Affiliation:
Department of Diagnostic Imaging, Sheba Medical Center, Tel Hashomer, affiliated with the Sackler School of Medicine, Tel Aviv University, Tel-Aviv 52662, Israel
Email: iriseshed@gmail.com
ORCID: https://orcid.org/0000-0002-4655-9606
Explor Musculoskeletal Dis. 2023;1:43–53 DOI: https://doi.org/10.37349/emd.2023.00008
Received: October 22, 2022 Accepted: February 04, 2023 Published: April 27, 2023
Academic Editor: Fernando Perez-Ruiz, Cruces University Hospital, Spain
The article belongs to the special issue Diffuse Idiopathic Skeletal Hyperostosis- A common but neglected disease
Diffuse idiopathic skeletal hyperostosis (DISH) is a systemic condition characterized by the new bone formation and enthesopathies of the axial and peripheral skeleton. The diagnosis of DISH currently relies upon the end-stage radiographic criteria of Resnick and Niwayama, in which bridging osteophytes are present over at least four thoracic vertebras. The pathogenesis of DISH is not well understood, and it is currently considered a non-inflammatory condition with an underlying metabolic derangement. However, an inflammatory component was suggested due to the similarities between DISH and spondyloarthritis (SpA) in spinal and peripheral entheseal new bone formation. Magnetic resonance imaging (MRI) is the imaging modality of choice in the diagnostic work-up and follow-up of patients with SpA, as well as in understanding its pathogenesis. The aims of the current review were to evaluate the current and future role of MRI in imaging DISH.
Diffuse idiopathic skeletal hyperostosis (DISH) is a bone-producing disease characterized by excessive new bone formation in the axial and peripheral skeleton. It usually affects the thoracic spine, while new bone formation involves entheseal sites, mainly those of the pelvis [1–3]. Although initially considered a radiographic rather than a clinical entity, various clinical symptoms have been reported in patients with DISH, including back pain, spinal stiffness [4], and dysphagia [5].
The diagnosis of DISH relies upon the radiographic criteria of Resnick and Niwayama, in which bridging osteophytes along the anterolateral aspect of the spine extend over at least four contiguous vertebral bodies in the absence of degenerative disc disease and inflammatory sacroiliac or facet joint changes [1]. These criteria represent a late, ankylotic, and end stage of DISH and although several alternative criteria have been suggested over the years, the Resnick and Niwayama criteria continue to be the ones most commonly used in current practice [6].
The pathogenesis of DISH is not well understood, and it is currently considered a non-inflammatory condition with an underlying metabolic derangement that results in new bone formation [7, 8]. However, an inflammatory component has also been suggested due to similarities between DISH and spondyloarthritis (SpA), which represents a group of rheumatic diseases of which ankylosing spondylitis (AS) is the prototype and which shares a tendency towards spinal and peripheral entheseal new bone formation similar to that of DISH [9]. The clinical presentation of DISH may mimic long-standing advanced AS with severe limitation of spinal mobility and postural abnormalities [10]. Moreover, individuals with DISH are similarly susceptible to unstable spinal fractures [11].
Magnetic resonance imaging (MRI) has been widely used in the diagnostic work-up and therapeutic follow-up of patients with SpA. Inflammation and its resultant structural damage can be readily identified in MRI studies of the sacroiliac joints (SIJ) and the spine, making MRI the current imaging modality of choice for the diagnosis and evaluation of SpA [12, 13]. Furthermore, imaging findings on MRI were employed for formulating several etiopathogenetic theories related to SpA [14, 15], as well as for deciphering the nature of the spectrum starting from inflammation and extending to structural progression [16–19].
It is thus reasonable to assume that MRI may also add valuable input to establishing the diagnosis and to conducting of research on DISH. Therefore, the aims of the current review were to evaluate the role of MRI in the imaging of DISH and to investigate the extent and nature of its contribution. The use of anonymized images was approved by the local ethics committee (9916-22-SMC). Patient consent was waived by the Sheba Medical Center’s institutional ethics committee due to the retrospective nature of the analysis.
