Affiliation:
1Department of Rheumatology, VieCuri Medical Centre, 5912BL Venlo, The Netherlands
2Department of Rheumatology, University of Medicine and Pharmacy “Iuliu Hatieganu”, 400012 Cluj-Napoca, Romania
Email: hoteaioana@yahoo.com
ORCID: https://orcid.org/0000-0003-2552-9835
Affiliation:
1Department of Rheumatology, VieCuri Medical Centre, 5912BL Venlo, The Netherlands
ORCID: https://orcid.org/0000-0002-1632-4232
Affiliation:
1Department of Rheumatology, VieCuri Medical Centre, 5912BL Venlo, The Netherlands
ORCID: https://orcid.org/0000-0003-1269-820X
Affiliation:
1Department of Rheumatology, VieCuri Medical Centre, 5912BL Venlo, The Netherlands
ORCID: https://orcid.org/0000-0002-0854-4374
Affiliation:
3Leerhuis, VieCuri Medical Centre, 5912BL Venlo, The Netherlands
ORCID: https://orcid.org/0000-0003-4329-2560
Affiliation:
1Department of Rheumatology, VieCuri Medical Centre, 5912BL Venlo, The Netherlands
ORCID: https://orcid.org/0000-0003-3700-6257
Affiliation:
1Department of Rheumatology, VieCuri Medical Centre, 5912BL Venlo, The Netherlands
4Department of Medical Cell BioPhysics (MCB), University of Twente, 7522NB Enschede, The Netherlands
ORCID: https://orcid.org/0000-0003-3026-3154
Explor Musculoskeletal Dis. 2023;1:97–105 DOI: https://doi.org/10.37349/emd.2023.00014
Received: March 19, 2023 Accepted: June 28, 2023 Published: August 30, 2023
Academic Editor: Peter Mandl, Medical University of Vienna (MUW), Austria
Aim: This study aims to assess outcomes of gout patients from the treat to target (T2T) perspective at 6 months and 12 months while using urate lowering therapy (ULT): allopurinol, febuxostat, and/or benzbromarone.
Methods: All gout patients visiting the Rheumatology department between 2015 to 2021 were identified from the digital hospital system. The diagnosis of gout was based on the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) 2015 classification criteria. Patient outcomes were predefined intention to treat (ITT) categories: category 1: patients with serum uric acid (sUA) ≤ 0.360 mmol/L (ACR target for gout); category 2: patients with sUA ≤ 0.300 mmol/L (ACR/EULAR target for severe gout); category 3: patients with sUA > 0.360 (failure to meet ACR target).
Results: Gout diagnoses were present in 1,186 patients: 986 (83.1%) males and 200 (16.9%) females. A follow-visit at 6 months was present in 76.9% (n = 856) out of 1,113 patients reaching sUA < 0.36 mmol/L, but 257 (23%) failed to reach the 0.36 mmol/L target. At 12 months, a follow-up visit was available in 792 (71.1%) patients, and from these, 710 (90%) had reached sUA < 0.36 mmol/L target. The use of benzbromarone was a strong predictor of reaching the sUA < 0.30 mmol/L target: odds ratio (OR) 3.2, 95% confidence interval (CI) (1.735, 6.017) at 6 months. Diabetic patients had the highest proportion of not reaching the target: 18%. Male patients needed higher dosages of allopurinol to reach the sUA target at 6 months compared to female patients.
Conclusions: This is a large study on a T2T approach based in a real-life clinical setting. Only 42% reached the sUA target at 6 months with allopurinol 300 mg quaque die (QD) monotherapy. About 77% of gout patients reach the predefined sUA target of 0.36 mmol/L at 6 months with the availability of three ULTs. There is still a significant unmet need in gout as many patients failed to achieve predefined sUA targets.
