Affiliation:
1Department of Medicine, Miller School of Medicine, University of Miami, Miami, FL 33136, USA
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0002-4186-2729
Affiliation:
2Department of Medicine, University of Massachusetts School of Medicine, Worcester, MA 01655, USA
3Corrona Research Foundation, Albany, NY 12110, USA
ORCID: https://orcid.org/0000-0002-2463-4847
Affiliation:
3Corrona Research Foundation, Albany, NY 12110, USA
4Department of Medicine, Columbia University, New York, NY 10032, USA
5CorEvitas, LLC, Waltham, MA 02451, USA
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0001-8338-027X
Affiliation:
2Department of Medicine, University of Massachusetts School of Medicine, Worcester, MA 01655, USA
5CorEvitas, LLC, Waltham, MA 02451, USA
ORCID: https://orcid.org/0000-0002-0170-2747
Affiliation:
3Corrona Research Foundation, Albany, NY 12110, USA
†These authors contributed equally to this work.
Email: jkremer@corrona.org
ORCID: https://orcid.org/0000-0001-6674-9901
Explor Musculoskeletal Dis. 2023;1:228–240 DOI: https://doi.org/10.37349/emd.2023.00025
Received: August 23, 2023 Accepted: October 16, 2023 Published: December 05, 2023
Academic Editor: Rebecca Grainger, University of Otago, New Zealand
The article belongs to the special issue Comorbidities in rheumatoid arthritis
Aim: To evaluate the association of disease activity with patient-reported subjective cognitive decline (SCD) in patients with rheumatoid arthritis (RA) stratified by age.
Methods: A cross-sectional analysis using data from the CorEvitas RA registry was utilized. The clinical disease activity index (CDAI) was used along with patient-reported problems thinking, age, and gender. The association of CDAI with patient-reported SCD was estimated using logistic regression models adjusted for sociodemographic characteristics, comorbidities, RA disease characteristics, and medication use. Additional models estimated and tested the moderating effect of patient age (< 55 years vs. ≥ 55 years).
Results: A total of 3,041 out of 33,537 patients (9.1%), reported SCD with a mean CDAI of 16.2 [standard deviation (SD): 12.5] vs. 10.1 (SD: 10.8) in those who did not. The adjusted odds ratio (OR) for low, moderate, and severe disease activity vs. remission was 2.17 [95% confidence intervals (CI): 1.88–2.50], 3.25 (95% CI: 2.82–3.75), and 3.84 (95% CI: 3.29–4.48) respectively. Age had a moderating effect with the association of disease activity and self-reported SCD more prevalent in those aged < 55 years. The ORs for low, moderate, and severe disease activity were 3.37, 5.59, and 5.76 respectively for age < 55 vs. 1.90, 2.67, and 3.37 respectively for age ≥ 55 (P = 0.0001). The patient global component of CDAI displayed the highest OR of risk for SCD broken out by quartiles (1, 1.62, 2.80, and 4.55).
Conclusions: Increasing disease activity is associated with a higher likelihood of patient-reported SCD. The effect was more pronounced in younger RA patients and patients with a higher patient global score.
Rheumatoid arthritis (RA) is a heterogeneous systemic, inflammatory polyarthritis with a significant impact on quality of life, morbidity, and mortality [1]. Reports of an association between so-called cognitive dysfunction and RA, as well as data on its prevalence, have been inconsistent. A few studies of a relatively small number of patients have reported a wide range of formally tested cognitive impairment ranging from 20% to 71% in adults with RA [2–5], but not seen in a recent study [6]. Patients with RA were found to either perform more poorly on a range of cognitive assessment tasks than healthy controls or perform at a level below the age-related norm [7].
The hypothetical pathogenic mechanisms of cognitive impairment in RA are unknown [8]. It has been speculated that systemic inflammation as well as acceleration of the atherosclerotic process [7, 9] may contribute to cognitive dysfunction in RA [10].
There is also limited data available in the literature regarding the overall prevalence, as well as the clinical and sociodemographic factors that may contribute to cognitive dysfunction in RA. While some studies found an association between cognitive dysfunction and disease activity [11, 12], others did not show this association [2, 4, 13].
It is quite important and relevant to this report to note that studies of healthy subjects have noted that subjective cognitive complaints can be a valid indicator of early cognitive impairment not detectable on neurocognitive testing [14, 15]. Subjective cognitive decline (SCD) has been defined by the Center for Disease Control as the self-reported experience of worsening or more frequent confusion or memory loss. It is a form of cognitive impairment and one of the earliest noticeable symptoms of Alzheimer’s disease and related dementias [16].
It is also apparent that completion of comprehensive cognitive testing is not likely to occur in the majority of patients in routine care reporting difficulty thinking. Formal cognitive testing presents challenges as its administration is costly, time intensive, and requires specialized personnel to administer and interpret test results.
