Affiliation:
1Ruhr-Universität Bochum, Am Marienhospital Herne, Hölkeskampring 40, 44625 Herne, Germany
2Rheumazentrum Ruhrgebiet, Claudiusstrasse 45, 44649 Herne, Germany
Email: David.kiefer@elisabethgruppe.de
ORCID: https://orcid.org/0000-0003-1602-7649
Affiliation:
3Case Western Reserve University, Cleveland, OH 44106, USA
ORCID: https://orcid.org/0000-0003-4704-8311
Affiliation:
1Ruhr-Universität Bochum, Am Marienhospital Herne, Hölkeskampring 40, 44625 Herne, Germany
2Rheumazentrum Ruhrgebiet, Claudiusstrasse 45, 44649 Herne, Germany
ORCID: https://orcid.org/0000-0002-9475-9362
Explor Musculoskeletal Dis. 2023;1:194–206 DOI: https://doi.org/10.37349/emd.2023.00022
Received: July 15, 2023 Accepted: September 12, 2023 Published: November 20, 2023
Academic Editor: Fernando Pérez-Ruiz, Cruces University Hospital, Spain
The article belongs to the special issue Diffuse Idiopathic Skeletal Hyperostosis- A common but neglected disease
Diffuse idiopathic skeletal hyperostosis (DISH) and axial spondyloarthritis (axSpA) are diseases with inflammatory involvement of the axial skeleton that can result in new bone formation that may lead to total ankylosis of the spine and functional impairment of different extent in individual patients. In these diseases, the new bone formation may lead to total ankylosis of the spine and impaired mobility with functional impairment. This review will highlight the similarities and differences of these two conditions. In axSpA, the genetic background with the association with human leukocyte antigen-B27 (HLA-B27) is known for 50 years, while in DISH, a genetic contribution is not yet proven. The phenotype of new bone formation and its anatomic features are different between these diseases. In axSpA symmetric, thin and marginal syndesmophytes representing an ossification of enthesitic inflammation at the area of the attachment of the annulus fibrosus that may extend to the adjacent deeper layers anterior longitudinal ligament and are typical, while in DISH the so-called “chunky bridging osteophytes” grow as an additional layer on the anterior longitudinal ligament. Besides distinct anamnestic and clinical features, magnetic resonance imaging is helpful differentiating the two diseases since inflammatory changes with the typical pattern of axSpA are reliably visualized. Similar in both diseases is the high prevalence of vertebral fractures, which are mainly caused by the local osteoporosis and decreased flexibility of the affected bones, and therefore may occur even after minor traumata. The presence of extraarticular manifestations like uveitis, inflammatory bowel disease or psoriasis are only linked to axSpA. In contrast, DISH is associated with obesity, diabetes mellitus, and other metabolic diseases. Although DISH and axSpA are distinct conditions, the cooccurrence of these two diseases exists in some patients. Various therapeutic options are becoming available for axSpA, but no therapy has been approved for DISH yet.
Diffuse idiopathic skeletal hyperostosis (DISH), initially known as ankylosing hyperostosis or Forestierʼs disease is characterized by excessive bone formation (hyperostosis) at entheses (where tendons and ligaments attach to bones) in the spine, and less often the ischial tuberosity, femoral trochanter, tibial tubercle and calcaneum [1–3]. The current name emphasizes that extraspinal hyperostosis may occur without appreciable spinal changes; therefore, a new name—DISH—is preferred. DISH can be confused with ankylosing spondylitis (AS) and related forms of spondyloarthritis (SpA) with involvement of the spine. There are no diagnostic criteria do not exist but imaging both with conventional radiographs or by magnetic resonance imaging (MRI) is similarly crucial for its identification. Although DISH and axial spondyloarthritis (axSpA) are distinct conditions, cooccurrence of these two diseases has been reported in some patients due to chance cooccurrence of two relatively common diseases [4, 5].