Although many subjects with DISH are asymptomatic, back pain and spinal stiffness are frequently reported [20]. Whether spinal ankylosis is of itself the source of pain, however, is open to question. While one study reported that the prevalence of back pain was similar for DISH and non-DISH [21], another study reported a comparatively lower prevalence among patients with DISH, and those authors subsequently suggested that the relatively increased spinal stability in DISH limits rather than induces the extent of pain [22]. Potential causes of pain due to DISH may be osteophytic pressure on neighboring organs, such as the anterior cervical spine, causing both pain and dysphagia in that region (Figure 1) [23].
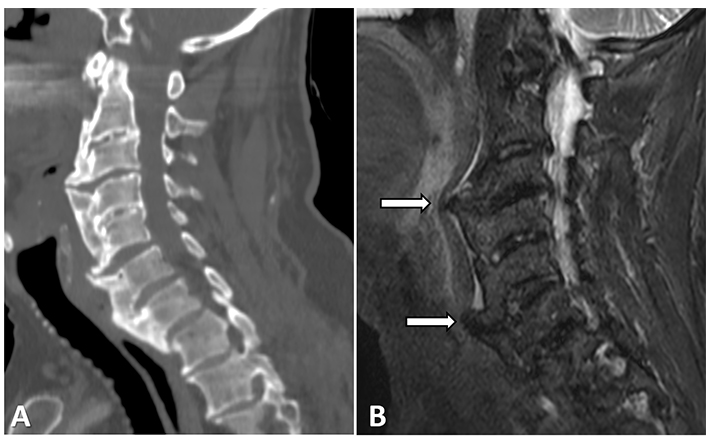
A 76-year-old male with DISH and dysphagia. A coarse and thick anterior osteophyte can be detected on both (A) the sagittal cervical spine computed tomography (CT) and on (B) sort tau inversion recovery (STIR) and is seen to be placing pressure upon the esophagus (arrows)
In the upper cervical spine, the protruding osteophytes and new bone formation in DISH subjects were reported to cause spinal cord compression and myelopathy [24, 25], while a combination of bridging osteophytes with an ossified posterior longitudinal ligament (OPLL) was reported to cause myelopathy of cervical (Figure 2) as well as thoracic spines [26–28]. Therefore, MRI may succeed in identifying the cause of back pain when other imaging modalities have failed.
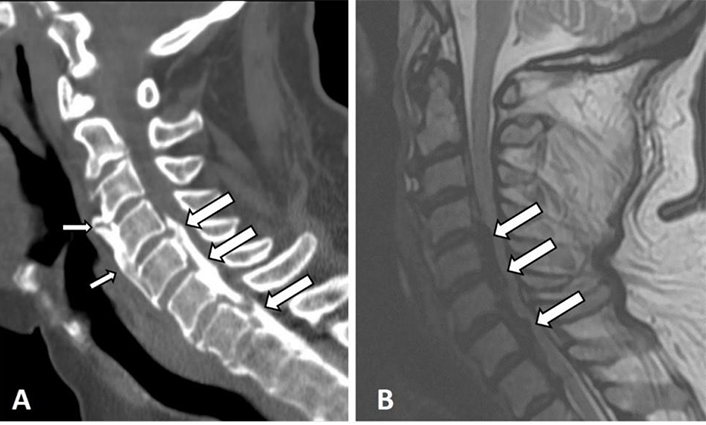
Sagittal cervical spine CT and T2-weighted (T2-W) MRI images of a 67-year-old male with DISH in the thoracic spine. Anterior flowing osteophytes between C3–5 (thin arrows) and an OPLL (thick arrows) causing stenosis of the spinal canal and myelopathy are depicted. Notice the difficulty in detecting the flowing osteophytes on MRI
It is worth noting that it is mostly the structural causes of nerve root compromise that are currently detected by MRI. Moreover, MRI is reportedly not sensitive enough for the detection of nonstructural causes for nerve root compromise and radiculopathy [29].