Gout is a highly prevalent auto-inflammatory disorder treated by general practitioners (GPs) and rheumatologists. In general, rheumatologists adhere to protocolized urate-driven pharmacotherapy aiming at a low serum urate concentration [1], whereas GPs commonly adhere to an attack-driven approach [2]. For an attack-driven approach or treat to avoid symptoms, commonly non-steroidal anti-inflammatory drugs (NSAIDs), colchicine, or prednisolone are prescribed. For a treat to target (T2T), i.e., urate-driven approach, in several countries like the Netherlands, Germany, and Spain, 3 urate lowering drugs are available: allopurinol, febuxostat and/or uricosuric benzbromarone. Allopurinol is the first choice xanthine oxidase inhibitor (XOi), while febuxostat is the alternative XOi. In the Netherlands, benzbromarone can be used as an add-on therapy in combination with XOi or as monotherapy, conform European League Against Rheumatism (EULAR) guidelines [2]. The primary outcome of this real-life urate-driven pharmacotherapy study is the percentage of patients reaching the American College of Rheumatology (ACR)/EULAR treatment levels for gout [serum uric acid (sUA) ≤ 0.36 mmol/L] and/or (sUA ≤ 0.30 mmol/L) after 6 months and 12 months respectively, in a setting with the availability of three urate lowering drugs. In addition, we studied the dosages used and factors for a successful treatment.
All gout patients diagnosed from January 2015 until March 2021 were identified from the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD10) coding system, and data were retrieved and entered anonymously in a Castor Electronic Data Capture (EDC) database by the first author. Patients’ medical data was used in agreement with the ethical consent form of the hospital.
The inclusion criterium was the ICD10 code M10 for gout. Diagnoses were based on identifying monosodium urate crystals via polarized light microscopy and/or meeting the ACR/EULAR 2015 classification criteria for gout [3]. There were no specific exclusion criteria for this study. Therefore, all patients who met the inclusion criteria were included in the analysis.
Patients’ data were collected and stored anonymized for analysis with SPSS version 26. We collected demographic data, clinical variables, and laboratory parameters at baseline after 6 months and 12 months of follow-up. Age, sex, family history of gout, duration of symptoms, body mass index (BMI), clinical comorbidities (cardiovascular disease, diabetes mellitus type 2, dyslipidemia, renal disease), and medication data were collected. The comorbidities were defined based on prespecified diagnoses as described in the medical files. Obesity was defined as a BMI over 30 kg/m2, and cardiovascular disease was considered as history of ischemic heart disease or hypertension (blood pressure > 140/90 mmHg; 18/11 kPa). Renal disease/disorder was defined as a glomerular filtration rate (GFR) of less than 60 mL/min. Laboratory assessment included sUA and creatinine, estimated GFR (eGFR), mL/min per 1.73 m2.
The main outcomes of this study were the efficacy of urate lowering therapy (ULT) achieving the ACR target of sUA ≤ 0.36 mmol/L (6 mg/dL) or the stricter sUA ≤ 0.30 mmol/L (5 mg/dL) respectively at 6 months and 12 months after initiation of the ULT. Some patients were seen only once at consultation and did not show up for follow-up visits, and therefore cannot be analyzed.
According to the sUA levels in patients with an intention to treat follow-up, we distinguished 3 patient groups:
Group 1: sUA ≤ 0.36 mmol/L but > 0.30 mmol/L (ACR/EULAR target for gout [1]).
Group 2: sUA ≤ 0.30 mmol/L (ACR/EULAR target for severe gout [1]).
Group 3: sUA > 0.36 mmol/L (failure to meet any ACR/EULAR target).
The dosage of ULTs that were analyzed were noted at 6 and 12 months. The numbers of patients and the comorbidities with particular reference to the clusters defined by Richette et al. [3] were noted. Additionally, we analyzed sex differences in outcomes.
Baseline characteristics and adherence to therapy are presented as mean ± SD values. Differences between groups were compared using an independent t-test for continuous variables and a chi-square test (χ2) for categorical variables. Associated factors for reaching the sUA target were analyzed using linear regression. The odds ratio (OR) was presented with a 95% confidence interval (CI), and P < 0.05 was considered statistically significant. Analysis was done using SPSS software version 26 and Graph Pad Prism 7.