DISH and axSpA are two musculoskeletal disorders that have partly distinct epidemiological and demographic profiles. DISH primarily affects older individuals, while axSpA affects a younger population. DISH has a prevalence ranging from 3.8% [6] to 30% [7–15] in people aged > 50 years. The prevalence of DISH is increasing with age [16]. Still, despite that DISH is more prevalent in older patients, it has also been described in patients < 50 years [16] and even cases of DISH at the age of under 40 have been reported [17, 18] which can make it sometimes difficult to differentiate it from axSpA. While in Caucasian and Asian populations the prevalence of DISH is higher in men, this ratio seems to be more balanced in Sub-Saharan Africans and African Americans [6, 19]. AxSpA is comprising from both non-radiographic (nr-) and radiographic (r-) axSpA, with an estimated prevalence between 0.1% and 1.4% in different parts of the world [20]. AxSpA mainly starts in the third decade of life, rarely (< 5%) with an onset older than 45 years, but in 10–20% of patients even between the ages of 10 years and 20 years [21]. The male/female ratio is estimated to be 2:1 in r-axSpA, with an equal sex distribution among patients with nr-axSpA, indicating that women (for still unclear reasons) develop chronic changes later and less frequently [22, 23].
DISH and axSpA share common clinical features, mainly back pain, but also impaired physical function, although there are also many differences in their clinical appearances and manifestations. Peripheral arthritis is very common in axSpA, but also found in DISH [24]. In both diseases, but especially in DISH, asymptomatic forms can be seen in clinical practice, being revealed by chance in performed imaging. Both DISH and axSpA can cause spinal stiffness and chronic pain, particularly in the lower back region. The pain in SpA is typically inflammatory in nature and may be accompanied by morning stiffness that improves with exercise, while DISH pain is usually mechanical and worsens with physical activity [23, 25]. While inflammatory back pain is a cardinal feature of axSpA and included in the classification criteria [26], DISH is not associated with typical inflammatory back pain [27]. Both conditions can lead to reduced spinal mobility, affecting the range of motion of the neck, back, and hips, resulting in impaired physical function [11, 28–30]. Chest expansion, typically reduced in advanced stages if axSpA, may also be reduced in up to 50% of patients with DISH [3] mainly due to the characteristic involvement of consecutive vertebral segments [31]. Peripheral manifestations like arthritis, dactylitis, and enthesitis are more common in SpA than in DISH. In both conditions, the affected joints are usually not affected by primary osteoarthritis [24, 32]. In DISH, enthesopathies at various sites including entheseal insertions of joint capsules, and tendons attachments are showing calcification and ossification [24]. Similar to axSpA, also Achilles tendon and plantar fascia are affected [2]. While in daily practice arthritis and enthesitis are more common in axSpA than in DISH, studies with small numbers of patients demonstrated the presence of inflammatory signal on power Doppler but also presence of erosions also in DISH-associated enthesopathies [33]. In addition, typical extra-musculoskeletal manifestations of axSpA, such as uveitis, psoriasis, and inflammatory bowel disease are not found in patients diagnosed with DISH. These extra-musculoskeletal manifestations can significantly impact the patient’s health-related quality of life (HRQoL), contributing to physical and psychological distress [23]. The cervical spine involvement of DISH may in rare cases develop giant osteophytes that induce dysphagia as well as other airway compressions which might result in sleep apnea, aspiration pneumonia and stridor and may lead to surgery [1, 27, 34]. Ozgocmen coined the term “DISHphagia” to describe the dysphagia observed in association with DISH [35, 36].
While physical function and spinal mobility are impaired by both diseases, reports on the impairment in quality of life are more frequent for patients with axSpA, while only small studies exist for DISH patients, indicating that HRQoL in those patients is not reduced in comparison to matched patients without DISH [30]. Progressive fusion of the spinal column and altered biomechanical characteristics increase the risk of vertebral fractures in both DISH and axSpA as compared to the general population. These fractures are more frequently accompanied with a higher risk of complications such as neurological deficits due to associated spinal cord injury [37–39]. Fractures may occur after even a minor or trivial trauma [38, 40]. A retrospective analysis has shown that 15% of patients with DISH presenting to a particular neurosurgical unit had serious neurologic manifestations requiring neurosurgical intervention [41]. The ossification of the ligamentum flavum and an association of ossification of the posterior longitudinal ligament (OPLL) with DISH may explain, in part, the occasional presence of spinal stenosis, spinal root compression with posterior segment involvement, and resultant neurologic findings in patients with DISH [42].
