Affiliation:
1Laboratorio de Neurobiología Celular, Unidad de Neurociencias, Fundación Instituto de Estudios Avanzados IDEA, Caracas 1080, Venezuela
Email: mlongart87@gmail.com
ORCID: https://orcid.org/0000-0003-2589-7638
Affiliation:
2Laboratorio de Fisiología y Biofísica, Centro de Biología Celular, Instituto de Biología Experimental-IBE, Facultad de Ciencias, Universidad Central de Venezuela, Caracas 1041, Venezuela
ORCID: https://orcid.org/0000-0003-1225-9098
Affiliation:
1Laboratorio de Neurobiología Celular, Unidad de Neurociencias, Fundación Instituto de Estudios Avanzados IDEA, Caracas 1080, Venezuela
ORCID: https://orcid.org/0000-0002-6024-8492
Affiliation:
1Laboratorio de Neurobiología Celular, Unidad de Neurociencias, Fundación Instituto de Estudios Avanzados IDEA, Caracas 1080, Venezuela
ORCID: https://orcid.org/0000-0002-4787-7809
Affiliation:
1Laboratorio de Neurobiología Celular, Unidad de Neurociencias, Fundación Instituto de Estudios Avanzados IDEA, Caracas 1080, Venezuela
ORCID: https://orcid.org/0000-0001-5757-2980
Explor Neurosci. 2022;1:31–53 DOI: https://doi.org/10.37349/en.2022.00003
Received: July 23, 2022 Accepted: August 15, 2022 Published: September 29, 2022
Academic Editor: Dirk M. Hermann, University of Duisburg-Essen, Germany
Neuregulins (NRGs) and their cognate ErbB receptors (ErbB2–ErbB4) constitute a vast group of proteins encoded by six different genes (NRG1–6) and many isoforms with critical roles in the development and functioning of the nervous system. NRGs are known to regulate important processes in the nervous system like neural development, neuronal differentiation, neurite outgrowth, and specification. These factors are involved in the regulation of neurotransmission pathways and the modulation of several forms of synaptic plasticity. Due to NRGs’ role in synaptic plasticity, defects in their normal functioning are translated into altered signaling networks, which have been linked to susceptibility to developing psychiatric disorders like schizophrenia (SZ), autism, depression, and bipolar disorders. Additionally, deviation of the NRG normal functioning is involved in neurological diseases like Alzheimer’s and Parkinson’s disease. Contrastingly, NRG/ErbB signaling is also involved in the recovery after traumatic brain injuries (e.g., ischemic stroke). The NRG/ErbB signaling complex is highly unusual because the ligands (mainly NRG1–NRG3, with their multiple isoforms) and receptors (ErbB2–ErbB4) can orchestrate vast signaling complexes, with a wide reach within the processes that govern the development and appropriate function of the nervous system. This may explain why NRGs and ErbB receptor genes have been linked to complex brain disorders, like SZ. This review, are discussed important aspects of NRG and their relevance for nervous system functioning, including 1) subcellular localization, 2) signaling pathways involved in neuronal functions, 3) effect on neurite development and synapse formation, 4) modulation of some mechanisms of synaptic plasticity [long-term potentiation (LTP), depotentiation, long-term depression (LTD)] and 5) roles of NRGs in some neurological diseases. This review intends to present a summary of the main findings about this family of proteins, which might position them as one of the master regulators of brain functioning.
Neuregulins (NRGs) represent a group of proteins structurally related to the family of the epidermal growth factor (EGF) with implications for the development and structural homeostasis of the nervous system. So far, six different genes have been reported and the first member, NRG1, was identified more than two decades ago [1, 2]. Other NRG members NRG2–NRG6 (reviewed in [3]) were later described, with each gene able to generate multiple isoforms by several mechanisms, mainly through differential splicing [2, 4, 5].
NRG unprocessed proteins are synthetized as transmembrane proteins and undergo proteolytic cleavage to produce soluble N-terminal moieties containing an EGF-like domain, which is conserved among all NRG members. Via this EGF-like domain, NRG interacts and activates ErbB receptors, thus initiating the specific (canonical and non-canonical) signaling pathways. One of the most studied members of this family is NRG1: its gene produces different types (NRG1 type I–VI) and about 33 isoforms, which can arise from the use of different initiation transcription sites and alternative splicing [2, 6–9].
NRG1 types are differentiated by their N-terminal domains. NRG1 type I, II, IV, V, and VI have an immunoglobulin (Ig) domain, which is able to interact with components of the extracellular matrix [e.g., heparan-sulfate proteoglycans (HSPGs)] to establish concentrations and distance over which NRG would act [10]. On the other hand, NRG1 type III distinctively presents a cysteine-rich domain (CRD), which functions as a second transmembrane domain, rendering a membrane-anchored isoform that can signal in an autocrine manner.
