Affiliation:
1Department of Orthopedic Surgery, University Medical Center Utrecht, 3584 CX Utrecht, The Netherlands
2Department of Radiology, University Medical Center Utrecht, 3584 CX Utrecht, The Netherlands
Email: N.I.Harlianto@umcutrecht.nl
ORCID: https://orcid.org/0000-0001-8196-3949
Affiliation:
1Department of Orthopedic Surgery, University Medical Center Utrecht, 3584 CX Utrecht, The Netherlands
ORCID: https://orcid.org/0000-0002-5961-0677
Affiliation:
1Department of Orthopedic Surgery, University Medical Center Utrecht, 3584 CX Utrecht, The Netherlands
ORCID: https://orcid.org/0000-0001-8105-6660
Explor Musculoskeletal Dis. 2023;1:84–96 DOI: https://doi.org/10.37349/emd.2023.00013
Received: April 07, 2023 Accepted: August 04, 2023 Published: August 29, 2023
Academic Editor: Reinhard Graf, Academic hospital Stolzalpe, Austria
The article belongs to the special issue Diffuse Idiopathic Skeletal Hyperostosis- A common but neglected disease
Diffuse idiopathic skeletal hyperostosis (DISH) can lead to dysphagia, airway obstruction, and unstable vertebral fractures. Surgery can be performed to relieve cervical compression or stabilize fractures of the spinal column, with or without decompression of spinal cord injuries. In this review, the peri-operative surgical techniques in cases with DISH are discussed, as well as the pre-operative and post-operative pearls and pitfalls. It is essential for spine surgeons, including orthopedic surgeons and neurosurgeons, to be aware of the considerations, anticipations, and approaches for the management of dysphagia, airway obstruction, and fractures in DISH patients in order to improve patient outcomes for this specific at-risk patient population.
Forestier and Rotes-Querol [1] first described a case of spinal enthesis and hyperostosis in 1950, which later came to be termed diffuse idiopathic skeletal hyperostosis (DISH). Classification criteria for DISH were established by Resnick and Niwayama [2] back in 1976, including presence of anterolateral ossification of at least four contiguous vertebrae; (relative) preservation of the intervertebral disc height; and the absence of apophyseal joint bony ankylosis or sacroiliac joint erosion. To this date, it is unclear what the precise pathophysiology underlying the anterolateral new bone formation and ectopic calcification in DISH is. Patients affected with obesity, hypertension, and metabolic syndrome are more at risk for the development of DISH [3–5]. These associations suggest the involvement of inflammatory and metabolic components in the process of new bone formation [3–5]. Moreover, increasing evidence is showing that patients with DISH have a relatively large burden of cardiovascular disease, including an increased risk for incident ischemic stroke [6, 7]. Extraspinal hyperostosis, including hypertrophic or atypical osteoarthritis, has also been reported in peripheral joints of DISH patients [3, 8]. Bone mineral density (BMD) may be inaccurately measured in DISH patients, as the local surplus of bone in the scanning field could overestimate the true BMD value [9], which is important for risk stratification of osteoporosis and fracture prevention.
Surgical treatment in DISH is most often performed for symptoms of DISH in the cervical region including dysphagia and airway obstruction, and also for fractures of the spinal column. The increased prevalence of metabolic comorbidities in patients with DISH warrants extra attention in the general work-up for surgery. DISH requires additional considerations regarding pre-, peri-, and post-operative management, as DISH is an important modifier for treatment strategy and clinical outcome success [3]. The aim of this narrative review is to provide an overview of pre-operative considerations, surgical management, complications, and post-operative management for the most common disorders in DISH.