We identified 1,186 patients with gout who met the 2015 ACR-EULAR classification criteria: 986 (83%) males and 200 (17%) females. Baseline characteristics are given in Table 1.
Demographics and baseline characteristics
| Patient characteristics | n (%) | Mean (SD) | Range |
|---|---|---|---|
| Sex | |||
| Male | 986 (83%) | N/A | N/A |
| Female | 200 (17%) | N/A | N/A |
| Age in years | N/A | 63.3 (14.1) | 17–99 |
| Symptom duration at presentation in years | N/A | 2.1 (4.8) | 1–36 |
| BMI (kg/m2) | N/A | 28.13 (5.01) | 15.62–59.77 |
| Tophi presence | 204 (17.2%) | N/A | N/A |
| Crystal proof via polarized microscope | 875 (82.2%) | N/A | N/A |
| Disease history | |||
| Gout in family | 78 (6.5%) | N/A | N/A |
| Cardiovascular disease | 908 (76.5%) | N/A | N/A |
| Dyslipidemia | 499 (42.1%) | N/A | N/A |
| Type 2 diabetes | 254 (21.4%) | N/A | N/A |
| Renal disease | 442 (37.2%) | N/A | N/A |
| Baseline serum urate (mmol/L) | N/A | 0.47 (0.12) | 0.13–0.99 |
n: number; N/A: not applicable
A microscopically crystal proven diagnosis of gout was made in 976 cases (82%) using compensated polarized light microscopy (CPLM) analysis. There were 204 patients (17%) classified as tophaceous; 210 (18%) were not microscopically crystal-proven patients but they did fulfill the ACR-EULAR classification criteria for gout [3]. Of 1,186 gout patients, 70% suffered from cardiovascular or other cardiovascular comorbidities: hypertension in 33%, dyslipidemia in 42%, renal disease (eGFR < 60 mL/min) in 37%, and type 2 diabetes in 21.4%.
All patients were given written dietary advice poor in purines, limited use of (malt) beers and fructose-enriched drinks, and, if possible, increased coffee use. The written dietary advice was also explained orally.
ULT escalation regimes were only prescribed by rheumatologists. No ULT prescription was given before the consultation. In 95% of patients, a urate lowering drug was prescribed at the baseline visit. Allopurinol was prescribed in 882 cases (87%), and adherence at 6 months was strict in 808/1,009 (80%) patients. Febuxostat was the 2nd choice alternative and thus started in 48 (4.7%). Benzbromarone was prescribed in 98 (8.2%) patients, indicated because of a previously proven and/or experienced allopurinol/febuxostat intolerance. The combination of allopurinol/febuxostat plus benzbromarone was prescribed in 70 patients (5.9%). Of these patients, 78% achieved the predefined target < 0.30 mmol/L at 6 months vs. 51% of patients who followed allopurinol in monotherapy (Figure 1).
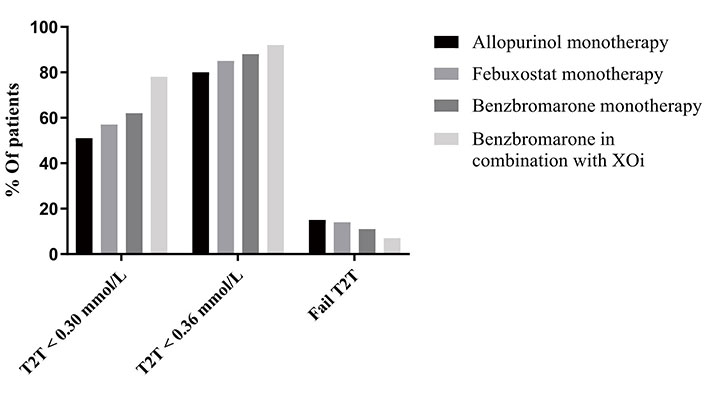
Outcome regarding achieving the predefine target of serum urate level at 6 months using the three urate lowering therapies in monotherapy or in combination setting
We started with 1,186 gout patients, and 73 (6.1%) were lost at 6-month follow-up (plus/minus 1 month); 50 males (79.3%) and 23 females. At the 6-month time slot (plus/minus 1 month), we had data from 1,113 patients (Figure 2):
Group 1: Treat to sUA between 0.30 and 0.36 mmol/L; n = 292 (26.2%); 241 males (82.5%) and 51 females (17.5%).