Different types of NRG1 are expressed within the nervous system (peripheral and central). NRG1 is also expressed outside the nervous system, mainly in the heart, liver, stomach, lung, kidney, spleen, and skin. In the brain, high expression levels of NRG1 are observed in the prefrontal cortex (PFC), hippocampus, habenula, amygdala, substantia nigra, striatum (dorsal and ventral), hypothalamus, spinal cord, and cerebellum [9, 11]. All six types of NRG1 are detectable in the brain, although their abundance might differ significantly and can be developmentally regulated and subjected to neuronal activity [9]. For instance, CRD NRG1 (type III) is one of the most predominant types expressed in the brain, while types I, II, and IV seem to have lower expression levels. Regarding NRG2, two types with different EGF-like domains (α y β) are described, and these comprise at least 10 isoforms generated by alternative splicing [12, 13]. NRG2 is expressed in the developing nervous system and other embryonic tissues (heart, lung, and bladder) [13, 14]. In the adult brain, the highest expression levels of NRG2 have been reported in the hippocampal dentate gyrus (granule cells), cerebellum, and olfactory bulb [12–15], other brain areas like the neocortex, hippocampal CA1 (CA1)–CA3 hippocampal neurons, and striatum show weaker expression [16]. In the case of NRG3, it has been found that this gene produces up to 15 different splice variants [4, 17–20], which are mainly diffused in the embryonic and adult brains. The main places for NRG3 expression are the spinal cord, anterior olfactory nucleus, cerebral and piriform cortex, vestibular nuclei, medial habenula, hypothalamus, thalamus, deep cerebellar nuclei, and hippocampus [21]. Five isoforms have been described for NRG4 [22, 23] and their expression appears more confined to peripheral organs (pancreas, skeletal muscle, and brown adipose tissue) [22, 24]. However, more recent findings have reported NRG4 expression in specific areas of the developing brain (e.g., cortex, hippocampus, cerebellum, olfactory bulb, midbrain, and brain stem) [25]. Another member of this complex family of proteins is NRG5, which is also known as tomoregulin or transmembrane protein with EGF-like and two follistatin-like domains 1 (TMEFF1) [5, 26], with five reported spliced isoforms [22, 23]. NRG5 is highly expressed in several brain areas including the olfactory bulb, amygdala, cortex (entorhinal, cingulate, motor, and somatosensory), hippocampus (CA3, CA1, and subiculum), locus coeruleus, substantia nigra pars compacta (SNpc), hypothalamic nuclei, and cerebellum [27]. To finish with the main members of this group, NRG6, also known as neuroglycan C (NGC), chondroitin sulfate proteoglycan 5 (CSPG5), or chicken acidic leucine-rich EGF-like domain containing brain protein (CALEB) [5, 28], is strongly expressed in the striatum, hippocampus, amygdala, and cerebral cortex. NRG6, on the other hand, shows a weaker expression in the substantia nigra, thalamus, pons, medulla oblongata, and cerebellum [29]. For a more extensive review see [20].
As we can see, the NRG family is highly complex and its range of action within the brain is extensive. Since NRGs, their receptors and their downstream signaling pathways are strongly involved in key processes governing the nervous system’s development and function. For instance, the balance of NRGs regulates several aspects of synaptic plasticity including long-term modifications in some brain areas [e.g, hippocampus and midbrain dopamine (DA) nuclei] and presynaptic or postsynaptic mechanisms. In the hippocampus, NRGs have multiple roles in the long-term regulation of excitatory/inhibitory balance. In the midbrain, NRG/ErbB signaling regulates long-term depression (LTD) and DA system activation [3]. ErbB4 is expressed in interneuron precursor cells during development and NRG1/ErbB4 signaling intervenes in circuitry assemblies such as axon development and the formation of new synapses [30–32]. On the other hand, NRG/ErbB signaling gives evidence that cognitive functions and behavior may be maintained by an appropriate NRGs/ErbB balance. Deviation of this balance can represent risk factors for Alzheimer’s disease (AD), schizophrenia (SZ), major depression, and other brain disorders [33, 34].
In this review, we discuss relevant aspects of the NRG family. We mainly focus on NRG’s role in brain processes related to NRG subcellular localization and the main signaling pathways by which these proteins act, NRG function in neurite development and synapse formation, the modulation of some mechanisms of synaptic plasticity long-term potentiation (LTP), depotentiation, LTD, and the role of these proteins in some neurological diseases and disorders.
Classically, it is well known that NRGs are transported from cell bodies towards axons and presynaptic terminals (see [15]), and interact with ErbB receptors (homo- or hetero-dimers) in paracrine or juxtacrine modes to activate specific signaling pathways, as described for NRG1 (see [3, 35–37]). However, their discovery in other structures like dendrites, which were demonstrated for NRG2 [15], started to change this classical paradigm. Following this idea, it was demonstrated that single-pass transmembrane NRG1 (type I and II) and NRG2 proteins, with an Ig-like domain, share similar subcellular distributions and ectodomain shedding properties [38]. These isoforms accumulate as unprocessed proforms in cell bodies and proximal dendrites [38]. NRG1 has been reported to accumulate postsynaptically at C-boutons, at cholinergic synapses between local interneurons, and motor neuron cell bodies [39]. The EGF-like domain of NRG is important for axonal clustering of ErbB4 [38], which is expressed in GABAergic interneurons at excitatory synapses [40, 41].
Interestingly, NRG2 is also expressed in ErbB4-positive GABAergic interneurons [42], suggesting autocrine signaling. The Ig-like domain enables NRGs to bind HSPGs, and this interaction is thought to promote local retention of the ectodomain in the extracellular matrix upon shedding [43]. Additionally, dual-pass transmembrane proteins, containing the CRD domain (NRG3 and CRD-NRG1), establish juxtacrine interactions with ErbB4 in axons of GABAergic interneurons. These NRGs, through juxtacrine interactions, form clusters and mutations in CRD-NRG1 and NRG3 that lead to the accumulation of immature proforms in neuronal somas and cause the loss of axonal puncta. The ectodomain shedding is mediated by metalloproteases and requires N-methyl-D-aspartate (NMDA) receptor (NMDAR) activity, and the mutations in CRD-NRG1 and NRG3 make these isoforms resistant to cleavage by β-secretase (BACE) [38].
It is important to highlight the relevance of NRG isoform diversity and its targeting of distinct subcellular compartments. Selective retention or delivery is a mechanism that regulates the subcellular localization of synaptic proteins [44, 45]. Specific subcellular localization is organized and regulated by specialized compartmentalization of the cytoskeleton. This process occurs through specific interactions with receptors, adaptors, and motor proteins [46, 47]. It has been reported that the wiring of specific microcircuits in the cerebral cortex is modulated by tyrosine kinase activity of the ErbB4 receptor [48], which is only expressed in some types of cortical interneurons and absent from pyramidal cells [32, 49]. At the synapses, subcellular localization of ErbB4 is observed both at presynaptic (axons) and postsynaptic (somatodendritic) compartments [32], where it balances the number of excitatory and inhibitory synapses established by different types of interneurons [31, 32, 50–52]. In this sense, the development of cortical circuits might be modulated by interactions of ErbB4 receptors (pre and postsynaptic) with specific synaptic partners [44, 45].