The rigid ankylotic spine of subjects with DISH is vulnerable to fracture even after seemingly low-energy trauma [30, 31]. There is a four-fold increased risk for fractures in an ankylosed spine compared to a non-ankylosed one, while associated spinal cord injury is seen in up to 58% of subjects with an ankylosed spine [32]. Hyperextension and displacement-type fractures are the ones most frequently observed in DISH, and they characteristically pass through the vertebral body [32, 33]. These fractures are associated with greater instability and several more complications that may be caused by a delay in diagnosis [32]. However, the interpretation of radiographic images can be challenging, and the detection of fracture was reportedly 65% at best with low inter-observer rates [34]. Consequently, both CT and MRI are often recommended for post-traumatic subjects with DISH [11], and MRI is specifically indicated when a neurological deficit is present (Figure 3) [35, 36].
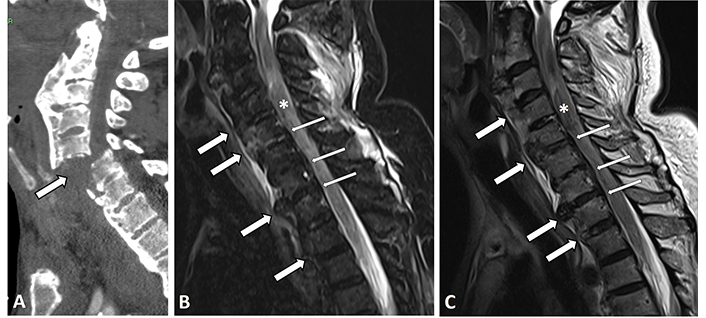
Sagittal CT image of the cervical spine of an 84-year-old male with DISH. (A) Displayed is a devastating extension dislocation-type fracture through C5–6 (thick arrows); (B, C) sagittal STIR and T2-W MRI images of the cervical spine of a 72-year-old male with upper limb parasthesis and weakness. Anterior flowing osteophytes (thick arrows) and OPLL (thin arrows) are depicted in both sequences, as is a thickened non-homogenous cord with blood residue contained within it (asterisk)
Both DISH and AS are bone-producing ankylosing diseases, and differentiating between them is usually based upon distinct clinical features, such as age and genetic predisposition as well as characteristic imaging features, e.g., vertical, symmetrical, and marginal slender intervertebral syndesmophytes in AS compared to chunky, thick, and horizontal osteophytes in DISH [9, 37]. However, the diagnosis of DISH is not always straightforward and a differential diagnosis of AS is sometimes suggested. This is probably due to the fact that both types of bone formation (syndesmophytes and bridging osteophytes) can be detected simultaneously both in DISH (Figure 4) and in AS, albeit with different ratios, with the majority being syndesmophytes in AS and the majority being osteophytes in DISH [37]. Moreover, the simultaneous occurrence of both conditions in the same patient has been reported [38].