Group 2: T2T sUA < 0.30 mmol/L; n = 564 (50.7%); 472 males (83.6%) and 92 females (16.3%).
Group 3: Failure to reach target 0.36mmol/L; n = 257 (23.1%); 223 males (86.7%) and 34 females (13.2%).
In groups 1 and 2, the adherence to ULT options was similar, and significant changes were not found between the proportion of patients receiving ULTs at 6 months vs. 12 months. At 6 months and 12 months, a substantial number of patients (n = 65 and n = 64, respectively) required a combination of ULTs to get to the stricter target sUA ≤ 0.30 mmol/L. At 12 months, allopurinol was used by 84.5% of patients, benzbromarone by 9.9%, and febuxostat by 8.8%, and the combination of XOi plus uricosuric was prescribed in 67/792 cases (8.4%).
The dose of allopurinol varied between 50 mg to 900 mg quaque die (QD). Allopurinol 300 mg QD was the predominant dose, and in 51% of patients, this dosage was still used at both 6 months and 12 months follow-up. With allopurinol 300 mg QD, the target sUA ≤ 0.36 mmol/L was reached in 42.2% (n = 123) of patients after 6 months of therapy. The stricter sUA ≤ 0.30 mmol/L target was achieved in 45.5% of cases (n = 255). With allopurinol 100 mg QD (n = 11), 3.8% of patients reached the ≤ 0.36 mmol/L target; and 2.6% (n = 15) reached the sUA ≤ 0.30 mmol/L target.
A strong correlation was found between the prescribed dose of allopurinol and achievement of reaching the stricter sUA ≤ 0.30 mmol/L target at 6 months: r = −0.1795, 95% CI (−0.2558, −0.101), P < 0.001. The use of benzbromarone was strongly associated with reaching the sUA ≤ 0.30 mmol/L target at 6 months: vs. no benzbromarone OR 3.2, 95% CI (1.735, 6.017).
At 6 months, 856 patients (77%) reached the serum urate ≤ 0.36 mmol/L target. Within this group, there was a positive correlation between the allopurinol dose at 6 months and the eGFR: r = 0.233, P < 0.0001. There was a negative correlation between allopurinol dose and serum urate level at 6 months: r = −0.153 (P = 0.0001). At 6 months, over 90% of patients were using allopurinol treatment, and it was noted that the eGFR in these patients had improved slightly but significantly: +8.6 mL/min per 1.73 m2 (P = 0.0005), 95% CI (1.423, 9.941). This was not observed in patients treated with febuxostat or benzbromarone. No difference between these groups was found in using corticosteroids or a potentially nephrotoxic medication, i.e., NSAIDs. There was no significant difference in GFR at baseline in the group of patients using benzbromarone vs. allopurinol.
Febuxostat was only dosed in 80 mg QD. In this group, 50% had a decreased renal function, and 33% was tophaceous. With febuxostat monotherapy (n = 54), 9.5% reached the ≤ 0.36 mmol/L target and 12.3% (n = 36) the ≤ 0.30 mmol/L target.
Prophylactic medication was advised and prescribed in 75.6% (647) of cases: predominantly colchicine in 66.1% (427) and/or prednisone in 39.6% (256).
Despite prophylactic treatment, gouty attacks were reported spontaneously by 7 (0.1%) patients on febuxostat and 59 (7.2%) on allopurinol during 6 months of ULT treatment.