While both DISH and axSpA are characterized by formation of new bone tissue in the axial skeleton, the underlying pathogenetic pathways seem to be different. In axSpA, up to 90% of the pathogenesis is genetically determined, with human leukocyte antigen-B27 (HLA-B27) being by far the most important risk factor [43, 44]. The chance of developing SpA in HLA-B27-positive individuals is about 5%, in HLA-B27-positive first-degree relatives from r-axSpA patients it is 12–27%, while in HLA-B27-negative relatives from r-axSpA patients it is less than 1% [45, 46]. The most recent reports show a very convincing evidence that HLA-B27 is directly involved in disease pathogenesis because it presents microbial peptides that act as a trigger for autoimmunity by activating CD8+ T-cells that cross-react with self-peptides and result in AS/SpA [47]. In addition, microbiomic dysbiosis, other genetic and epigenetic factors, and entheseal stress and micro-damage influence disease induction and progression.
Gut bacteria seem to be an important environmental factor, since axSpA can occur in the context of reactive arthritis, inflammatory bowel disease (IBD) and clinically relevant or subclinical colitis [23, 48]. Although its exact role has not yet been identified, there is an increasing interest to investigate the role of microbiota in the pathogenesis of SpA [49, 50].
DISH is considered a metabolic condition, as multiple associations between DISH and metabolic syndrome, cardiovascular disorders, high body mass index (BMI) and type 2 diabetes mellitus (DM) have been regularly reported [15, 51, 52]. DISH is associated with overweight and obesity [51, 53–55] in particular in patients with obesity measured by waist circumference [51, 56]. Furthermore, association between impaired glucose tolerance and/or type 2 DM and the prevalence of DISH, has been found [11, 51, 57]. While type 2 DM has been reported to be associated with DISH, such association has not been found in patients with type 1 DM [58]. In contrast to DISH, axSpA is not associated with metabolic disorders, and hence, its metabolic markers are usually within normal limits.
From the laboratory analyses, elevated C-reactive protein (CRP) levels is found in only about 60% of r-axSpA patients and in about 33% of patients with nr-axSpA [59], while a significant number of patients with axSpA have clearly inflammatory symptoms despite normal levels of acute phase reactants [60]. CRP and erythrocyte sedimentation rate (ESR) levels are not significantly different in DISH and controls [61]. Associations between DISH and dyslipidaemia or hyperuricaemia are also reported [52, 62]. In comparison, HLA-B27 plays no role in the pathogenesis of DISH [63].
DISH and axSpA are the prototypes of hyperostotic diseases of the axial skeleton [64]. The bone proliferation leads in the long term to ankylosis of the spine and functional impairment. Besides the axial skeleton, peripheral involvement of joints, entheses and ligaments with or without structural changes are common in both entities.
In axSpA, sacroiliitis, spondylitis, and spondylarthritis are the main inflammatory manifestations in the axial skeleton [65], which may lead to new bone formation occurring as syndesmophytes and ankylosis in the vertebral column and may lead to the development of a “bamboo spine” [66]. Peripheral manifestations are mainly found in the root joints, the hips and the shoulders, and frequently affect entheses such as the heel, the knee and the elbow.
In DISH, structural changes are typically characterized by calcification and ossification of ligaments and entheses. The condition usually affects the axial skeleton, in particular at the thoracic segment though also other portions of the spine are often involved. Like in axSpA, in DISH peripheral joint, tendinous and/or entheseal involvement may occur [24].
Typical for DISH are the so-called “chunky bridging” osteophytes’ which grow as an additional layer on the anterior longitudinal ligament mostly starting at the thoracic spine. Interestingly, whereas the hyperostosis in the cervical spine is symmetrically distributed anterior to the vertebral bodies without a flowing pattern, the involvement in the thoracic spinal exhibits an asymmetrical flowing pattern because the vascular system (the descending aorta) acts as a natural barrier against new bone formation in DISH [67]. There is also a significant ventral displacement of the trachea and esophagus that may explain the mechanism of dysphagia and airway obstruction that can occur in some patients with DISH.