We aimed to evaluate the rate of patient-reported difficulty thinking and confusion (SCD) and its association with validated disease activity metrics broken out by age.
A cross-sectional cohort study was conducted within the CorEvitas RA registry (formerly Corrona), a US-based registry that was initiated in 2001 and has been previously described in detail [17]. The CorEvitas RA registry is a multicenter, longitudinal, and observational cohort enrolling patients across the US. Patients have been recruited from 211 private and academic practice sites across 43 states, with 911 participating rheumatologists. As of December 31, 2021, data on 57,543 patients with RA, as defined by the latest ACR diagnostic criteria, have been collected. The CorEvitas database includes information on 452,967 patient visits and 225,180 patient-years of follow-up observation time. Both a clinical disease activity index (CDAI) and patient self-reported “difficulty thinking and confusion” are collected at the time of each visit. For all patients with follow-up, the mean duration of patient follow-up is 4.75 years (median, 3.42 years). Data are collected from both patients and their treating rheumatologists by using structured case report forms and include information on disease activity as measured by a CDAI, disease duration, comorbidities, use of medications, and patient-reported outcome data. Follow-up assessments are requested as often as every 6 months and completed during routine clinical encounters.
We included participants with a diagnosis of RA aged ≥ 18 years who had at least one visit between January 1, 2011 to December 1, 2019. The latest visit in the time frame was used for analysis. The data collection used for this investigation was obtained starting in January 2011 as patient-reported fatigue and physician-reported prednisone doses became available starting at this time. We ended the data analysis period in December 2019 to exclude the coronavirus disease (COVID) era. Patients needed to have both a documented CDAI value and a response to the difficulty-thinking/confusion question. Only patients with complete data on all reported parameters were included in the final analysis.
Patient race and ethnicity were self-reported at the time of enrollment on questionnaires indicating Asian, Black, Hispanic ethnicity, and White. A complete array of other ethnic options was also available at the time of enrollment.
All participating investigators were required to obtain full board approval for conducting research involving human subjects. Sponsor approval and continuing review were obtained through a central Institutional Review Board [IRB, New England Independent Review Board (NEIRB), No. 120160610]. For academic investigative sites that did not receive a waiver to use the central IRB, approval was obtained from the respective governing IRBs, and documentation of approval was submitted to the sponsor prior to initiating any study procedures. All registry subjects were required to provide written informed consent prior to participating. Only de-identified patient data were used in this study. Patients were not involved in the design, conduct, reporting, or dissemination plans of this research.
The primary outcome was SCD defined by a patient-reported indication of difficulty thinking/confusion response on a questionnaire administered at the time of their clinic visit. CDAI, measured at the same visit was the primary dependent variable predicting the outcome of difficulty thinking/confusion. CDAI is calculated by adding the sum of tender joint count (TJC) and swollen joint count (SJC) as well as patient global assessment (PGA), and evaluator global assessment (EGA) using a 10 cm visual analogue scale [18]. Age was considered as a potential moderating factor.
Age, gender, race, education, exercise, alcohol intake, insurance type, disability, non-steroidal anti-inflammatory drug use (NSAID), narcotic medications, depression, Health Assessment Questionnaire-Disability Index (HAQ-DI), patient fatigue, and global assessment are patient-reported measures included in the analysis. Physician-reported measures include tender and swollen joints, duration of RA, history of diabetes mellitus (DM), concomitant fibromyalgia, prednisone use, and dose, opioid use, and methotrexate (MTX) use as well as present and past biologic disease-modifying anti-rheumatic drug (DMARD) use.
Patient demographics, clinical characteristics, and medication use were compared between those reporting cognitive dysfunction vs. those who did not. Means of continuous measures were tested using t tests and categorical measures were tested using Fisher’s exact tests.
The primary association of interest was between patient-reported SCD and disease activity (based on CDAI categories of remission (CDAI ≤ 2.8), low (2.8 < CDAI ≤ 10), moderate (10 < CDAI ≤ 22), and high (CDAI > 22). Unadjusted and adjusted odds ratios (ORs) for risk of self-reported cognitive dysfunction by CDAI categories were estimated with 95% confidence intervals (CI) using logistic regression models using a binary indicator for SCD as the outcome and four categories of CDAI as the primary predictor. A series of adjusted regression models were estimated illustrating the cumulative impact of covariates on the estimated association of interest. Model 1 adjusted for patient demographics and behavior. It was adjusted for age, insurance, body mass index (BMI), education, race, disability, smoking status, duration of RA, history of DM, presence of fibromyalgia, exercise frequency, and alcohol consumption. Model 2 used all the covariates in model 1 and additionally adjusted for medication use (prednisone use, NSAID use, narcotic use, current MTX use, current biologic/targeted synthetic DMARD use, and the number of prior biologics); models 3–5 were exploratory analyses designed to assess the additional impact on the association of controlling for reported depression and patient-reported fatigue. Model 3 used all the covariates in model 2 and added patient-reported depression; model 4 used all the covariates in model 2 and added patient-reported fatigue. Finally, model 5 used all the covariates in model 2 and added both depression and fatigue.