NRG1 and NRG3 show similar subcellular distributions, with NRG1 present in axons of peripheral and central (hippocampus) neurons [15, 38, 53, 54], while NRG3 is enriched in axonal varicosities and synaptic puncta that contact the dendrites in interneurons [38, 55]. In pyramidal cells, sorting NRG1 and NRG3 to different compartments is involved in excitatory and inhibitory synaptogenesis in different populations of cortical interneurons [56]. Regarding the differential subcellular localization of NRGs in pyramidal cells, it has been shown that NRG1 is spatially restricted to the perisomatic compartment, and NRG3 is highly enriched in the neuropil. While NRG1 is enriched in postsynaptic compartments of inhibitory synapses, targeting the soma, NRG3 is mostly restricted to excitatory presynaptic terminals contacting interneurons [56]. Given all this evidence, we could say that differential subcellular sorting of different NRGs, ErbB receptors, and other synaptic proteins might represent a novel strategy to orchestrate the assembly of complex brain circuitries.
Some of the most important neural functions like neuronal differentiation, survival, proliferation, and migration are affected by NRG/ErbB signaling pathways [5, 57]. NRGs regulate numerous neurodevelopmental and activity-dependent processes. There is evidence showing that important aspects of the central nervous system (CNS) synaptic plasticity, a cellular process believed to represent a substrate for cognitive processes, such as learning and memory, is regulated by NRGs [5, 58]. For example, NRG/ErbB4 signaling in parvalbumin-positive (PV+) GABAergic interneurons regulates glutamatergic synaptic plasticity in the hippocampus [59, 60] and critical period plasticity in the visual cortex [61, 62].
Specific signaling mechanisms start with the binding of NRG to the specific ErbB receptor, through the extracellular EGF-like domain. ErbB receptors are part of the EGF receptor (EGFR) family and comprise four members (EGFR/ErbB1, ErbB2, ErbB3, ErbB4). Each receptor, except for ErbB2, binds to an exclusive set of ligands causing dimerization, activation, and phosphorylation of the receptor intracellular domains (ICDs), followed by the creation of docking sites for adaptor proteins. ErbB2 is the preferred heterodimerization partner for the other ErbB proteins, because of its strong kinase activity (see [2, 20]). The above-described process represents the classical or canonical signaling pathway. More specifically, NRG binding induces conformational changes of the receptor subunits, activates cross-phosphorylation, and initiates the recruitment of proteins with phosphotyrosine or Src homology-2 domain (SH2) binding domains. These adaptor/docking/effector proteins trigger multiple signaling pathways including phosphatidylinositol 3-kinase (PI3K)-AKT-mammalian target of rapamycin (mTOR)-ribosomal protein S6 kinase (S6K) and Ras-Raf-mitogen-activated protein kinase (MEK)-extracellular signal-regulated kinase (ERK) [2, 20], protein kinase C (PKC)-phospholipase C (PLC), and kinases like c-Abl, c-Jun N-terminal kinase (JNK), cyclin-dependent kinase 5 (CDK5), Kyn, and proline-rich tyrosine kinase 2 (Pyk2) [63–65]. On the other hand, neuronal growth and survival involving protein synthesis are regulated by activation of PI3K-Akt-mTOR and glycogen synthase 3 kinase (GS3K) pathways, with ErbB3 having the highest prevalence for PI3K docking sites [66]; thus, activation of the PI3K-Akt-mTOR might be due to the stimulation of ErbB3-containing dimer partners, like ErbB2/ErbB3 and/or ErbB3/ErbB4 (see [20]).
Ras-Raf-MEK-ERK is another pathway commonly stimulated by NRGs/ErbB, which allows the recruitment of growth factor receptor-bound protein 2 (GRB2) to the ErbB subunits with activated phosphotyrosine residues. The binding of GRB2 to ErbB can be mediated by the interaction with the Src homolog and collagen homolog adaptor protein, through the tyrosine phosphorylated residues in ErbB. Then, the ErbB-GRB2 partner recruits and activates the Son of Sevenless (SOS), a guanine nucleotide exchange factor, which fosters guanosine triphosphate (GTP) availability to bind Ras. In consequence, Ras activation activates c-Raf, MEK1/2, and ERK1/2. The subsequent phosphorylation of ERK1/2 translocates this kinase to the nucleus, activates transcriptional factors (like Elk1), and induces transcription of regulatory genes for cell growth and survival. The remaining fraction of active cytoplasmic ERK1/2 can phosphorylate actin, a cytoskeletal protein that intervenes in the regulation of cytokines, cell motility, cell division, vesicle, and organelle movements, among others. NRG also regulates PLC-PKC, Abl, JNK, CDK5, Kyn, and Pyk2 kinases, these pathways regulate gene expression, by controlling the activity of transcriptional factors such as c-Fos, Elk1, signal transducer and activator of transcription (STAT), c-Jun, and c-Myc [20, 63–65].
NRGs/ErbB-dependent effects can be also mediated through non-classical pathways, these include the “non-canonical forward ErbB signaling” and the “NRG1 backward signaling”. The non-canonical pathway initiates with proteolysis of ErbB4 by γ-secretase in the membrane-bound fragment, causing a release of the ErbB4 ICD (ErbB4-ICD), which can be translocated to the nucleus to regulate gene expression [67, 68]. Additionally, processing of the extracellular domain (ECD) of ErbB4 by tumor necrosis factor alpha converting enzyme (TACE) releases a soluble protein called ecto-ErbB4, which can bind to membrane-bound NRG1 (e.g., immature pro-NRGs or the membrane-bound NRG1 type III). The binding of ecto-ErbB4 to NRGs can either block NRG canonical pathway or elicit the already mentioned “NRG1 backward signaling” [69, 70]. This alternative signaling pathway is initiated with the action of γ-secretase, which performs a proteolytic cleavage of the ICD of NRG1 (NRG1-ICD), it is then released and translocated to the nucleus. Following nuclear translocation, the interaction of NRG1-ICD with transcription factors (e.g., Eos) allows it to exert its regulating effects over different genes, among which it is worth highlighting the regulation of the post synaptic density (PSD) proteins genes [2, 71]. Similarly to NRG1, NRG3 can also exert back signaling actions with its C-terminal domain (See Figure 1) [70].