In axSpA the structural changes of the axial skeleton usually start in the sacroiliac joint (SIJ) and lumbar spine [32]. Sacroiliitis in axSpA is the key features of the disease and typically also present in early stages [68], and in later stages the frequently thin, marginal and often symmetrical syndesmophytes are characteristic [24]. In DISH, pelvic enthesopathies are considered to be highly characteristic of DISH mostly seen as an ossification or calcification of the entheses [69, 70]. Similar to axSpA, the SIJ may also be involved in DISH, with enthesopathies of the anterior and posterior ligaments and capsule, as well as bridging, fusing enthesophytes within the joints mimicking joint ankylosis of axSpA [64, 71, 72].
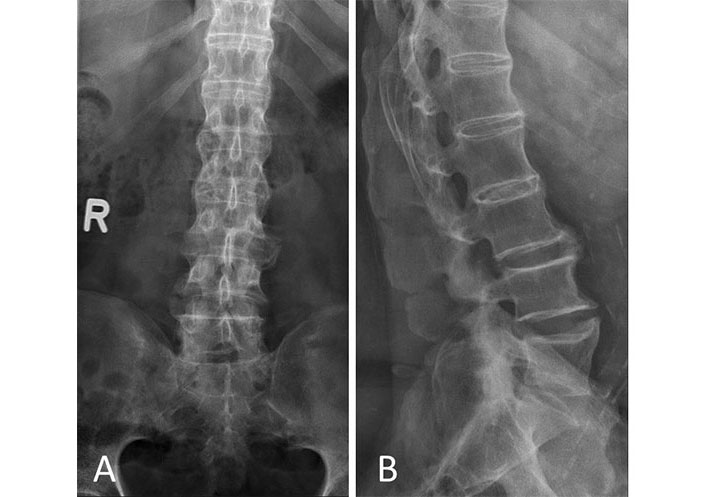
Different imaging techniques are relevant for diagnosis, classification, assessment of disease activity and structural damage, and prognosis of patients with DISH and axSpA. Conventional radiography (CR) mainly is still the initial imaging modality and the gold standard for the assessment of structural changes in both DISH and axSpA (Figures 1 and 2). With CR, typical findings in axSpA are sclerosis, erosions, and pseudowidening joint, progressing later to ankylosis of the SIJ and spinal involvement that can result in formation of bony bridges (syndesmophytes) and spinal fusion [24, 32]. For the classification of axSpA CR of the SIJ is used to grade structural damage according the New York criteria [73]. Furthermore, CR is used in clinical trials to calculate the modified stoke AS spinal score (mSASSS), a scoring method for grading the radiographic changes in the spine [74]. CR in patients with DISH can show the characteristic flowing osteophytes along the anterior aspect of the vertebral bodies and ossification of the anterior longitudinal ligament. It may also visualize hyperostosis at the sites of entheses, such as the iliac crest and ischial tuberosity [24]. For clear identification of DISH, CR of the thoracic spine is used for the classification in order to prove the occurrence of the Resnick criteria [31].
X-ray of a patient with AS. (A) Anterior-posterior with syndesmophytes and sacroiliitis; (B) lateral view with syndesmophytes. R: right side
X-ray. (A) Anterior posterior view; (B) lateral view. Patient with DISH showing characteristic flowing osteophytes and ossification of the anterior longitudinal ligament. R: right side
In axSpA, computed tomography (CT) scans of the SIJ and spine have been found to be superior to CR for the detection of structural changes because of the multidimensional imaging of anatomic structures [75–77] but is mainly used for research purposes. For the diagnosis of DISH, the use of CT is superior to CR with less intra- and inter-observer error compared to CR and may also have advantages at early disease stages [16, 78].