Age (< 55 vs. ≥ 55) was considered as a potential moderator—estimating the association of SCD and CDAI separately for different age groups. To test whether the ORs for SCD by CDAI differed by age, the interaction of age and CDAI was included in all models, and separate estimates of ORs for disease activity were estimated in each age group based on the models with the interaction term. The unadjusted model is:
log(π/(1 – π)) = β0 + β1 × Agelt55 + β2 × LDA + β3 × moderate + β4 × severe + β5 × Agelt55 × LDA + β6 × Agelt55 × moderate + β7 × Agelt55 × severe
Where π is the probability of SCD; Agelt55 is an indicator (0/1) of age < 55; and low disease activity (LDA), moderate, and severe are indicators (0/1) of CDAI levels (age > 55 and CDAI remission are reference groups).
The interaction was tested by comparing the models with and without the interaction terms using a likelihood ratio test.
Age was first examined in multiple categories (< 45, 45–55, 55–65, 65–75, and > 75) and a clear-cut point at 55 was determined and used for clarity (see Table S1).
Additional exploratory analyses were conducted using logistic regression to estimate the association of SCD with the individual components of the CDAI (tender and swollen joints, patient, and physician assessment of global disease activity, each divided into quartiles). Adjusted ORs and 95% CI were estimated (see Table S2).
The primary analysis was carried out in the population of patients with complete data (no missing values) on all covariates (covariates used in model 5). The complete data population was compared to the population missing any covariate values (Table S3). In addition, the unadjusted association of SCD and CDAI was similar using the complete data population (only those with no missing covariate data) vs. using all patients with SCD and CDAI regardless of any missing covariate data.
A total of 41,302 patients were identified for analysis using the latest visit for each RA patient in the period January 1, 2011 to December 31, 2019. Forty thousand, four hundred and nine patient visits had complete information on CDAI, self-reported SCD, age, and gender. There were 33,537 patients with complete data (no missing values) on all covariates.
Baseline characteristics of the complete and missing data population showed no meaningful differences other than slightly higher disease activity and a parallel increase in self-reported cognitive dysfunction (Table S1). The comparison of the clinical characteristics and demographics of those with and without reported SCD in the complete data population are seen in Tables 1 and 2. There were 3,041 patients with reported SCD and 30,496 without. Those who reported cognitive dysfunction were more likely to be women (84.1% vs. 76.5%, P < 0.001), disabled (28.7% vs. 12%, P < 0.001), and have a higher CDAI value (mean score 16.16 ± 12.50 vs. 10.11 ± 10.76, P < 0.001; Tables 1 and 2). Patients reporting SCD had a greater history of fibromyalgia (15% vs. 4.7%, P < 0.001), patient-reported depression (37.4% vs. 8.4%, P < 0.001), and patient-reported fatigue (mean score 63.03 ± 27.29 vs. 35.7 ± 29.6, P < 0.001).
Baseline characteristics with SCD and no SCD
| Variable | No SCD* (n = 30,496) | SCD (n = 3,041) | P+ | ||
|---|---|---|---|---|---|
| Mean | ± SD | Mean | ± SD | ||
| Age | 62.52 | ± 13.37 | 60.66 | ± 14.19 | < 0.001 |
| BMI | 29.54 | ± 7.21 | 30.76 | ± 7.94 | < 0.001 |
| Duration of RA (years) | 12.92 | ± 10.76 | 11.18 | ± 10.12 | < 0.001 |
| CDAI | 10.11 | ± 10.76 | 16.16 | ± 12.50 | < 0.001 |
| 28 TJC | 3.02 | ± 5.21 | 5.41 | ± 6.84 | < 0.001 |
| 28 SJC | 2.26 | ± 3.86 | 2.93 | ± 4.30 | < 0.001 |
| PGA | 30.74 | ± 26.58 | 51.5 | ± 27.03 | < 0.001 |
| EGA | 17.57 | ± 19.49 | 26.66 | ± 23.23 | < 0.001 |
| Fatigue VAS | 35.7 | ± 29.60 | 63.03 | ± 27.29 | < 0.001 |
| HAQ | 0.76 | ± 0.71 | 1.38 | ± 0.72 | < 0.001 |
* SCD determined with patient self-report of difficulty thinking and confusion; + means compared using t tests, categorical variables compared using Fisher’s exact test. HAQ: Health Assessment Questionnaire; VAS: Visual Analog Scale; SD: standard deviation