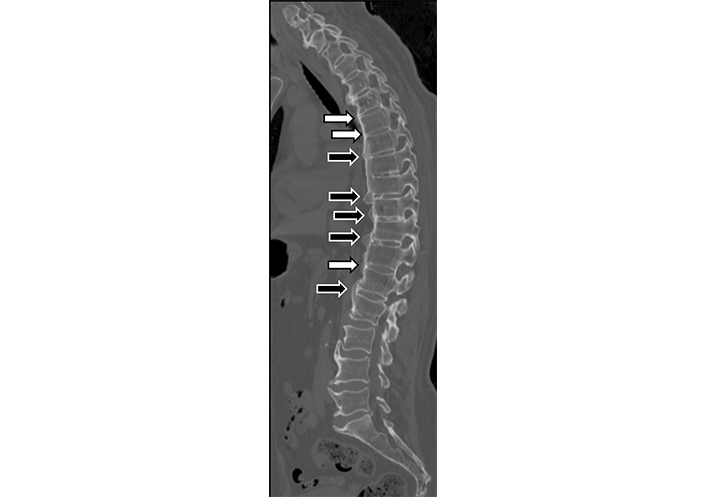
Sagittal thoracic and lumbar spine CT image of a 74-year-old male with DISH. Both thick horizontal osteophytes (black arrows), as well as thin vertical syndesmophytes (white arrows), are present, although in different frequencies
The MRI characteristics of the involved spine and SIJ in SpA have been well documented. They include acute lesions, such as anterior spondylitis inflammatory lesions, and periarticular osteitis in the SIJ, as well as structural lesions, such as erosions, fat metaplasia, sclerosis, and ankylosis in the vertebral corners or the SIJ [39, 40].
The presence of periarticular bone marrow edema (BME), or osteitis, which is highly suggestive of sacroiliitis with or without characteristic structural lesions, is part of the Assessment in SpondyloArthritis International Society classification criteria for axial SpA. It is now known that periarticular SIJ BME is not pathognomonic to sacroiliitis and that its detection alone does not necessarily imply that diagnosis [41]. Indeed, BME has also been described in the MRI examinations of patients with SIJ-related mechanical degeneration [42, 43].
There are two studies that demonstrated the presence of known SpA-related active and structural MRI lesions in the spine and in the SIJ of subjects with DISH [44, 45]. Both studies describe some degree of BME in their SIJ, however, BME highly suggestive of sacroiliitis was rarely seen [44], and when it was present, it was located mainly in the upper parts of the joint [45]. It is also our clinical impression that BME in DISH is seldom seen and that it is not similar in either amount or distribution to that appearing in SpA (Figure 5), thus allowing for the distinction between the two pathological entities based upon a characteristic appearance of SpA [46, 47]. This clinical impression warrants further confirmation.
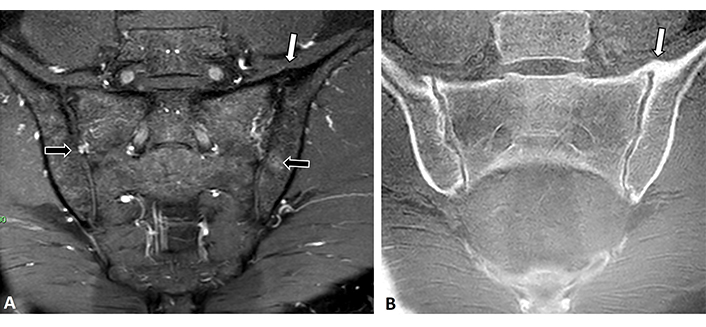
MRI of the SIJ of a 60-year-old male with DISH. (A) Coronal oblique T2-W and (B) ultra-short echo-time-weight (USTE-W) images of the SIJ. An anterior bridging osteophyte is seen on the left sacroiliac joint (white arrows) as is some very minor BME on the right sacral and left iliacal parts of the joints (black arrows)
In the spine, anterior vertebral BME corners (anterior spondylitis) as well as fat metaplasia in the corners of the vertebras have been described in subjects with DISH (Figure 6) [44, 45, 48]. Their frequency in DISH, however, was much lower compared to that of SpA [45].