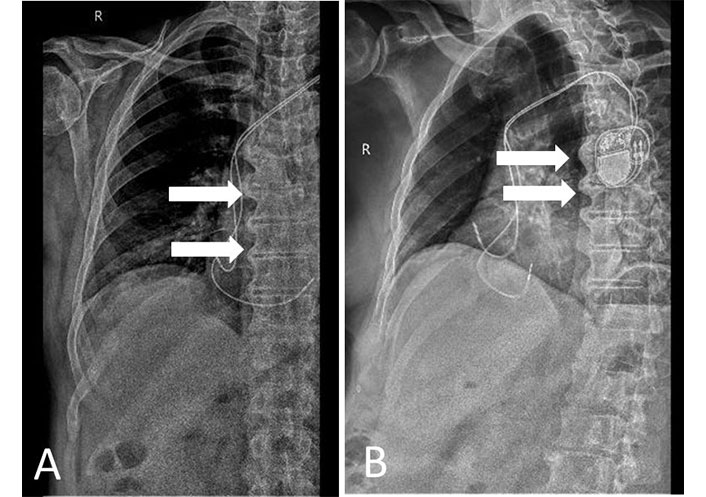
Both for classification and diagnosis MRI has become the most important tool for axSpA. In contrast to CR and CT, MRI of the axial skeleton and peripheral joints can show active inflammation in the form bone marrow edema (BME) as a sign of osteitis. Besides BME, MRI is capable of detecting enthesitis, synovitis and capsulitis. Inflammation of the SIJ is the key feature of axSpA may be visualized with MRI (Figure 3). Typical findings of disease activity when using spinal MRI in patients with axSpA are spondylitis (Figure 4), inflammation of the facet joints and aseptic spondylodiscitis but inflammation of costosternal and costovertebral joints are also commonly seen. To increase specificity for clinical trials Assessment of SpA international Society (ASAS) recently published the definition of a positive MRI of the SIJ and spine in axSpA [79]. Furthermore, MRI is also capable for the detection of structural changes such as erosions [80]. The contextual interpretation of BME and erosions are of high relevance in differential diagnostic consideration, and also in the differentiation to DISH. To strengthen specificity for axSpA the ASAS MRI working group has recently published new definitions for MRI lesions in the SIJ and spine [81].
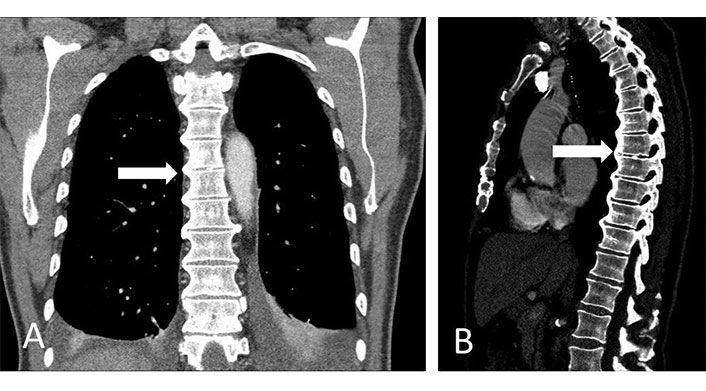
CT spine. (A) Coronal view; (B) sagittal view. Patient with DISH showing characteristic flowing osteophytes and ossification of the anterior longitudinal ligament
MRI turbo inversion recovery magnitude (TIRM) sequences. (A) Lateral view; (B) SIJs coronal view. Patient with axSpA and BME showing spondylitis and sacroiliitis
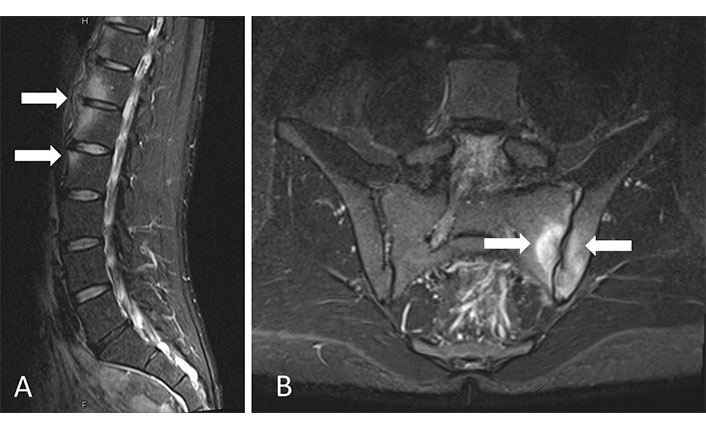
Some preliminary MRI studies have demonstrated BME in MRI of SIJ and spine MRI in patients with DISH suggesting a local inflammatory process (Figure 5) [82]. Comparing axSpA and DISH, patients with axSpA were more likely to present non-corner ankylosis, facet joint ankylosis, upper corner fat infiltration and SIJ BME, however, these features were not completely absent in DISH [83]. However, the presence of BME in DISH needs further investigation.
