenous and intrathecal | I/II | Recruiting |
EVs can be classified based on their size and cellular origin as exosomes, microvesicles, and apoptotic bodies. Exosomes (70–150 nm in diameter) are formed as intraluminal vesicles by the late endosomal compartment and are released into the extracellular environment when multivesicular bodies (MVBs) fuse with the plasma membrane. Microvesicles (100–1,000 nm) are formed and released by budding from the cell plasma membrane, and apoptotic bodies (> 500 nm) are formed as fragments of apoptotic cells [8, 9]. The term “EVs” is generally used to refer to all experimentally-obtained extracellular vesicles due to the lack of technology to separate given subtypes [76]. It is widely assumed that therapeutic actions of EVs mainly derive from exosome-sized EVs which are also referred to as small EVs [8, 66, 76]. MSC-EVs have been reported in preclinical models to protect against more than 30 different diseases [8, 66, 77]. In ischemic stroke, MSC-EVs have been shown by others and us to induce neuroprotection and promote neurological recovery and brain remodeling in rodents following systemic delivery [45, 49, 58, 59, 69, 78–83]. While initial studies have been performed in young mice and rats [45, 49, 69, 78–83], additional studies recently examined the MSC-EV effects in aged mice, rats, and non-human primates [58, 59, 84–87]. In aged (15–24-month-old) male and female mice exposed to intraluminal MCAO, our group recently showed that MSC-EVs, when systemically administered immediately after reperfusion or with 6 h delay, induced neuroprotection, reduced functional neurological deficits, and reduced brain leukocyte infiltrates [58]. In aged (19–20-month-old) male Sprague-Dawley rats exposed to permanent distal MCAO, we showed that systemically administered MSC-EVs promoted motor-coordination recovery, reduced brain macrophage infiltrates in periinfarct tissue, and increased periinfarct angiogenesis and neurogenesis [59]. In aged (16–26-year-old) rhesus monkeys (Macaca mulatta) with cortical cold injury, systemic MSC-EV administration was found to improve fine motor and coordination recovery, reduce microglial-mediated neuroinflammation, and enhance oligodendrocyte-mediated myelin maintenance [84–86]. In a single study using middle-aged (12-month-old) mice subjected to thromboembolic MCAO, Webb et al. [87] observed no protective effects of systemically administered MSC-EVs on neurobehavioral recovery, infarct volume, and peripheral immune response. The lack of neuroprotection by MSC-EVs in this latter study might be attributed to the heterogeneity of MSCs, as we previously demonstrated that MSC-EV preparations exhibited considerable donor-to-donor variability in terms of anti-inflammatory and neuroprotective properties [45]. The joint evidence of these studies provides important information that supports the clinical translation of MSC-EVs. Cell-free MSC-EV therapies have numerous advantages over MSC treatments. First, MSC-EVs are much easier to handle since they can be sterilized by filtration. Secondly, the biological activity of EVs can be predicted more precisely than that of MSCs, since they hardly respond to environmental changes. Finally, in contrast to cells EVs lack endogenous tumor transformation potential. These traits make MSC-EVs particularly attractive as stroke treatments. Mechanistically, the neuroprotective and neurorestorative actions of MSC-EVs in ischemic stroke are closely related to their potent immunomodulatory and anti-inflammatory effects.
Immunomodulatory and anti-inflammatory actions of MSC-EVs were clinically documented in our worldwide first case report of MSC-EV application in a human patient with steroid-refractory acute graft-versus-host disease (GvHD), in which MSC-EV treatment effectively reduced the GvHD symptoms and modulated the patient’s immune response by reducing the levels of proinflammatory cytokines and increasing the levels of anti-inflammatory cytokines [88]. Our preclinical animal studies evaluating the therapeutic efficacy of MSC-EVs in ischemic stroke have also correlated the MSC-EV-induced neuroprotection and neurological recovery to their immunomodulatory activities [45, 58, 59, 69]. As discussed above, immune responses and neuroinflammation are hallmarks in ischemic stroke pathophysiology. Notably, MSC-EVs effectively reduce poststroke microglial-mediated neuroinflammation and promote microglial polarization from the M1 to the M2 phenotype [59, 85, 89–92], and reduce brain infiltration of leukocytes, namely of PMNs, monocytes/macrophages, and lymphocytes [45, 58, 59, 93, 94], as summarized in Figure 1. As a result, the decreased expression of pro-inflammatory factors and increased anti-inflammatory mediators are noted in the ischemic brain [89–91, 93–95]. Besides, MSC-EVs successfully reverse poststroke lymphopenia in peripheral blood [45, 69, 94]. The mechanisms underlying MSC-EV-induced immunomodulation are not fully elucidated. We have previously shown that PMNs play a pivotal role in the MoA of MSC-EVs in ischemic stroke [45]. PMN depletion using anti-Ly6G delivery mimicked the effects of MSC-EVs on reducing neurological deficits and brain ischemic injury as well as on reducing brain-infiltrating leukocyte counts after intraluminal MCAO; however, MSC-EVs did not have any synergistic effects regarding further reduced stroke injury and brain immune cell infiltrates in PMN-depleted mice [45]. Interestingly, MSC-EVs did not influence peripheral blood immune response in sham-operated mice [45], indicating that the immunomodulation of MSC-EVs is pathophysiology-dependent. In addition, MSC-EVs did not influence the PMN frequencies and activation states in the peripheral blood of both young and aged stroke mice, but they did so in the ischemic brain [45, 58]. Given that intravenously infused MSC-EVs firstly encounter peripheral blood immune cells and PMN depletion abolishes the neuroprotective effects of MSC-EVs, PMNs might be the site of immunomodulatory action of MSC-EVs in ischemic stroke [45, 49, 58]. Alternatively, MSC-EVs may target macrophages/microglia, as supported by the findings that MSC-EVs promote macrophage/microglia polarization from the M1-like to the M2-like phenotype and reduce the macrophage/microglia-associated brain inflammatory response [59, 85, 89–92].