Comparison of patients with SCD and no SCD
| Variable | No SCD* | SCD | P+ | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Sex | Male | 7,152 | 23.5 | 484 | 15.9 | < 0.001 |
| Female | 23,344 | 76.5 | 2,557 | 84.1 | ||
| White | No | 5,231 | 17.2 | 554 | 18.2 | 0.138 |
| Yes | 25,265 | 82.8 | 2,487 | 81.8 | ||
| College completed | No | 12,472 | 40.9 | 1,372 | 45.1 | < 0.001 |
| Yes | 18,024 | 59.1 | 1,669 | 54.9 | ||
| Smoker in 3 categories | Never | 16,032 | 52.6 | 1,428 | 47.0 | < 0.001 |
| Previous | 9,751 | 32.0 | 962 | 31.6 | ||
| Current | 4,713 | 15.5 | 651 | 21.4 | ||
| Private insurance | No | 10,257 | 33.6 | 1,197 | 39.4 | < 0.001 |
| Yes | 20,239 | 66.4 | 1,844 | 60.6 | ||
| Medicare | No | 16,615 | 54.5 | 1,584 | 52.1 | 0.011 |
| Yes | 13,881 | 45.5 | 1,457 | 47.9 | ||
| Medicaid | No | 28,852 | 94.6 | 2,688 | 88.4 | < 0.001 |
| Yes | 1,644 | 5.4 | 353 | 11.6 | ||
| No insurance | No | 30,128 | 98.8 | 2,995 | 98.5 | 0.145 |
| Yes | 368 | 1.2 | 46 | 1.5 | ||
| History of DM | No | 27,452 | 90.0 | 2,664 | 87.6 | < 0.001 |
| Yes | 3,044 | 10.0 | 377 | 12.4 | ||
| History of fibromyalgia | No | 29,052 | 95.3 | 2,586 | 85.0 | < 0.001 |
| Yes | 1,444 | 4.7 | 455 | 15.0 | ||
| Disabled | No | 26,832 | 88.0 | 2,169 | 71.3 | < 0.001 |
| Yes | 3,664 | 12.0 | 872 | 28.7 | ||
| MTX use | No | 15,035 | 49.3 | 1,601 | 52.6 | < 0.001 |
| Yes | 15,461 | 50.7 | 1,440 | 47.4 | ||
| Prednisone use | No prednisone | 24,087 | 79.0 | 2,231 | 73.4 | < 0.001 |
| Dose < 10 | 4,775 | 15.7 | 553 | 18.2 | ||
| Dose ≥ 10 | 1,634 | 5.4 | 257 | 8.5 | ||
| Alcohol use | Non-drinker | 16,735 | 54.9 | 1,968 | 64.7 | < 0.001 |
| Occasionally | 5,445 | 17.9 | 520 | 17.1 | ||
| 1–3 Per week | 5,950 | 19.5 | 408 | 13.4 | ||
| 1–2 Per day | 2,173 | 7.1 | 133 | 4.4 | ||
| 3 Or more daily | 193 | 0.6 | 12 | 0.4 | ||
| Exercise | No | 12,924 | 42.4 | 1,655 | 54.4 | < 0.001 |
| 1–2 Times/week | 5,423 | 17.8 | 417 | 13.7 | ||
| 3–4 Times/week | 5,574 | 18.3 | 374 | 12.3 | ||
| 5–6 Times/week | 2,343 | 7.7 | 157 | 5.2 | ||
| Daily | 1,347 | 4.4 | 133 | 4.4 | ||
| Not sure | 2,885 | 9.5 | 305 | 10.0 | ||
| Number prior biologic/TS DMARDs | None | 10,546 | 34.6 | 1,040 | 34.2 | < 0.001 |
| 1 | 9,558 | 31.3 | 838 | 27.6 | ||
| 2 | 4,867 | 16.0 | 466 | 15.3 | ||
| 3+ | 5,525 | 18.1 | 697 | 22.9 | ||
| Use of biologic/TS DMARDs | No | 14,763 | 48.4 | 1,508 | 49.6 | 0.215 |
| Yes | 15,733 | 51.6 | 1,533 | 50.4 | ||
| Use of NSAIDs | No | 14,695 | 48.2 | 1,473 | 48.4 | 0.791 |
| Yes | 15,801 | 51.8 | 1,568 | 51.6 | ||
| Narcotic pain med | No | 26,396 | 86.6 | 2,201 | 72.4 | < 0.001 |
| Yes | 4,100 | 13.4 | 840 | 27.6 | ||
| Depression | No | 27,949 | 91.6 | 1,904 | 62.6 | < 0.001 |
| Yes | 2,547 | 8.4 | 1,137 | 37.4 | ||
* SCD determined with patient self-report of difficulty thinking and confusion; + means compared using t tests, categorical variables compared using Fisher’s exact test. TS DMARDs: targeted synthetic DMARDs
The overall rate of SCD was 9.1% (95% CI: 8.8–9.4). It increased from 3.1% for patients in remission to 17.0% for those with severe disease (Table 3). The estimated OR in the series of models that add progressively more variables in a stepwise manner as seen in Table 4. In unadjusted estimates, the OR for reported SCD were 2.69 (95% CI: 2.35–3.09) for LDA category, 4.78 (95% CI: 4.17–5.49) for moderate disease activity category, and 6.53 (95% CI: 5.66–7.53) for high disease activity compared to remission. With adjustment for confounders in model 1 (Table 4), the OR decreases slightly to 2.21 (95% CI: 1.92–2.54) in low disease, 3.41 (95% CI: 2.96–3.92) for moderate disease, and 4.08 (95% CI: 3.52–4.74) for severe disease. In model 2 (Table 4) adjusting for medication use, the OR was 2.17 (95% CI: 1.88–2.50) for LDA, 3.25 (95% CI: 2.81–3.75) for moderate disease activity, and 3.83 (95% CI: 3.29–4.47) for high disease activity (Table 4).