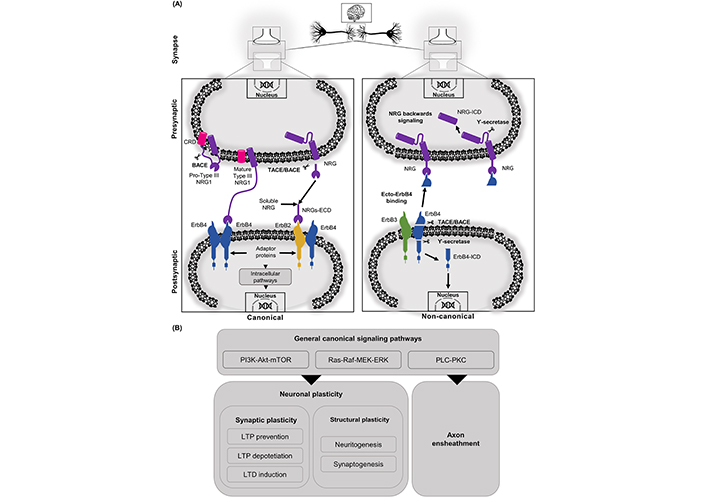
NRGs related signaling cascades can be activated through canonical or non-canonical pathways. A. In the canonical pathway, the ErbB receptors mainly participate interacting with a cleaved matured soluble ECD. Another way to activate ErbB4 is through juxtacrine interaction with the CRD domain of NRG1 type III. In the non-canonical pathway, NRGs/ErbB-dependent effects can be also mediated through non-classical pathways (“non-canonical ErbB signaling” and the “NRG1 backward signaling”). In this non-canonical pathway, processing of the ECD of ErbB4 by TACE/BACE releases a soluble protein called ecto-ErbB4, which can bind to membrane-bound NRG1. NRGs/ecto-ErbB4 can either block the NRG canonical pathway or elicit the already mentioned “NRG1 backward signaling”. This alternative signaling pathway is initiated with the action of γ-secretase, which performs a proteolytic cleavage of the ICDs of NRG1 (NRG1-ICD) and/or ErbB4 (ErbB4-ICD), which are then released and translocated to the nucleus to exert their regulating effects over different genes; B. among the signaling cascades found in canonical NRG/ErbB signaling is PI3K-Akt, Ras-Raf, PLC-PKC. Those signaling cascades activate nuclear factors regulating neuronal plasticity. In synaptic plasticity, NRG/ErbB signaling regulates LTP and LTD. NRGs can also regulate structural plasticity processes like neuritogenesis, synaptogenesis, and axon ensheathment
Given all these observations from studies with NRG1, it would be interesting to further study the possibility that some NRGs could act as transcription factors for key neuronal processes. This possibility could also involve other transcription factors interacting with NRGs. This is a very exciting field that would be necessary to explore further.
One well-studied effect of NRGs is the control over the development of axons and dendrites. For instance, cultures of hippocampal neurons treated with NRG1 induced axonal elongation and branching of GABAergic interneurons [32]. NRG1 also increases dendritic arborization of hippocampal neurons which express wild-type ErbB4 (but not the deficient ErbB4) by activation of the ErbB4-PI3K downstream pathway [72]. Important experiments with ErbB4 or conditional double ErbB2/ErbB4 mutant mice, which were heart rescued, exhibited cortical and hippocampal neurons with normal dendritic morphology [30]. Also, mutant mice for NRG1 type III showed cortical neurons with disturbances in basal dendrites and axon formation, and these effects are thought to be mediated by NRG1 back signaling [59, 73].
NRG1 type III enhances the outgrowth of both dendrites and axons at the early stages of differentiation in glutamatergic synapses from ErbB4-expressing GABAergic interneurons. In these neurons, ErbB4 exhibit a transient developmental expression in the axons which progressively decreases until becoming undetectable after one week in culture, while it remained high in the soma and dendrites through the in vitro development [49, 74]. Interestingly, the NRG1 type III-ECD regulates axonal extension and the NRG1 type III-ICD is necessary for dendritic outgrowth [59]. It is important to mention that a soluble form of NRG2 was detected in GABAergic interneurons, produced by proteolytic activity and NMDAR activation [42, 75]. It would be interesting to investigate whether or not this soluble NRG2 have similar functions in other neuronal types.
NRG1 and ErbB receptors have a key role in synapse formation, as well as in other mechanisms that contribute to the synapses. NRG1 signaling regulates oligodendrocyte development and axon myelination [76, 77], axon pathfinding [78], and the expression of neurotransmitter (NT) receptors [79]. The PSD is an electron-dense area just beneath the postsynaptic membrane and is a specialized zone harboring proteins mainly involved in the trafficking and insertion of ion channels, receptors, and scaffolding proteins, relevant to the synapses. PSD-95 and ErbB4 receptors are both located in the postsynaptic density and are found to interact through PSD-95, disks-large and zonula occludent-1 (PDZ) domains [40]. NRG1 and ErbB4 are essential for the growth, density, and maturation of dendritic spines and the stabilization of synaptic complexes. Interestingly, mice lacking NRG1-ErbB2/ErbB4 signaling show impairment in spine maturation and defective interactions of postsynaptic scaffold proteins with glutamate receptors (Glu-R) [30, 72, 80, 81]. NRG1 promotes dendritic spine growth through kalirin-7 (member of Rac-GEFs), with disrupted-in-schizophrenia 1 (DISC1) enhancing kalirin-7 binding to PSD-95 [82].