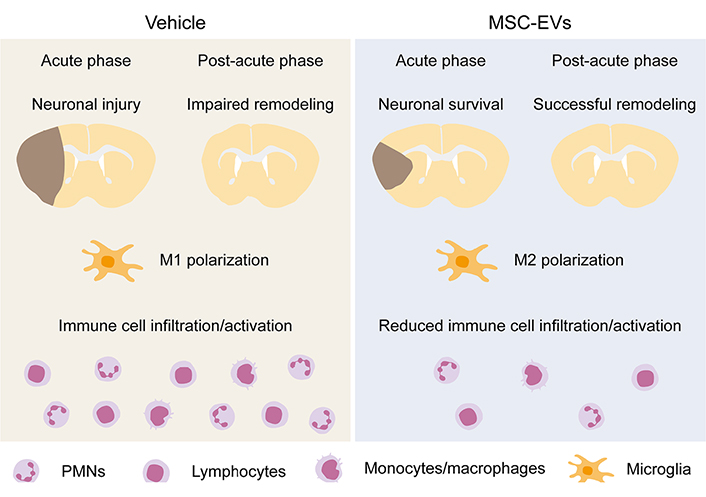
Immunomodulatory effects of MSC-EVs in the ischemic brain. MSC-EVs induce ischemic neuroprotection in the acute stroke phase and promote brain tissue remodeling in the post-acute stroke phase. As underlying mechanisms, immunomodulatory effects involving M2 microglia polarization and reduced brain immune cell infiltration and activation play a decisive role. (This figure is built upon graphic elements of previous publications [45, 58] with self-owned copyrights.)
There is meanwhile growing evidence that systemically administered MSC-EVs reduce ischemic injury and promote neurological recovery in animal stroke models. Studies in young mice and rats [45, 49, 69, 78–83] have meanwhile been complemented by studies in aged mice [58], rats [59], and non-human primates [84–86]. Considering the low immunogenicity potential of MSCs and MSC-EVs and an own worldwide first case report of MSC-EV application in a steroid-refractory human GvHD patient, in which MSC-EV treatment was well tolerated and effectively reduced the patient’s GvHD symptoms [88], MSC-EV treatment in humans is likely safe. Indeed, clinical phase I/II studies demonstrated the safety of intravenous transfusion of allogeneic MSCs in human stroke patients [96, 97]. Currently, there is only one registered clinical trial on MSC-EVs for ischemic stroke (Table 1), but others will undoubtedly follow. A major risk with respect to the clinical translation of MSC-EVs is the heterogeneity of MSCs [98] and MSC-EV products [99]. The extent of MSC-EV heterogeneity is partially dependent on donors, tissue origins, cell culture conditions, EV isolation, and storage conditions [76]. We have previously demonstrated that even within the same manufacturing workflow, MSC-EV preparations obtained from three different healthy donors exhibited considerable heterogeneity in terms of neuroprotection and immunomodulatory properties in mice exposed to intraluminal MCAO [45]. Hence, the development of potency assays for MSC-EV products is an essential task for translational projects [66, 99]. Potency testing of biologics including MSC-EV products is highly recommended for early clinical trials and becomes mandatory for phase III clinical trials in human patients. By fulfilling these needs, we should become able to successfully translate MSC-EVs to clinical practice.
BBB: blood-brain barrier
EVs: extracellular vesicles
GvHD: graft-versus-host disease
MCAO: middle cerebral artery occlusion
MoA: mechanism of action
MSC: mesenchymal stromal cell
MSC-EVs: mesenchymal stromal cell-derived extracellular vesicles
PMNs: polymorphonuclear neutrophils
tPA: tissue-plasminogen activator
CW drafted the manuscript. All authors revised it and approved the submitted version.
DMH and BG hold U.S. and E.U. patents on extracellular vesicles for the treatment of inflammatory conditions (US9877989B2; EP2874634B1).
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Supported by German Research Foundation (389030878, 405358801) and Federal Ministry of Education and Science (3DOS; 161L0278B). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