Rates of SCD* and CDAI category
| CDAI | SCD | |
|---|---|---|
| n | Rates | |
| Remission | 8,962 | 273 (3.1%) |
| Low | 1,1871 | 926 (7.8%) |
| Moderate | 8,104 | 1,059 (13.1%) |
| High | 4,600 | 783 (17.0%) |
| Overall | 33,537 | 3,041 (9.1%) |
LDA: 2.8 < CDAI ≤ 10; moderate disease activity: 10 < CDAI ≤ 22; high disease activity: CDAI > 22. * SCD determined with patient self-report of difficulty thinking and confusion
Association of SCD* and CDAI (OR and 95% CI estimated using logistic regression)
| CDAI | Unadjusted | Model 1 | Model 2 | Model 3* | Model 4* | Model 5* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Remission | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| Low | 2.69 | 2.35–3.09 | 2.21 | 1.92–2.54 | 2.17 | 1.88–2.50 | 1.96 | 1.70–2.26 | 1.25 | 1.08–1.45 | 1.22 | 1.05–1.42 |
| Moderate | 4.78 | 4.17–5.49 | 3.41 | 2.96–3.92 | 3.25 | 2.81–3.75 | 2.78 | 2.41–3.22 | 1.53 | 1.31–1.78 | 1.46 | 1.25–1.71 |
| Severe | 6.53 | 5.66–7.53 | 4.08 | 3.52–4.74 | 3.83 | 3.29–4.47 | 3.19 | 2.72–3.73 | 1.50 | 1.26–1.77 | 1.42 | 1.20–1.69 |
Exploratory analysis: model 1: age, insurance, BMI, education, race, disabled, smoking status, duration of RA, history of diabetes, history of fibromyalgia, exercising, and drinking; model 2: model 1 + prednisone use, NSAIDs use, narcotic use, number prior biologics, current MTX use, and current biologic/TS DMARDs use; model 3: model 2 + patient-reported depression; model 4: model 2 + patient fatigue; model 5: model 2 + patient-reported depression and patient fatigue. * SCD determined with patient self-report of difficulty thinking and confusion. Ref: OR = 1
The Table 5 shows estimated unadjusted rates of reported SCD by different CDAI groups within each age group. In the age group of < 55 years, the reported SCD was 2.44% in the remission group and increased to 20.66% in severe disease. In patients ≥ 55, it was 3.25% in patients in remission and increased to 15.45% in severe disease. Unadjusted rates of self-reported SCD in moderate and severe disease groups are higher in younger vs. older patients (18.0% and 20.7% vs. 11.2% and 15.5% respectively). The absolute risk (change in rates of cognitive dysfunction in different CDAI disease categories) is also greater in younger (< 55 years old) vs. older (≥ 55 years old; Table 5).
Percentage of SCD* by disease activity groups (CDAI) stratified by age groups
| Age | CDAI | |||||
|---|---|---|---|---|---|---|
| Remission | Low | Moderate | High | Overall | ||
| Age < 55 | n | 2,291 | 2,819 | 2,226 | 1,384 | 8,720 |
| SCD | 56 (2.44%) | 268 (9.51%) | 401 (18.01%) | 286 (20.66%) | 1,011 (11.59%) | |
| Age ≥ 55 | n | 6,671 | 9,052 | 5,878 | 3,216 | 24,817 |
| SCD | 217 (3.25%) | 658 (7.27%) | 658 (11.19%) | 497 (15.45%) | 2,030 (8.18%) | |
The percentages are unadjusted. * SCD determined with patient self-report of difficulty thinking and confusion
The interaction analyses of age with SCD using multiple categories of age are seen in Table S2. It can be seen that the cutoff of patients < 55 displayed the highest association with risk and this age was therefore used as a cutoff.