The presence of anterolateral ossification or osteophytes may obstruct and impinge the esophagus and/or airway, which can result in symptoms of dysphagia and dyspnea. Symptom severity for dysphagia may range from some difficulty to swallow solids to the inability to ingest liquids for the most severe cases. Two systematic reviews have been performed including a total of 623 DISH patients with dysphagia and/or airway obstruction reported between 1980 and 2021 [10, 11]. The prevalence is estimated to be 7:100,000 and the mean age of presentation is 67.3 years, ranging from 35 to 91 years in literature [11]. Diagnosis is most frequently established using plain cervical radiographs, followed by cervical computed tomography (CT) and barium swallow radiographs. Symptoms usually include weight loss, neck pain, limited range of motion, and dysphonia [10, 11]. To date, no guidelines have been established for treatment. Non-surgical treatment includes dietary measures, nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, anti-reflux drugs, muscle relaxants, and postural changes. Surgery is usually indicated after conservative treatment of dysphagia has failed, or if symptoms progress [11, 12]. Symptom progression may include the onset of neurologic symptoms or worsening of dysphagia, e.g., from solid to liquid dysphagia [11]. Some authors recommend surgery even in the case of mild dysphagia symptoms as DISH is known for its progressive nature [12]. Surgery aims to alleviate the mechanical pressure of the osteophytes on the soft tissues, trachea, and esophagus [13].
In patients with DISH, traumatic vertebral fractures are frequently observed after minor trauma, as the energy distribution of traumatic energy is altered in the fused spine in DISH resulting in high local mechanical stress peaks [14, 15]. Hence, the ankylosed spine has been recognized as an important modifier for patient outcomes following vertebral fractures. Vertebral fractures in DISH are most commonly hyperextension fractures (type B3 fractures according to the AO Spine Classification) followed by displacement fractures (type C fractures according to the AO Spine Classification) [15–17]. For reference, in non-ankylosed spines, the proportion of hyperextension fractures is rare, being less than 1% in some cohorts [18]. Furthermore, injuries at the level of the cervical spine are more frequent compared to thoracolumbar fractures, with the spine levels of C5–7 most often affected [17].
Trauma patients with DISH are more likely to have worse clinical outcomes compared with trauma patients without DISH, including increased complications and mortality. Neurological deficit at the time of admission is present in a high percentage of DISH patients with vertebral fractures (around 40%, ranging from 21% to 100% in various cohorts) [15, 16]. Extended delay of clinical presentation may be observed in patients with DISH due to three main causes. First, because patients with DISH may suffer from pre-existing back pain making recognition more difficult. Second, a fracture can result from minor/trivial trauma, which may, upon presentation, lead to a low clinical suspicion of vertebral fractures. Finally, delays may also occur because vertebral fractures in the ankylosed spine are more easily missed on imaging, due to subtle abnormalities or the overprojection of newly formed bone [15, 18, 19]. Presentation delays are reported to range from 15–41% in various cases in the literature [17].
Compared to spinal fractures in non-DISH patients, mortality is also increased in patients with an ankylosed spine, ranging from 0 to 32% at the timepoint of 1 year post-surgery. In the review of Rustagi et al. [16] 84% of all trauma patients suffered from complications, which most frequently included pneumonia and respiratory failure [17].
Patient comorbidities are also important to take into account whether surgical treatment should be initiated or not. In the review by Westerveld et al. [15], medical comorbidities were attributed as the leading factor of opting for conservative treatment in 46% of vertebral fractures.
Conservative treatment is in certain cases used to bridge the time between admission to surgery, and most frequently consists of halo immobilization in the case of cervical fractures, and immobilization with plaster jackets or braces for thoracic and lumbar fractures [17, 20].
Surgery was initially initiated only for cases with progressive neurological symptoms, or in cases of inadequate reduction or instability of the fracture hampering patient mobilization due to pain [15]. Current management strategies usually recommend surgery as first-line treatment, depending on the fracture classification and type of patient. Surgical treatment aims to stabilize the spine and prevent (further) neurological damage. Furthermore, immediate surgery prevents prolonged bedrest and hospitalization of non-surgically treated patients. Nonetheless, recommendations have been put forth regarding treatment algorithms, where surgery within 24 h is recommended in patients with incomplete spinal cord injuries (SCI). In cases of complete SCI decompressive and stabilizing surgery should be performed when the patient is physiologically and hemodynamically stable [19, 21]. A summary of the pre-, peri, and post-operative considerations is provided in Figure 1.