Experiments that measured PSD-95/GluA1-positive puncta and miniature excitatory postsynaptic current (mEPSC) frequency showed that the formation and maturation of excitatory synapses in GABAergic interneurons were promoted by NRG/ErbB signaling. The results also demonstrate that this pathway is supported by an increase of PSD-95 puncta (number and size) and mEPSC frequency, indicating that NRG not only stimulates the formation of new synapses but also strengthens the existing ones [48, 81]. In experiments using a treatment with ecto-ErbB4, both the number and size of excitatory synapses were diminished, suggesting that endogenous NRG1 may be critical for basal synapse formation [48, 81]. Indeed, in glutamic acid decarboxylase 65-kilodalton isoform (GAD65)+ interneurons, overexpression and inactivation of ErbB4 altered two markers of excitatory axon terminals (synaptophysin and vGlut1) [72]. Also, in hippocampal PV+ interneurons, in vivo genetic ablation of ErbB4 reduced mEPSC frequency and vGlut+ terminal densities, providing strong evidence that ErbB4 is important in excitatory synaptogenesis in interneurons [31, 32]. Furthermore, in PV+ fast-spiking neurons, in the PFC, ErbB4 is only required for maturation (but not for formation) of glutamatergic synapses (in vivo) [52]. These ErbB4 effects on excitatory synapses may be due to PSD-95 stabilization [31], which is involved in the maturation of glutamatergic synapses [83]. Some experiments suggest that presynaptic differentiation may be regulated by postsynaptic ErbB4 through transsynaptic interaction with transmembrane NRG1 [72]. Interestingly, ErbB4 is also able to promote GABAergic synaptogenesis and maintenance in pyramidal neurons. Precisely, ErbB4-chandelier cells develop fewer synapses onto the axon initial segments (AISs) of hippocampal pyramidal neurons in vivo. Additionally, NRG1 type III overexpression in pyramidal neurons increased the density of interneuron axonal boutons of chandelier cells, an effect that may be mediated by the interaction of presynaptic ErbB4 with NRG1 type III [32]. NRG1 has been also shown to stimulate neurite outgrowth of excitatory neurons in the hippocampus and cerebellum [84–86]. Moreover, conditional deletion of ErbB2 and ErbB4 in single and double mutant mice affected excitatory synapse formation in pyramidal neurons (hippocampal and cortical) by reducing dendritic spine density [30]. Indeed, ErbB4 knockdown in hippocampal slices impaired spine formation [81]. More recently, reports have shown that axonal NRG1 type III regulates glutamate synapse formation and receptor trafficking of Glu-R2 subunit in central synapses (hippocampal-accumbens) modulating glutamatergic transmission [87].
Another member of this family involved in GABAergic synaptogenesis is NRG2, which is accomplished through a forward signaling mechanism, while its reverse signaling contributes to the maturation of glutamatergic synapses. For instance, in newborn granule cells, NRG2 is necessary for GABAergic synapse formation but not for its maintenance. Also, in these granule cells, NRG2 is crucial for the maturation of glutamatergic synapses [88].
Synaptic plasticity is an important function of the CNS, involving the ability to perform fine adjustments in the strength of the synaptic transmission. Memory and learning are thought to rely on synaptic plasticity [89], depending on antithetic processes LTP/LTD for memory formation and removal, respectively [90, 91]. In hippocampal NMDAR-dependent LTP/LTD, memory formation proceeds through various regulatory mechanisms [92]. LTP/LTD at Schaeffer collateral (SC)-CA1 hippocampal synapses (SC-CA1) are operated through postsynaptic functional regulation of NMDAR and AMPA receptor (AMPAR) [93]. Besides, LTP can be reversed or depotentiated by a brief protocol of subthreshold theta pulse stimulation (TPS), or theta-burst stimulation (TBS) to mimic natural activity patterns, only when delivered during the short labile period after LTP induction. LTP depotentiation is a mechanism preserving synaptic homeostasis at hippocampal SC-CA1s [94].
Some circuits in the PFC, hippocampus, and the midbrain DA nuclei show synaptic plasticity modulated by NRGs at the presynaptic and/or postsynaptic terminals of glutamatergic, GABAergic, cholinergic, and dopaminergic (DAergic) synapses [3, 20]. In these circuits, the interaction of scaffolding proteins, like PSD-95, is important for correct signaling (see Figure 2) [40, 95]. In the postsynaptic compartment, NRGs modulate NMDA, AMPA, and mGluRI receptors, which in turn determine the synaptic plasticity processes. In the presynaptic compartment, NRG modulates NT release. In general, it seems that NRG/ErbB exerts a tonic regulatory control to maintain cognitive functions and behaviors through different mechanisms (reviewed in [3]).
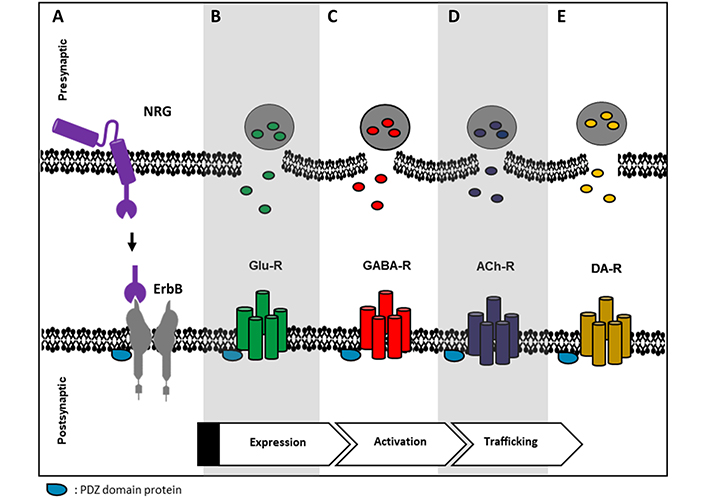
Representative image of possible relations between ErbB receptor and some NT receptors. A. NRG binds to its cognate ErbB receptor, PDZ domain-containing proteins are common scaffolding proteins that can connect ErbB receptors with some NT receptors like B. Glu-R; C. γ-aminobutyric acid (GABA), GABA receptor (GABA-R); D. acetylcholine (Ach) receptor (ACh-R) and E. DA receptor (DA-R). ErbB receptors can regulate these NT receptors at different levels, e.g., expression, activity, and/or trafficking
NRG1 is functionally associated with the hippocampal SC-CA1 [11, 96], where LTP can be stimulated by TBS or high-frequency stimulation (HFS) [20]. NRG impairs LTP at SC-CA1 in hippocampal slices, either by preventing HFS-induced LTP [41] or by reversing TBS-induced LTP. This prevention, also known as depotentiation, is due to the reduction of surface AMPAR expression without affecting NMDAR function or surface expression [97]. The presence of NRG/ErbB signaling inside the CA1–CA3 circuit is revealed by evidence like a) changes in ErbB4 levels alter dendritic spine size and AMPA synaptic currents [81], b) magnitude increments of TBS-induced LTP by ErbB inhibition [97], c) unchanged pair pulse facilitation of glutamatergic synaptic in response to NRG, d) PSD localization of ErbB4 [41] and Src-dependent functional upregulation of NMDA inhibited by NRGβ1/ErbB4 during TBS [98].