The estimated ORs in each model with age as an interaction (moderating) factor in the logistic regression model and estimates of OR by disease activity are shown within each age group in Tables 6, 7, and 8. The interaction of age and disease activity is significant in unadjusted and adjusted models (P ≤ 0.0004). The Figure 1 illustrates differences in OR estimates by age group using model 2 (Table 4).
Estimated ORs of SCD* based on logistic regression models (age ≥ 55)**
| CDAI | Unadjusted | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Remission | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| Low | 2.33 | 1.99–2.73 | 1.94 | 1.66–2.27 | 1.90 | 1.62–2.23 | 1.73 | 1.48–2.04 | 1.11 | 0.94–1.30 | 1.08 | 0.92–1.28 |
| Moderate | 3.57 | 3.20–4.39 | 2.80 | 2.38–3.29 | 2.67 | 2.27–3.14 | 2.29 | 1.94–2.70 | 1.27 | 1.07–1.51 | 1.21 | 1.01–1.44 |
| Severe | 5.44 | 4.61–6.42 | 3.59 | 3.03–4.26 | 3.37 | 2.83–4.01 | 2.80 | 2.35–3.35 | 1.31 | 1.09–1.58 | 1.24 | 1.03–1.51 |
* SCD determined with patient self-report of difficulty thinking and confusion; ** log(π/(1 – π)) = β0 + β1 × Agelt55 + β2 × LDA + β3 × moderate + β4 × severe + β5 × Agelt55 × LDA + β6 × Agelt55 × moderate + β7 × Agelt55 × severe, where π is the probability of SCD; Agelt55 is an indicator (0/1) of age < 55; and LDA, moderate, and severe are indicators (0/1) of CDAI levels (age > 55 and CDAI remission are reference groups)
Estimated ORs of SCD* based on logistic regression models (age < 55)**
| CDAI | Unadjusted | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Remission | Ref | Ref | Ref | Ref | Ref | Ref | ||||||
| Low | 4.19 | 3.13–5.62 | 3.33 | 2.48–4.47 | 3.29 | 2.45–4.43 | 2.95 | 2.18–3.97 | 1.86 | 1.37–2.51 | 1.80 | 1.33–2.45 |
| Moderate | 8.77 | 6.59–11.68 | 5.80 | 4.33–7.75 | 5.59 | 4.17–7.48 | 4.79 | 3.56–6.43 | 2.56 | 1.90–3.45 | 2.45 | 1.81–3.32 |
| Severe | 10.40 | 7.74–13.97 | 6.03 | 4.46–8.15 | 5.76 | 4.25–7.81 | 4.76 | 3.50–6.48 | 2.21 | 1.62–3.02 | 2.11 | 1.54–2.90 |
* SCD determined with patient self-report of difficulty thinking and confusion; ** log(π/(1 – π)) = β0 + β1 × Agelt55 + β2 × LDA + β3 × moderate + β4 × severe + β5 × Agelt55 × LDA + β6 × Agelt55 × moderate + β7 × Agelt55 × severe, where π is the probability of SCD; Agelt55 is an indicator (0/1) of age < 55; and LDA, moderate, and severe are indicators (0/1) of CDAI levels (age > 55 and CDAI remission are reference groups)
An interaction of age and CDAI groups based on logistic regression models
| Logistic regression models | P |
|---|---|
| Unadjusted | < 0.0001 |
| Model 1 | < 0.0001 |
| Model 2 | < 0.0001 |
| Model 3 | < 0.0001 |
| Model 4 | < 0.0003 |
| Model 5 | < 0.0004 |
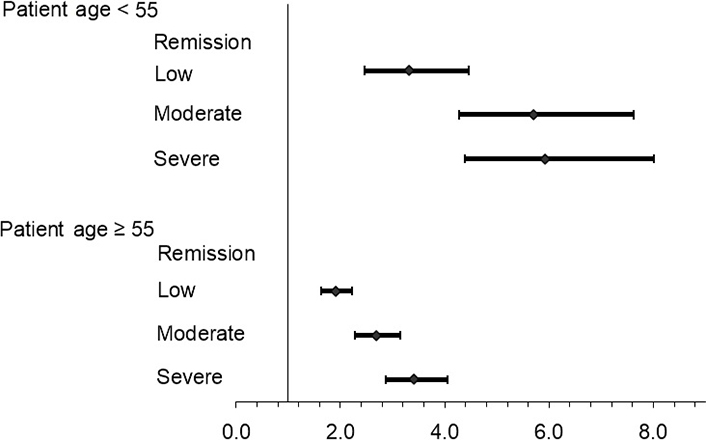
In the exploratory analysis, models 3–5 examined the impact of adjusting for patient-reported depression and patient fatigue on the association (separately and together, Tables 4, 6, 7, and 8). While the estimated ORs for SCD are somewhat reduced, the association with disease activity remains highly significant along with the moderating effect of age. The moderating effect of age is also seen in Figure 1.