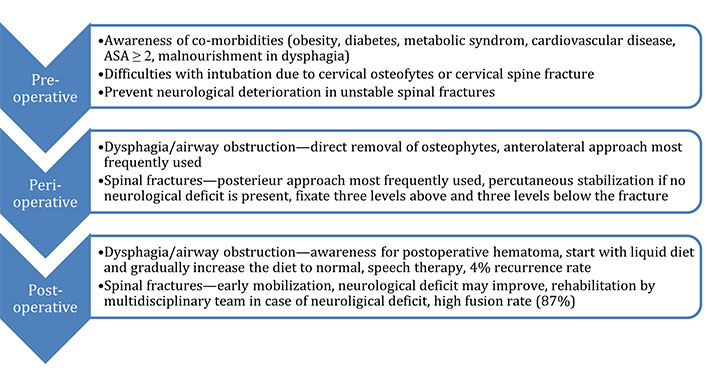
The pre-, peri- and post-operative considerations in the surgical treatment of patients with DISH
Patients with DISH have an increased risk for cardiovascular disease, obesity, type 2 diabetes, and metabolic syndrome [4, 5], and are most likely classified according to the American Society of Anesthesiologist Classification (ASA) as ASA 2 or higher. For the spine surgeon, it is important to be aware that patients with metabolic syndrome have an increased risk for complications following surgery [22, 23]. Blood pressure regulation as well as adequate glucose regulation are essential to minimize complications, including poor wound healing after surgery [24]. Similarly, anterolateral bone formation at the level of the cervical spine, may result in compression of the esophagus and trachea. Therefore, patients with cervical DISH may be malnourished as a result of swallowing dysfunction, for which preoperative nutritional support may improve muscle mass and wound healing after surgery and reduce complication risk, including ulcers and infections [25].
In patients with cervical DISH, passing devices through the esophagus and airway may prove difficult, as a result of narrowing of these structures due to compression of expanding cervical osteophytes. There have been cases described with difficult tracheal and fiberoptic intubation, which may result in failure to secure the airway for which an emergency tracheotomy would be required [26–30]. In cervical spine surgery for dysphagia, eight percent of DISH patients required a tracheostomy to secure the airway [11]. Limiting movement during airway management is important in order to prevent potential cervical injury, including fractures, secondary neurological injury or esophageal perforation [29]. Furthermore, endoscopy, laryngoscopy and transesophageal echocardiography may also cause perforation [31, 32].
For surgical management, close collaboration between specialists from neurosurgery and orthopedic surgery is recommended to optimize the surgical process and improve patient care. Ideally, spine surgery is performed by dual-attending surgeons from both neurosurgery and orthopedic surgery, with the inclusion of otolaryngology specialists at the level of the cervical spine [33]. All treating physicians should be involved in the surgical planning for DISH patients, which includes the diagnostic aspects, consideration of surgical approaches, method of osteophyte resection, and awareness of the role of each specialty within the care team, especially in complex cases. It is also important to anticipate the different anatomy which is likely be encountered intraoperatively, and discuss beforehand which aspects of surgery are more prone to error and complications, and require additional care and considerations. It is mandatory to plan preoperatively and to take patient positioning into account, to avoid complications as overstraining and implant failure.
Surgery in patients with DISH is performed both in supine and prone position. For the removal of cervical osteophytes the supine position is most commonly used. Cervical lordosis of the spine is increased by the use of extra supporting cushions between the shoulders to accentuate lordosis and provide better access. Slight head rotation can be hampered in cases with extensive ossification.
Patient positioning is an important aspect to consider when trauma patients present with unstable vertebral fractures, to prevent secondary neurological damage. Hence, manual in-line stabilization is usually recommended in the case of vertebral fractures to prevent further displacement of the fracture [29]. In case of a fracture of the thoracolumbar spine, most often the patient is placed in prone position to allow for a posterior approach [34, 35]. For surgery that requires (antero)lateral exposure, the supine and prone position could also be used [35]. Specifically for the cervical spine, hyperextension of the neck should be avoided to prevent further injury. Moreover, it is important to take existing spinal deformities into account, which should be adapted to when positioning the patient intraoperatively.