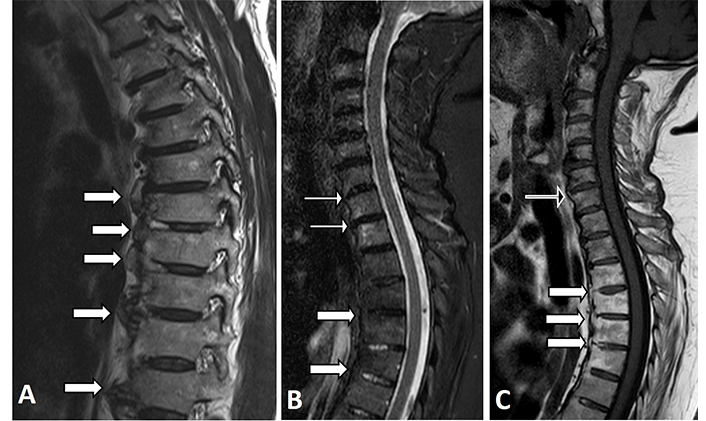
Thoracic spine MRI images of two patients with DISH. (A) Sagittal T1-W MRI image showing the most common presentation of flowing osteophytes with a regular bone marrow signal (thick white arrows); (B) sagittal STIR and (C) T1-W MRI images of another patient in which the fatty normal marrow can be seen in the upper thoracic vertebras (thick white arrows), while some mild BME can be seen in the endplates of the cervical vertebral corners (thin white arrows in Figure 6B), and a corner containing mild fat metaplasia can be detected at the base of an osteophyte of C7 (black arrow in Figure 6C)
These findings are commonly seen in the elderly population, and they may be attributed to mechanical changes and advanced age and not necessarily to DISH [49, 50]. Either way, the presence of only a few vertebral BME and fat corners on an MRI should neither establish the diagnosis nor differentiate between SpA or DISH, while the presence of many vertebral BME corners (above 3 BME corners and 5 fat corners) would be more strongly suggestive of SpA [40].
Contrast enhancement is a characteristic finding on MRIs of enthesopathy and inflammation. Since both DISH and SpA are enthesopathies, albeit with different pathogenesis, it is possible that contrast injection may help in differentiating between them. However, the role of MRI contrast material injection in differentiating between DISH and SpA has not yet been evaluated.
In a study that compared whole-body MRI findings between subjects with DISH and those with AS, lesions located in the posterior corners of the thoracic spinal vertebras (either BME or fatty lesions) as well as intervertebral and facet joint ankylosis were rarely found in the ones with DISH [45]. The depiction of those lesions highlights the most promising feature of the future role of MRI in differentiating between the two entities.
Erosions of the SIJ are quite specific to SpA and were not characteristically detected on CT in subjects with DISH [51]. Thus, the presence of erosions on MRI may help in ruling out a diagnosis of DISH. Indeed, CT is currently the gold standard imaging modality for the detection of erosions, although new erosion-sensitive sequences on MRI show comparable ability for its detection [52, 53].
Bridging osteophytes appear in subjects with DISH not only along the spine but anterior to the SIJ as well [51, 54, 55]. Nevertheless, the identification of osteophytes or syndesmophytes on MRI is difficult and easy to miss, both in the spine and in the SIJ (Figure 7) [56]. Therefore, CT rather than MRI would be the imaging modality of choice for their detection.
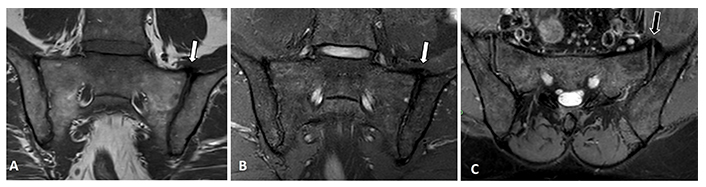
MRI images of the sacroiliac joints of a patient with DISH. (A) Coronal oblique T1-W and (B) T2-W with fat suppression and (C) an axial oblique T2-W with fat suppression MRI sequences. An anterior bridging osteophyte can be seen although not easily detected in the left SIJ on the semi coronal plane (white arrows), while it is somewhat easier to detect on the axial oblique plane (black arrow)
MRI characteristics differentiating between DISH, SpA, and degenerative spinal disease are summarized (Table 1).