The association of the individual components with SCD broken down into quartile values is seen in Table S3. It is evident that there is a marked association with the patient global component of the CDAI that is significantly greater than the other 3 components.
Our study is by far the largest investigation that provides information on the rate of self-reported SCD marked by difficulty thinking and confusion in RA associated with a validated disease activity metric. We were able to examine the effects of disease activity metrics on this phenomenon in this large cohort over a much longer interval than has previously been reported. We found an overall prevalence of self-reported SCD of 9.1% with marked increases in this prevalence in patients with higher CDAI categories of disease.
In this large US observational study, we also sought to characterize the association of a validated measure of disease activity with patient-reported SCD in patients with RA stratified by age. Increasing disease activity as measured by CDAI was associated with a higher likelihood of reporting these problems after adjusting for multiple, simultaneously measured potential confounders. To our surprise, the impact of disease activity on SCD was more pronounced in younger (< 55 years) than older (≥ 55 years) RA patients.
The patient-reported global disease evaluation score was significantly associated with self-reported SCD to a greater extent than other components. To our knowledge, this is the first study to examine the overall prevalence of these patient-reported cognitive complaints from a very large database along with a strong association with provider-measured disease activity after adjustment for multiple simultaneously measured potential confounders. It is also the first study to report that self-reported cognitive complaints are more common in younger patients in association with disease activity.
A similar correlation between high disease activity and cognitive dysfunction as evaluated by objective tests has been inconsistently reported [7, 11, 12]. In a recent systematic review of cognitive impairment in RA, a relationship between disease activity and reduced performance on cognitive function tests was observed in small numbers of patients [7]. A large longitudinal population-based study that evaluated different types of arthritis found that the presence of joint disorders, especially RA, at midlife, was associated with a worse cognitive status later in life, but patients with RA were not broken out from the larger cohort [18]. High cumulative RA disease activity as measured by disease activity score 28 (DAS28) was associated with objectively measured cognitive impairment in a cohort of 464 patients from Thailand [11] that did not include analysis of medications. Lee et al. [12] reported that cognitive dysfunction was related to disease activity and increasing age in a smaller observational study of 70 patients.
We report that SCD was more likely to be associated with higher disease activity in younger patients. The reasons for the differences in the distribution of these complaints with age from the study by Lee et al. [12] are uncertain and may be associated with the smaller number of patients in that report. It is also possible that our report of a greater prevalence of SCD in a younger population was made possible because of the much larger population of patients studied. It is possible that there could be a greater awareness and recognition of cognitive challenges in a younger population, although this is speculative.
The association between disease activity and cognitive complaints was not found in some other studies [2, 13, 19, 20]. Shadick et al. [13] reported that higher disease activity as measured by DAS28 showed a non-statistical trend of associations with self-reported poor concentration although it is possible that this finding may have been affected by differences in the DAS28 metric vs the CDAI, as well as differences in statistical power. Shin et al. [4] studied 115 RA patients and assessed cognitive dysfunction using a battery of standardized neuropsychological measures. In this cohort, the severity of RA as measured by the RA disease activity index (RADAI), a patient-assessed measure of disease activity, and C-reactive protein (CRP), were not significant predictors of cognitive impairment [4] although validated disease activity metrics were not reported.
In an exploratory analysis, we found that the impact of adjusting for patient depression on the association of disease activity and SCD was not likely to be meaningful compared to the impact of patient fatigue on this association. The association of severe disease (compared to remission) decreased by approximately 17% with adjustment for patient depression (OR decreased from 3.83 to 3.19, Table 4), while adjusting for patient fatigue decreased this association by about 61% (OR decreased from 3.83 to 1.50, Table 4). Patient depression has a complex causal association with disease activity in RA [21–24], but whether as a confounder or a mediator, we did not find a large impact of self-reported depression on the association of disease activity and SCD. However, adjusting for patient fatigue—likely a proxy for disease activity in this analysis—has had a large impact on this association. Future longitudinal studies should examine patient fatigue as a potential mediator of patient-reported SCD.
It is possible that cognitive complaints may be associated with clinical features such as pain, fatigue, and sleep disturbance, as reported by research in chronic pain populations [25], or psychological comorbidities such as depression or anxiety [26]. Shin et al. [20] found that higher levels of depression and fatigue were associated with perceived cognitive dysfunction after adjusting for disease severity as measured by RADAI in 120 patients with RA. It should be noted that increased depression in the setting of chronic disease is well-reported [27].
Our study has some limitations. Although we are reporting data on patients’ SCD, we did not perform formal neurocognitive testing. It is possible that there may be a different sensitivity for reporting cognitive problems in a formal cognitive assessment questionnaire [20, 28]. Although we are reporting strong correlations across time in a very large number of patients, we have not demonstrated a direct causative effect of disease activity on these subjective complaints. It is also possible that a single measure of these difficulties does not capture a chronic state or that patient self-reporting of cognitive issues can be related to other non-inflammatory factors not captured.