Fluoroscopy is frequently used intraoperatively to assist with visualization during cervical osteophyte removal, mainly to prevent damage to the vertebral body and disc space in DISH [12, 36]. In spinal fractures, visualization of the spinal column is needed in percutaneous fracture stabilization. Fusion length and neurological symptoms were comparable in series comparing open and percutaneous screw fixation [37]. Penetrating endplate screws may also be an alternative option for patients with DISH, with significantly lower screw loosening rates compared to percutaneous fixation (3% vs. 49%). Though the available evidence is low and more larger studies are needed to reaffirm these findings [38].
Surgery for DISH is mainly considered for the removal of symptomatic cervical osteophytes and the treatment of unstable spinal fractures (Figure 2A and B). For the resection of spinal osteophytes various surgical approaches can be used. Osteophyte resection can be performed using a combination of osteotomes, punches, rongeurs, and high-speed drill with a diamond or matchstick burr [12, 36]. The use of curved chisels has been associated with lower operation times (on average 66 min of total operation time) compared to the high-speed burr [12].
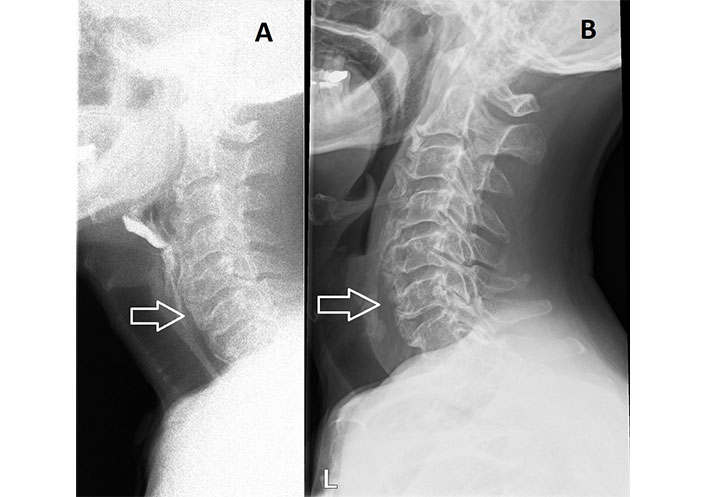
Cervical DISH. A. Preoperative barium swallow radiography showing extensive cervical DISH; B. postoperative lateral radiograph following partial anterior resection of levels C4–6
During osteophyte resection, close attention should be given to spare the annulus fibrosus in order to keep the vertebral disc intact. Damage to the intervertebral disc may result in motion segment instability, deformity, radiculopathy, and pain [39]. Furthermore, resection of the vertebral body itself should be avoided, therefore the spine surgeon must be able to differentiate between the vascular vertebral corpus and avascular overgrowth of osteophytes during surgery which can be difficult at times without adequate visualization. Minimizing the damage to the vascular vertebral body will also result in less bleeding during the operation [12]. In the case of vertebral thoracolumbar fractures, the most commonly used technique for spine stabilization includes open reduction and internal fixation, which is done by spanning at least three spine levels above and below the fracture to withstand the increased leaver forces as a result of the ankylosed spine (Figure 3A–C). Multilevel fixation may not always be necessary at the level of the cervical spine (Figure 4A–C). A summary is provided in Table 1.