MRI characteristics of the SIJ and the spine in patients with DISH vs. SpA and degenerative disease
| MRI lesions | DISH | SpA | Degenerative changes | |
|---|---|---|---|---|
| SIJ | Periarticular BMESuggestive of SpA | RareUpper part of SIJ | CommonAlong entire SIJ | RareUpper part of SIJ |
| Erosions | Rare | Common | Rare | |
| Bone production | Bridging osteophytes anterior to SIJ | Intra-articular bridging osteophytes | Small periarticular osteophytes | |
| Periarticular fat metaplasia | Rare | Common | Rare | |
| Spine | Bone production | Thick, bridging horizontal osteophytes | Thin, slender, vertical syndesmophytes | Horizontal osteophytes |
| BME corners | Present but uncommon | Common | Present but uncommon | |
| Fat corners | Present, not rare | Common | Present, not rare | |
DISH mainly affects the elderly (men more than women) and its prevalence increases with age [20, 57]. Thus, differentiating between DISH and degenerative disc disease as a cause of clinical presentation is a common diagnostic challenge.
The classification criteria of Resnick and Niwayama aim at differentiating between DISH and degenerative and inflammatory diseases of the spine [1]. The first criterion of four or more flowing osteophytes differentiates DISH from spondylitis deformans in which spinal osteophytes are increasingly more prevalent with age due to degenerative disc disease [58]. The second criterion relates to disc height preservation and the absence of degenerative disc-related changes, and it aims to differentiate DISH from vertebral osteochondrosis thought to result from age-related disc dehydration [58]. However, the Resnick and Niwayama criteria establish the diagnosis of DISH in its end stage when intervention is of little use [59]. The rate of new bone formation in DISH and AS was reported to be quite similar, with approximately 10 years of progression for a single bridging osteophyte to develop [37, 60]. That means that using the current Resnick and Niwayama classification criteria will delay the identification of DISH by at least 10 years. Other classification criteria were suggested for the purpose of earlier diagnosis of DISH [61–63], however, there is currently no consensus on which diagnostic criteria should be applied [61]. It was suggested that MRI may be of assistance in identifying earlier signs of DISH and, indeed, it is our impression that in some patients with DISH, as in those with extra-spinal enthesitis [64], inflammation can already be detected at the base of an osteophyte or in its soft tissue surrounding. However, there are currently no published reports on early MRI signs of DISH. Prospective studies targeting a population at risk of developing DISH, such as patients with the metabolic disease [7], are warranted and may help in characterizing early, preliminary stages of DISH as well as in deciphering its etiopathogenesis.
MRI is currently the recommended imaging modality for the clinical evaluation of symptomatic painful or post-traumatic patients with DISH. MRI may assist in differentiating between DISH and AS when other imaging modalities fail. Establishing a correlation between imaging characteristics of DISH with its clinical manifestations may facilitate early diagnosis of DISH as well as promote the understanding of the pathogenesis of DISH.
AS: ankylosing spondylitis
BME: bone marrow edema
CT: computed tomography
DISH: diffuse idiopathic skeletal hyperostosis
MRI: magnetic resonance imaging
OPLL: ossified posterior longitudinal ligament
SIJ: sacroiliac joints
SpA: spondyloarthritis
IE: Conceptualization, Investigation, Writing—original draft, Writing—review & editing.
The author declares that she has no conflicts of interest.
The use of anonymized images was approved by the local ethics committee (9916-22-SMC) and this study complied with the Declaration of Helsinki.
Patient’s consent was waived by the Sheba Medical Center’s institutional ethics committee due to the retrospective nature of the manuscript.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Netanja I. Harlianto ... Jorrit-Jan Verlaan
Bruna Parreira ... Jácome Bruges-Armas
Fernando Pérez-Ruiz ... Ana María Herrero-Beites
David Kiefer ... Xenofon Baraliakos
Fabiola Atzeni ... Reuven Mader
Greta Pellegrino ... Piercarlo Sarzi-Puttini