LTD in synaptic strength is believed to promote forgetting [99]. In the hippocampus from murine models, LTD can be induced by low-frequency stimulation (LFS). This process has been studied through an LTD experimental protocol dependent on NMDAR at excitatory synapses in hippocampal SC-CA1 [100–102]. It is harder to induce LFS-induced LTD in older rats than in younger ones [103–105]. However, recently it was reported that NRG1 markedly facilitates the LFS-induced LTD in SC-CA1 of adult animals but not in young ones, through the increased transmission of hippocampal LTD mediated by GABA subtype A (GABAA) receptor and involving NMDAR activity [106]. It is believed that NMDAR-dependent LTD underlies memory flexibility, which implies new memories substituting old ones [107]. Instead, mGluR-dependent LTD is stress-related [108], and is frequent in brain dysfunctions, such as autism, AD, memory deficits, learning and memory in the aging brain, and drug addiction [109–112].
This evidence supports an important role of NRGs/ErbB receptors in the LTP/LTD modulation. Thus, we can say that NRGs/ErbB signaling is fundamental for cognitive processes and other complex behaviors, rendering a proper brain function. In consequence, a dysregulated synaptic plasticity depending on NRGs/ErbB might be part of the mechanisms contributing to different neurological and psychiatric disorders.
Neurological diseases are heterogeneous disorders that affect the central and peripheral nervous systems, being the cause of disability and death [113]. Synaptopathy is a common feature of different neurodegenerative conditions, involving pathophysiological mechanisms based on the deterioration of synaptic strength [114]. NRGs are known to have a central neuroprotective role and could be a diagnostic marker in neurological diseases. In Table 1, a summary of the relationship between NRGs and neurological diseases is presented.
NRG and neurological diseases
| Disease | Aging | AD | PD | BT | SZ |
|---|---|---|---|---|---|
| Main hallmarks | Gradual loss of cognitive functions. Developmental abnormalities in cortical circuitry | Neuritic plaques. Neurofibrillary tangles.Accumulation beta-amyloid peptide (Aβ) | Degeneration of DAergic neurons.Deposition of α-synuclein | A brain injury that disrupts normal cellular and tissue function (b) | Chronic mental illness presents highly heritable genetic factors. Likely a consequence of neurodevelopmental disorders |
| Role of NRG | Neuroprotection, related to maximum lifespan in model systems | Neuroprotection. Decrement of Aβ peptide. Regulation of α-7 nAChR | Protection of DAergic neurons, elevation of DA levels (a) | Neuroprotection. Anti-inflammatory responses. Anti-apoptotic.Beneficial effects on endothelial cells and BBB permeability | Genetic association (NRG and ErbB). High levels of NRG1 contribute to hippocampal synaptic plasticity dysfunction (c) |
| Main affected areas | Cortex, PFC | Hippocampus and cerebral cortex | Substantia nigra, hypothalamus | Any area | Cerebral cortex, PFC, hippocampus |
Summary of the neurological diseases discussed in the review, showing their main features and how NRG is involved in those diseases. (a) In PD, ErbB4 is overexpressed and occurs with an excessive release of NRG; (b) BT: traumatic brain injury, stroke, etc.; (c) SZ: NRG1 concentration in serum is modulated by antipsychotic treatments and could be a therapeutic target. BBB: blood-brain barrier; nAChR: nicotinic ACh-R; BT: brain trauma; PD: Parkinson’s disease
It is known the importance of NRG/ErbB signaling in the developing, adult, and aging brain, specifically in the PFC, this area is involved in the organization of higher cognitive functions including memory, decision making, and attention. PFC develops and decays across the lifespan, and is dysregulated in disorders commonly associated with aging [115–117]. Some studies have shown that the consequences of altered NRG/ErbB signaling levels are dependent on the timing of the perturbation, suggesting that the expression of members of the NRG/ErbB network undergoes tight temporal regulation across the lifespan [118–120].
NRG/ErbB signaling plays a vital neuroprotective role in the aging brain [121, 122] and some studies have found that NRG1 and ErbB4 expression levels remain high in some species of adult rats Hibiscus glaber (H. glaber) [11] and correlate with maximum lifespan in rodent species [123]. A more recent study has found that NRG and ErbB genes display distinct expression profiles and that splice isoforms of these genes are differentially expressed across the murine lifespan [124]. These findings may suggest that NRG1 may be related to sustained neuron integrity in species that have long lifespans and that this factor expression may be an important component of longevity. Mouse models with disrupted genes for NRG and/or ErbB show developmental abnormalities in cortical circuitry, which may be relevant to developmental, psychiatric, and age-related disorders [125, 126].