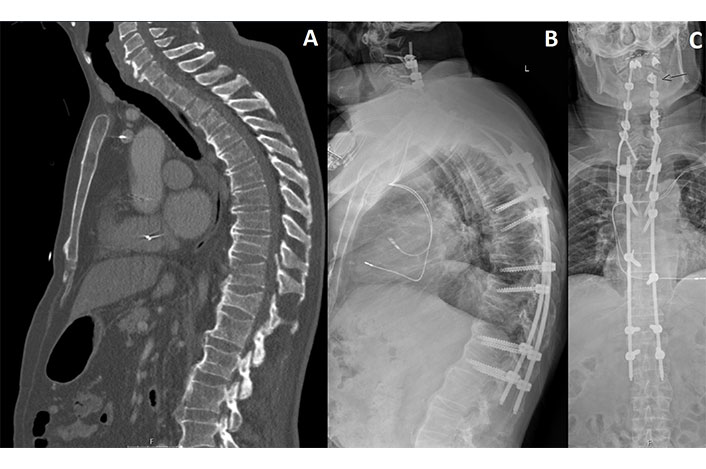
Thoracic DISH following trauma. A. Preoperative sagittal CT showing vertebral burst fractures of T8 and T11, and a compression fracture of T12; B. postoperative lateral radiograph following spinal fusion of C2–L1 with laminectomies of C3–7 and percutaneous fixation of T6–L1; C. anterior-posterior radiograph showing luxation of the C2 pedicle screw
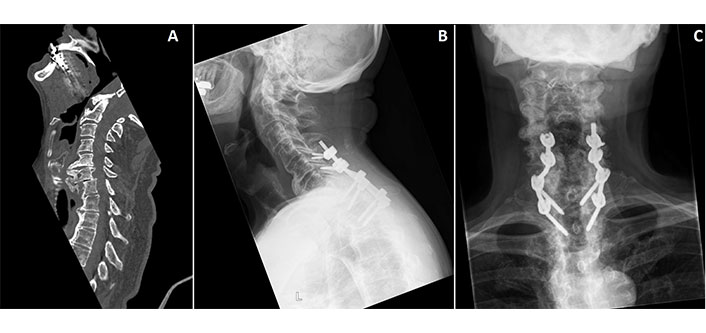
Cervical DISH following trauma. A. Preoperative sagittal CT showing a vertebral fracture of the C7 corpus, transverse process and facet joint; B. postoperative lateral radiograph following posterior fusion of C5–T2; C. postoperative anterior-posterior radiograph following posterior fusion of C5–T2
Surgical approaches and indications
| Indication | Surgical approach | Recommendation | |
|---|---|---|---|
| Dysphagia | Open | Anterolateral approach | The preferred approach given the ease of osteophyte removal and extended exposure from C2–T1 |
| Transoral approach | Indicated when exposure to the lower clivus or level C1 is needed | ||
| Vertebral fractures | Open | Posterior approach | The preferred approach for the cervical and thoracolumbar spine |
| Combined anterior and posterior approach | Recommended in patients with unstable B- and C-type cervical fractures | ||
| Anterior approach | Generally not recommended due to high failure rates | ||
| Minimally invasive | Posterior approach | Suitable for neurologically stable patients with vertebral fractures | |
The Smith-Robinson procedure, also known as the “classic” anterolateral approach, is a surgical approach that exposes the anterior vertebral bodies from C2–T1, and is the most commonly used approach for anterior cervical discectomy and fusion (ACDF) procedures. This approach can be easily used for osteophyte removal, with low risk for morbidity. Multilevel osteophyte resection can be easily performed, and this approach can be combined with other anterior procedures, including fusion, corpectomies, and discectomies when necessary. The osteophyte resection can also be combined with spinal fusion in some instances, usually when there is degenerative disease present or concerns exist for underlying instability. Plate fixation is generally used in the presence of radiculopathy and instability [40]. The structures at-risk during the surgical approach include the esophagus recurrent laryngeal nerve, sympathetic nerves, and carotid arteries [41].
General complications of this approach include dysphagia (5.3%), esophageal perforation (0.2%), recurrent laryngeal nerve palsy (1.3%), infection (1.2%), and hematoma (1%) [42].
Surgery for the anterior craniocervical junction can be performed with the transoral approach, which provides exposure to lower clivus, C1, and C2 [43, 44]. This approach is less frequently used in spine surgery. There have been few reported DISH cases with dysphagia treated with the transoral approach, for which osteophytes were at the C1 level. C1 has been identified as the cause of impingement for only 0.7% of cases, as most levels responsible for dysphagia symptoms are C3 and C4 (68%) [11]. This approach has been associated with increased mortality and morbidity and should be reserved for specific cases that cannot be approached in different way [45]. Complications from this approach include pulmonary complications (4.8%), infection (3.6%), and death (2.4%) [44–46].