In our study, 257 patients (23%) did not reach the 0.36 mmol/L target at 6 months. At the 6-month visit, 73% of patients still adhered to ULT medication. In this category, the lowest percentage of uricosurics was used (5.2% vs. 12.7% in category 2). A significant difference we found was a lower starting dose of allopurinol of 100 mg in category 3 compared to categories 1 and 2 (P < 0.0001). In this category, 3.4% had decreased renal function, and 27% had diabetes. Here we included 104 (40%) patients who chose a “treat to avoid symptoms” target in a shared decision-making process, specifically focusing on treating the attacks. These patients were treated with allopurinol 100 mg QD, and this dose decreased the sUA level by 0.19 mmol/L.
Regarding comorbidities in our cohort, decreased renal function was negatively associated with reaching sUA ≤ 0.30 mmol/L target at 6 months [OR 0.692; 95% CI (0.098, 0.035)]. A history of diabetes was also negatively associated with sUA ≤ 0.30 mmol/L target at 6 months [OR 0.652; 95% CI (0.001, 0.139)]. There was no correlation between the presence of tophi, initial sUA level, and the sUA ≤ 0.30 mmol/L target at 6 months.
In our study, 216 (18.2%) patients have no comorbidities. Looking into different clusters of patients based on the presence of specific comorbidities, i.e., patients with fewer comorbidities, obese gout patients, patients with predominance of diabetes, dyslipidemia, and patients with cardiovascular and renal failure, as described previously by Richette et al. [3], we observed that the effectiveness of ULT at lowering sUA did not differ between the defined clusters (data not shown).
Diabetic patients, however, present the highest proportion of patients not getting to target (18%).
The mean age was 66.2 years for male vs. 75.2 years for female gout patients. The mean sUA level at initial presentation was 0.51 mmol/L in males vs. 0.47 mmol/L in females (P = 0.67). After 6 months and 12 months of treatment, there was no significant difference between the sexes regarding the mean sUA level or the reached sUA target: 70.0% of females reached < 0.36 mmol/L vs. 72.3% of males (P = 0.42). To reach the predefined target, females needed a lower dose of allopurinol: on average, 238 mg QD in females vs. 334 mg QD in males (P = 0.0001; Figure S1).
This real-life hospital-based study shows that up to 77% of gout patients can reach predefined targets when three urate lowering drugs are available. With allopurinol 300 mg QD monotherapy, only 42% will achieve predefined sUA targets. In a minority, a combination of XOi and benzbromarone is needed. Combination therapy was more effective than monotherapy if used in an add-on strategy. Diabetes and decreased renal function negatively influence reaching predefined sUA targets. Interestingly, if one aims at specific biochemical urate targets, females require a lower daily XOi dose than males. Regarding unmet needs, still about 1 in 10 cannot be treated to the stricter 0.30 mmol/L target when only 3 urate drugs are available.
Our real-life results of over 1,100 patients with a T2T strategy showed that it is feasible in about 80% to get to a more lenient 0.36 mmol/L target, comparable or somewhat less when compared with a real-life study from Norway [4], with only 2 urate lowering drugs, i.e., allopurinol and febuxostat, and the British nurse-led gout study in which 95% reached the 0.36 mmol/L target [5]. The presented results align with previous Dutch work showing that a strict urate-driven therapy using a urate-driven approach reached the biochemical sUA < 0.36 mmol/L target in about 85% of patients [6, 7].
In the present population using a shared decision-making process, the majority chose to be treated to target, and interestingly indeed, this turned out to be feasible in this population. Still, about 10% of gout patients fail to reach this stricter target without a third urate lowering drug option, such as benzbromarone. However, more than 70% of patients treated with an escalating dosing scheme of allopurinol may well reach the stricter serum urate targets. Remarkably, allopurinol 300 mg QD will suffice only in 51% of real-life gout patients. Our patients showed adequate adherence and a similar survival curve of ULTs compared to other data on real-world gout populations [8, 9]. Patients starting with a higher dose of allopurinol reach the target sUA level more often (groups 1 and 2) than those starting with a lower dose (see group 3). Similar results were also shown in a recent study that a higher initiation dose of allopurinol is favorable for reaching and maintaining the sUA target [10].