There is increasing evidence that mechanical stress and microtrauma of cartilage/bone at weightbearing parts of the skeleton [84] might be possibly initiating factor for the onset and progression of axSpA [85, 86]. Inflammation in the bone can be followed by new bone formation as part of a repair process. However, the complex pathogenic process between inflammation and transformation to structural changes in axSpA is still incompletely understood [87].
New bone formation and the rate of progression vertebral bony bridging is quite similar between DISH and axial SpA [64, 88]. The pathways of radiographic progression are under investigation for axSpA but so far there is no clear biologic explanation for radiographic progression in DISH.
The management of axSpA comprises pharmacological and non-pharmacological treatment options. Recently, the ASAS-European League Against Rheumatism (EULAR) recommendations for the management of axSpA has been updated [89]. Besides non-pharmacological treatments such as physiotherapy and physical activity, the first line treatment option are still non-steroidal anti-inflammatory drugs (NSAIDs) followed by biologic disease-modifying antirheumatic drugs (bDMARDs) like tumor necrosis factor inhibitors (TNFi) and interleukin-17 inhibitors (IL-17i) and targeted synthetic (ts)-disease-modifying antirheumatic drugs (DMARDs; JAK-inhibitors) [90].
Due to the lack knowledge about underlying pathologies and the lack of clinical trials, there are no recommendations or guidelines for non-pharmacological and pharmacological treatment of DISH [91]. Although there is no evidence for the efficacy of NSAIDs, they might be used for back pain or for symptomatic peripheral enthesopathies. So far, no further treatment options like the use of DMARDs, biologic- or ts-DMARDs are approved for the management of DISH.
DISH and axSpA have distinct epidemiological and demographic profiles. Both diseases share some clinical features, such as spinal stiffness and chronic pain, but differ in their underlying pathology and age of onset. DISH and axSpA are diseases defined by osteoblastic features of the axial skeleton that may lead to ankylosis and functional impairment, DISH typically is first to be detected in the thoracic spine while axSpA starts in the SIJ and lumbar spine. While both diseases may present peripheral manifestations, extraarticular manifestations like uveitis, IBD or psoriasis are only linked to axSpA. In contrast to axSpA, DISH is associated with obesity, DM and other metabolic diseases. In axSpA, there are various treatment options in the management in axSpA while no therapy has been approved for DISH yet.
AS: ankylosing spondylitis
ASAS: Assessment of SpondyloArthritis international Society
axSpA: axial spondyloarthritis
BME: bone marrow edema
CR: conventional radiography
CT: computed tomography
DISH: diffuse idiopathic skeletal hyperostosis
DM: diabetes mellitus
DMARDs: disease-modifying antirheumatic drugs
HLA-B27: human leukocyte antigen-B27
MRI: magnetic resonance imaging
nr-: non-radiographic
r-: radiographic
SIJ: sacroiliac joint
SpA: spondyloarthritis
DK, MAK and XB: Writing—original draft, Writing—review & editing.
The authors declare that they have no conflicts of interest.
The manuscript does not require ethical approval in accordance with the Article 15 of the Professional Code of Conduct for Physicians of the Westphalia-Lippe Medical Association.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Iris Eshed
Netanja I. Harlianto ... Jorrit-Jan Verlaan
Bruna Parreira ... Jácome Bruges-Armas
Fernando Pérez-Ruiz ... Ana María Herrero-Beites
Fabiola Atzeni ... Reuven Mader
Greta Pellegrino ... Piercarlo Sarzi-Puttini