The posterior approach is the most commonly used approach in trauma surgery for DISH, and provides the surgeon with multiple fixation points and excellent exposure to the spinal canal if decompression of the spinal cord is needed. The posterior approach is the first choice to treat fractures of the cervical, thoracic and lumbar spine [19]. The posterior approach is used in 11–100% of the cervical spine reports and 64–100% of the thoracolumbar spine reports [21, 47–52]. Further advantages of the posterior approach include concurrent deformity correction and the possibility to decompress the spinal cord over multiple levels. The posterior approach is also effective for fractures of the cervical spine [47, 53], and this method results in lower complication rates and length of stay compared to the combined approach, though these differences were not statistically significant [54].
The anterior approach has been described in various series and small cohorts, resulting in limited evidence. The anterior approach was used in 11–68% of the cervical spine fractures and 25% of the thoracolumbar spine fractures [48–50, 52, 55]. The anterior approach has been associated with longer operation times and increased estimated blood loss and has been related to post-operative complications like displacement of titanium plates and screw loosening, as well as internal fixation failure [55, 56]. In the study by Einsiedel et al. [57] anterior surgery alone resulted in implant failure in 50% of cases. Hence, this approach is not recommended in DISH patients with vertebral fractures in their series.
The combined anterior and posterior surgical approach is recommended in patients with cervical fractures, in particular unstable B- and C-type fractures [19]. The combined approach was used in 12–46% of the cervical fractures and 12% of the thoracolumbar fractures [21, 48, 50–52, 55]. Treatment most frequently used includes long posterior stabilization, in combination with anterior fixation [58].
Even though surgery is prolonged and more extensive compared to anterior or posterior alone, the combined approach provides more spinal stability [59]. However, patients with DISH have the tendency to form new bone and thus might not need this extensive spinal stability.
A recent meta-analysis found no differences in complications, mortality, neurological outcomes between the combined approach and the posterior approach alone in cervical fractures [60]. The less invasive and shorter intervention with the posterior approach is therefore preferred. For thoracolumbar fractures, when fracture reduction is insufficient following posterior instrumentation, a combined approach may be a possible solution to prevent implant failure and pseudoarthrosis. Anterior plating and expandable cage systems may, in these cases, provide excellent spinal stability [19].
Minimally invasive spine surgery for fracture treatment has been reported by a few small sample studies and is usually dependent on the severity of injury, surgeons preference, and geographical variation. Minimally invasive spine surgery can be performed for the posterior surgical approach, and may be a viable alternative to open surgery in patients with stable vertebral fractures. Minimally invasive surgery is most frequently performed for patients with hyperextension fractures [61–63].
Kohler et al. [61] reported 48 patients receiving posterior minimally invasive spine surgery. Perioperative outcomes were improved in the minimally invasive group, and they recommended minimally invasive surgery for neurologically intact patients with unstable spine injuries with minimal displacement.
In the series by Sedney et al. [62] and Moussallem et al. [63] patients receiving percutaneous stabilization had lower blood loss, shorter operative times and less often need for transfusion compared to the open group. However, length of stay and mortality did not differ between groups [62], but perioperative complication rates were lower compared to the open group [63]. Minimally invasive surgery may be a promising technique for patients with thoracolumbar fractures without neurological deficit.
Following surgery, a liquid diet is usually maintained, with a soft diet on the first post-operative day, gradually until a regular diet can be tolerated based on clinical symptoms. Generally, no cervical collar is required following surgery and patients can mobilize as tolerated [64]. It is important to post-operatively monitor hematoma formation [11], as this may compress the airway. Drains can be removed the day after surgery in case of minimal production.