Interestingly, we found that allopurinol, not benzbromarone nor febuxostat, significantly improved the eGFR at 6 months. Several studies discuss the effect of allopurinol on kidney function [11–13]. We found an improvement of +8.6 mL/min per 1.73 m2 within 6 months of allopurinol treatment, which is in line with the increase in GFR mentioned in other studies [13–15]. This effect may be induced via improved endothelial function or arterial stiffness in chronic kidney disease [16]. Others have suggested xanthin oxidase- and oxidative stress inhibition as drivers for improved renal function [4]. One may also consider a nephrotoxicity of intra-arterial intra-glomerular hyperuricemia [17]. But there is still no proven etiopathogenic relationship between the use of xanthin-oxidase inhibition and improved renal function. Others showed that using ULT in a T2T manner could help improve renal function in patients with gout and chronic kidney disease stage 3 [18]. All real-life gout populations consist of patients with many comorbidities, and clustering of gout patients based on the associated diseases has been proposed previously by Richette et al. [3]. Our data indicate that this subdivision did not help predict which patients reach predefined biochemical sUA targets.
This study does show that with shared decision-making, explaining the importance of aiming for commonly used urate targets, and adherence to a strict protocolized treatment, most gout patients in real life can be treated to target indeed. Patient factors that may need consideration in an individual escalating ULT regime are diabetes and diminished renal function; factors that may need consideration of lowering dosages of ULT drugs are high creatinine clearance and female sex.
In real-world rheumatological practice, with shared decision-making, there is a small but significant patient group (9%) choosing a treat to avoid symptoms strategy; these patients refuse ULTs for different reasons, and/or have a poor tolerance of the available ULTs.
Our study has some limitations being a retrospective study in a real-life clinical setting. We had limited data regarding the patient’s previous medication use and other medication that could influence renal function, like NSAIDs and diuretics. The large number of patients using first choice allopurinol compared with second choice febuxostat made it difficult to compare these two ULTs in terms of effectiveness. Clinicians were free to choose their ULT regimen; for instance, febuxostat escalation to 120 mg was done less frequently (0.51% of cases) compared to reports from other centers. The patients were managed by 4 rheumatologists with potentially different treatment preferences.
In conclusion, with three ULT options available, a considerable proportion of gout patients (77%) can reach predefined targets with treatment according to guidelines. However, a significant percentage of patients (23%) fail to achieve targets while adhering to protocolized treatment. These data show that more than 3 urate lowering therapies are needed to achieve the predefined targets in all our gout patients, as its prevalence is still increasing with advancing ages of our West-European patient populations.
ACR: American College of Rheumatology
CI: confidence interval
eGFR: estimated glomerular filtration rate
EULAR: European League Against Rheumatism
GFR: glomerular filtration rate
NSAIDs: non-steroidal anti-inflammatory drugs
OR: odds ratio
QD: quaque die
sUA: serum uric acid
T2T: treat to target
ULT: urate lowering therapy
XOi: xanthine oxidase inhibitor
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/100714_sup_1.pdf.
IH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing—original draft. TLJ: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing—review & editing. TG, ME and AC: Resources, Validation. FVO: Formal Analysis. MJ: Methodology, Resources, Validation, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The study was approved by the institutional review board of VieCuri Medical Center (2021-009). The research of this article meets the requirements of the Declaration of Helsinki.
The VieCuri organization waived for anonymous use of retrospective data from the gout patient population.
Not applicable.
Not applicable.
IH was supported by a grad founded by EULAR-Eular scientific training grant for young fellows. The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