We found a statistically significant correlation with prednisone treatment and reporting of SCD. Eighteen point five percent of the total of 26.5% of patients on prednisone were on a dose of less than 10 mg daily at the time they reported SCD. We believe that continued long-term observations of the effects of differing doses of glucocorticoid daily dosing and patient perceptions of SCD are warranted.
We also report for the first time that the patient global component of the CDAI score has a marked association with reporting of SCD when broken out into quartiles. We believe that further investigation into the components of standardized physician and patient-derived metrics would be appropriate to confirm that patient global and pain reporting severity could be more closely linked with perceived cognitive challenges than a complete CDAI score. We have been interested in the individual components of standardized metrics like the CDAI and specifically the contribution of patient characteristics like the PGA to a final CDAI metric score [29]. We believe that it is likely that the patient global evaluation represents a range of patient characteristics that go well beyond actual RA disease activity [29].
Formal neuropsychological evaluation would be ideal for most patients with subjective cognitive complaints, but for a variety of reasons these tests are not typically obtained in routine clinical care. While self-reported measures of cognitive difficulties may have limitations, these measures can indeed capture a patient’s experience of cognitive performance and can have the potential to identify cognitive changes not yet detected with conventional neuropsychological assessments [14–16]. In this retrospective analysis, we found a strong association between this distressing personal complaint with a simultaneously measured and validated clinical disease activity metric. Patients with RA who report SCD are more likely to have simultaneously measured greater disease activity than those who do not report this problem.
Even in the absence of formal cognitive testing, a longitudinal study of normal elderly has in fact shown that self-reported cognitive complaints are a valid indicator of formally diagnosed cognitive impairment [30]. Moreover, the importance of patient-reported subjective cognitive complaints as a diagnostic criterion has previously been recognized [31] and formally defined by the CDC [16].
In conclusion, our study adds important new evidence regarding the prevalence and associations of patients’ own perception of cognitive difficulties (SCD). We found a strong association with disease activity in younger patients after extensive modeling that persisted after adjustment for a variety of other variables. Clinicians should be aware of the strong association of cognitive difficulties with increased disease activity in their RA patients which is strongly associated with fatigue and the global DAS. The prevalence of this phenomenon is not rare and is quite likely to be encountered by all clinicians treating RA. These individuals may require more patience in the clinic setting and should not be viewed as being difficult. They may be referred for formal cognitive evaluations. Future investigations will need to focus on the implications of patient-reported cognitive difficulties that can impact the personal, professional, and interpersonal function of patients with RA.
BMI: body mass index
CDAI: clinical disease activity index
CI: confidence intervals
DAS28: disease activity score 28
DM: diabetes mellitus
IRB: Institutional Review Board
LDA: low disease activity
MTX: methotrexate
NSAID: non-steroidal anti-inflammatory drug use
OR: odds ratio
PGA: patient global assessment
RA: rheumatoid arthritis
SCD: subjective cognitive decline
SD: standard deviation
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/100725_sup_1.pdf.
The authors would like to thank all the participating investigators and patients in the CorEvitas Rheumatoid Arthritis Registry who contributed data for this study.
OP, DAP, and JMK equally contributed to: Conceptualization, Data curation, Formal analysis, Writing—original draft, Writing—review & editing, Supervision. GR: Conceptualization, Formal analysis, Writing—original draft, Writing—review & editing, Supervision. LRH: Conceptualization, Data curation, Writing—original draft, Writing—review & editing, Supervision.
DAP has equity interests and is an employee of CorEvitas (formerly known as Corrona), is a member of the Board of Directors of the Corrona Research Foundation (CRF), and has received honoraria/consulting fees from Novartis, Sanofi, Genentech, Roche, and Abbvie. GR is a consultant for Corrona Research Foundation and CorEvitas, LLC. LRH is an employee and stockholder of CorEvitas and has received honoraria/consulting feeds from AbbVie, Bristol-Myers Squibb, and Roche, on the speaker’s bureau for Bristol-Myers Squib, and received grant funding from Pfizer. JMK is the president of the Corrona Research Foundation where he serves in a non-remunerative position. He is also a consultant for CorEvitas.
Sponsor approval and continuing review were obtained through a central IRB (New England Independent Review Board, NEIRB No. 120160610) and complied with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
Data are available from CorEvitas, LLC through a commercial subscription agreement and are not publicly available. Additional aggregated data are available from the authors.
This study was funded by the Corrona Research Foundation. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3071
Download: 23
Times Cited: 0
Gloria Candelas Rodríguez, Virginia Villaverde
Manolya Ilhanli, Ilker Ilhanli
Uğur Özkan ... Murat Birtane
Diego Benavent, Chamaida Plasencia-Rodríguez
Zehra Irshad, Nicola J. Gullick