For patients who suffered from dysphonia pre-operatively, speech therapy may be an effective option during rehabilitation, to improve voice quality [65]. Post-operative patient care can also involve the expertise of dieticians to optimize intake and food consistency during rehabilitation. After surgery, dysphagia may persist, albeit less severe compared to before surgery, for which swallowing exercises and expertise from speech-language pathologists may be beneficial. Generally, dysphagia symptoms significantly approve after osteophyte removal, with a success rate of 95.5% [11]. Post-operative deterioration of the symptoms of transient dysphagia can occur as a result of post-operative swelling, usually resolving within a few weeks [11]. There have been a few cases of recurrence of dysphagia in DISH patients following surgery, which is estimated at 4% after a mean follow-up of 3.7 years (range: 0.4–9.0 years), including 1.7% of osteophyte regrowth occurring [11]. While there is no standardized guideline for recurrence prevention, a few cases have been described treated with post-operative radiotherapy (low doses of 5 × 2Gy) and/or prophylactic indomethacin to prevent recurrent bone growth [66]. The evidence of efficacy of these additional treatments is low and therefore currently not recommended following surgery.
Early mobilization following surgery for vertebral fractures is an important aspect of patient rehabilitation. Weight bearing progression after surgery should be gradually increased as deemed necessary.
Fusion rates in cohorts of vertebral fracture patients with an ankylosed spine are generally high, ranging from 87% to 100% [16]. Reoperation rates following stabilization ranged from 0 to 14%, mainly for debridement after infection, or refixation after implant failure [16, 50].
In general, improvement in neurological outcomes varied widely between 6% and 66% at latest follow-up in different patient cohorts [15, 16]. After long-term follow-up, chronic pain may persist in surgically treated DISH patients following trauma and/or fracture, and these patients are at an increased risk for developing pseudoarthrosis [15, 16].
Partial or even complete SCI may be occur directly after spine trauma or sometimes develop in the work-up towards stabilization. Following SCI, rehabilitation is an important for patient recovery, though it is usually a long and arduous process. Complications associated with SCI include neurogenic bowel and bladder, increased risk for urinary tract infections, thrombosis, orthostatic hypertension, autonomic dysreflexia, as well as mental disorders [67]. The importance of early rehabilitation should be stressed for patients to maintain muscle strength, normal BMD, and to prevent joint contractures and further deterioration of the cardiovascular and respiratory systems [68].
Activity based interventions may also be considered. These effective therapies include functional electric stimulation to improve upper extremity independence, transcranial magnetic stimulation to improve patient walking speed and lower extremity function, and robotic assisted treadmill training to improve lower extremity function [69].
Surgical treatment for patients with DISH is most commonly performed for dysphagia, airway obstruction, and traumatic vertebral fractures. Treating physicians should be aware of the pitfalls during pre- and perioperative management in the work-up for surgery, including difficult intubation, patient comorbidities, and increased risk of neurological complications and mortality when treating fractures. Various treatment options are available, ranging from nonsurgical management, to surgery using various approaches with fusion, fixation, and/or decompression. It is essential for spine surgeons, including orthopedic surgeons and neurosurgeons to plan and be aware of the considerations, anticipations, and approaches for the management of dysphagia, airway obstruction, and fractures in DISH patients in order to improve patient outcomes for this special “at-risk” patient population.
BMD: bone mineral density
CT: computed tomography
DISH: diffuse idiopathic skeletal hyperostosis
SCI: spinal cord injuries
NIH: Conceptualization, Writing—original draft, Writing—review & editing, Project administration, Investigation. JSK: Conceptualization, Writing—original draft, Writing—review & editing. JJV: Conceptualization, Writing—original draft, Writing—review & editing, Supervision.
The authors declare that they have no conflicts of interest.
Not applicable.
Informed consent to participate in the study was obtained from all participants.
Informed consent to publication was obtained from relevant participants.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Iris Eshed
Bruna Parreira ... Jácome Bruges-Armas
Fernando Pérez-Ruiz ... Ana María Herrero-Beites
David Kiefer ... Xenofon Baraliakos
Fabiola Atzeni ... Reuven Mader
Greta Pellegrino ... Piercarlo Sarzi-Puttini