AD is a neurodegenerative disease [113, 127], which mainly affects the hippocampus and cerebral cortex, and exhibits pathological features like neuritic plaques and neurofibrillary tangles. These features are both related to the accumulation of abnormally processed Aβ. This implies modification in the cytoskeleton, which means hyperphosphorylation of the microtubule-associated Tau protein in neurons [128–131]. Due to the plethora of functions of the NRG family and its receptors, various groups have tried to discern their role in the disease [57, 128]. In this pathology, NRG1 expression is the location and interactome-dependent [132, 133], and evidence shows both favorable and/or harmful effects. In an animal model of AD, mutants for the amyloid precursor protein (APP), showed a recovery of impaired neuronal differentiation after NRG1 treatment, suggesting favorable effects of NRG1 on synaptic plasticity and neuroprotection [121]. Indeed, the neurotoxic effects in SH-SY5Y cells and primary cortical neurons as a consequence of APP-CT31 (a 31 amino acids cytoplasmic terminal fragment from APP) expression are attenuated by NRG1 [134]. Aβ exerts effects on the generation of reactive oxygen species and expression of proinflammatory cytokines [57, 128, 131–133]; decreasing neuroplasticity due to its accumulation [130, 135]. In a mouse model, Aβ load is decreased by the ectodomain of NRG1 type I and type III, through the activity of the Aβ-degrading enzyme neprilysin (NEP) [122]. Deletion of ErbB4 in PV neurons attenuates Aβ-mediated toxicity by signals downstream of ErbB4 mediated by JNK [136]. NRG1 has been found in blood and cerebrospinal fluid of patients and animal models for AD [133, 137, 138]; however, studies, where NRG concentration in blood and cerebrospinal fluid could be correlated with hallmarks of this disease, will be required to demonstrate its relevance as a reliable marker for AD.
Taking into consideration much evidence that implicates additional roles for cholinergic systems in the overall brain homeostasis and plasticity, the cholinergic hypothesis of AD is important to consider. The cholinergic system occupies a central role in ongoing research related to normal cognition and age-related cognitive decline. In AD occurs a progressive loss of neocortical cholinergic innervation and death of forebrain cholinergic neurons [139]. Interestingly, NRG is expressed in cholinergic neurons throughout the rodent brain [140] and induces an increase of ACh-induced inward currents through α-7 nicotinic ACh-Rs in interneurons of the hippocampus [141]. NRG1-type III is also required for axonal targeting of α-7 nicotinic ACh-Rs [141]. Additionally, NRG1 and ErbB4 were found in neuritic plaques in the hippocampus of the AD brain [96]. Recent studies have shown that NRG1 is significantly downregulated in the hippocampus of AD patients, and NRG1 alleviated cognitive impairment and neuropathology in an AD mouse model [122, 142, 143]. This evidence, altogether suggests an important regulatory role of NRGs/ERbBs over the cholinergic system, which further supports a neuroprotective role for NRGs regarding AD.
PD is a neurological disease that is mainly observed in the degeneration of DAergic neurons (in the SNpc), and the deposition of α-synuclein in different areas of the brain [144]. As a consequence, patients suffer from behavioral neurodegenerative disorders and cognitive and motor dysfunctions [145, 146]. DAergic neurons are one of the most affected in this disease and, using animal models of PD, it has been demonstrated that NRG1 protects DAergic neurons (in vivo and in vitro) [147, 148] and induces ErbB4 phosphorylation and elevation of DA levels [148, 149]. NRG1 stimulates mGluR1 synthesis, potentiates mGluR1-mediated currents in DAergic cells, stimulates the downstream PI3K-Akt-mTOR pathway, and, as a consequence, promotes DA synthesis [150]. This pathway is affected in patients with PD, in which it has been also shown overexpression of ErbB4. Other studies have compared cell-cell communication in normal and PD conditions, showing a greater release of NRG from diseased DAergic neurons towards other neuronal and non-neuronal cells, in comparison to healthy DAergic neurons [151].
BT includes all neurological diseases caused by any injury to the head that disrupts normal cellular or tissue function within this organ, leading to multiple neurological problems [152, 153]. A growing body of evidence has shown that BT also promotes regeneration to adapt and recover from the effect of neuronal injuries, which include apoptotic inflammatory processes and cell death. The NRG1/ErbB4 signaling is one of the main mechanisms that are being studied after BT since it has a great impact on neurons, microglia, and macrophages [154]. Using rat models for stroke, the transient middle cerebral artery occlusion (tMCAO), where NRG1 was administered before tMCAO, cortical damage was reduced compared to those that did not receive NRG1 [128]. In vitro and in vivo experiments have demonstrated the protective effect of NRG1β1 in oligodendrocytes. The effect appears to be mediated through ErbB4-dependent PI3K-Akt activation and downstream effects with B cell lymphoma-2 (Bcl-2) and Bcl-2-associated death promoter (Bad) [155, 156], as well as nuclear translocation of the nuclear factor-kappa (NF-kappa) subunit mediated by the anti-inflammatory responses of NRG1 [157]. Synaptic activity leads to activation of ErbB4 and this activity is enhanced by NRG1/ErbB4 [154]. NRG1-ErbB4 has also been shown to prevent neuronal cell death during recovery after BT. Additionally, the BBB is affected after BT, exposing the brain to inflammation. In adult rodents, NRG1 crossed the intact BBB activating midbrain ErbB4 receptors by phosphorylation, increasing DA levels in the substantia nigra and striatum [154, 158], and exerting beneficial effects on endothelial permeability and BBB permeability after BT [159]. This evidence shows the potential of NRG as a neuroprotective factor to induce neurological recovery after brain injury.
The extensive and complex NRG/ErbB signaling networks have a broad range of critical neuronal circuits within the brain, affecting both the developing and adult brain. In this sense, it is feasible to assume that NRGs and ErbBs may also contribute to neuropsychiatric diseases. NRGs and ErbBs have been linked to the susceptibility to develop SZ and other psychiatric disorders, including bipolar disorder and major depression. Using genetic linkage analysis, it was identified a locus in chromosome 8p21-p22, and together with fine mapping and haplotype-association analysis in schizophrenic patients from Iceland, led to the identification of NRG1 as a candidate gene for SZ [160]. Other studies with a Chinese family showed additional SZ-associated single nucleotide polymorphisms (SNPs) in NRG1 [161]. It is important to mention that meta-analyses studies in various populations [162–165], including genome-wide association studies (GWASs; [166–169] support the genetic association between NRG1 and SZ, reviewed in [5]).
SZ is a chronic mental illness of the CNS, presenting highly heritable genetic factors, but still without a complete and detailed pathological description [128, 135, 170]. It has been demonstrated that NRG1 plays a crucial role in SZ pathogenesis (reviewed in [58]). Since NRG1 and ErbB4 signaling regulate synaptic plasticity, among other developmental processes in the CNS, it is believed that a neurobiological defect in NRG is related to SZ [2]. Different researchers propose that this disease is a consequence of neurodevelopmental disorders [128, 170–173].
GWAS has demonstrated the direct relationship of NRG haplotypes with SZ [2, 135, 170]. As well as the association of SNPs and alleles within the region, which independently or in combination, increase the risk of transitioning to psychosis [2, 170]. Importantly, almost all NRG1 SNPs associated with SZ map to non-coding DNA sequences. An exception to this is represented by a coding exon for a transmembrane domain, found in families from the Central Valley of Costa Rica, where Val is replaced by Leu in NRG1 [174]. NRG/ErbB4 receptor signaling pathways in GABAergic interneurons and DAergic neurons contribute to the modulation of glutamatergic synaptic plasticity [60, 175], which likely will affect behavior in psychiatric disorders, such as SZ. The altered activity in the PFC of the schizophrenic brain has been associated with the glutamatergic and GABAergic pathways presenting anomalies in plasticity, with NRG1 acting as a sub-regulator of the synaptic transmission dependent of NMDARs [176]. Given the prevalence of the NRG1 type III [177], expressed in the prefrontal dorsolateral cortex, as a risk factor to develop SZ, murine models were created, in which the importance of this NRG1 haplotype as a modulator of schizophrenic behavior was studied [178]. Some studies reveal the possible mechanisms of action to explain the production of SZ symptoms through the deregulation of NRG signaling [179]. Also, it is important to note that high levels of NRG1 alter the signaling of 2-arachidonoylglycerol (2-AG) in schizophrenic patients and contribute to the dysfunction in synaptic plasticity of the hippocampus. The high levels of NRG1 promote the degradation of 2-AG, limiting the LTD in inhibitory synapses dependent on the cannabinoid mechanism [180]. On the other hand, it has also been shown that antipsychotic treatments work by modulating the concentration of NRG1 in serum. Indeed, clozapine increases the concentrations of some NRG1 isoforms in schizophrenic patients [181]. In summary, evidence suggests that NRG, and their cognate ErbB receptors, are highly involved in complex brain disorders and neurological diseases. Therefore, NRGs/ErbBs and their downstream signaling pathways may provide therapeutic targets for specific neurological diseases, psychiatric disorders, and traumatic brain injuries.
NRGs are factors with a clear relevance in nervous system development and functioning. In this sense, these proteins regulate processes such as cell (neuronal and glial) differentiation, neurite (axonal and dendrite) outgrowth and specification, and synapse formation. In the adult brain, NRGs modulate neuronal excitability, neurotransmission, and other aspects related to synaptic plasticity. Given the crucial role of NRG1 in axons, dendrites, spines, and synapses, this factor might act as a master regulator of synaptic plasticity processes. Indeed, studies have revealed that NRG1/ErbB signaling networks are involved in the assembly of neural circuitry. Reviewing the evidence suggesting that there must exist an optimal level of NRG/ErbB signaling in the brain, it can be hypothesized that deviation from this balance or optimal NRG/ErbB level would greatly impair brain functions. As discussed in the present review, NRG/ErbB signaling is involved in synapse formation, connecting circuits, and promoting the formation and maturation of excitatory synapses in GABAergic interneurons. NRG is also involved in the regulation of other stages of the glutamatergic circuit assembly including, pyramidal neuron radial migration, neurite development, and spines (formation and density). NRGs and their receptors create complex networks with diverse signaling pathways, modulating numerous functions in the developing and adult nervous system. Therefore, it is not surprising that genetic association studies are providing evidence suggesting that genes encoding for NRG and ErbB receptors are associated with complex brain disorders and neurological diseases. In this line of thought, NRGs, ErbBs, and their downstream signaling pathways could be therapeutic targets for specific neurological diseases and psychiatric disorders. These findings highlight the importance of the NRGs/ErbB family of proteins, and that they might be one of the master regulators in brain functioning. Ongoing and future research about NRG functioning will be crucial to unveil important and still unresolved issues in brain functioning.
ACh-R: acetylcholine receptor
AD: Alzheimer’s disease
APP: amyloid precursor protein
Aβ: beta-amyloid peptide
BACE: β-secretase
BBB: blood-brain barrier
BT: brain trauma
CA1: hippocampal CA1
CNS: central nervous system
CRD: cysteine-rich domain
DA: dopamine
DAergic: dopaminergic
ECD: extracellular domain
EGF: epidermal growth factor
ERK: extracellular signal-regulated kinase
GABA: γ-aminobutyric acid
Glu-R: glutamate receptor
GRB2: growth factor receptor-bound protein 2
ICD: intracellular domain
Ig: immunoglobulin
JNK: c-Jun N-terminal kinase
LFS: low-frequency stimulation
LTD: long-term depression
LTP: long-term potentiation
MEK: mitogen-activated protein kinase
mEPSC: miniature excitatory postsynaptic current
mTOR: mammalian target of rapamycin
NMDA: N-methyl-D-aspartate
NMDAR: N-methyl-D-aspartate receptor
NRGs: neuregulins
NT: neurotransmitter
PD: Parkinson’s disease
PDZ: post-synaptic density-95, disks-large and zonula occludent-1
PFC: prefrontal cortex
PI3K: phosphatidylinositol 3-kinase
PKC: protein kinase C
PLC: phospholipase C
PSD: post synaptic density
PV+: parvalbumin-positive
SC: Schaeffer collateral
SNPs: single nucleotide polymorphisms
SZ: schizophrenia
TACE: tumor necrosis factor alpha converting enzyme
TBS: theta-burst stimulation
ML designed the outline of the manuscript, wrote the abstract, part of sections 1, 2, and 3, contributed to
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
