Affiliation:
2Department of Nephrology, Division of Internal Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA
Email: SreedharMandayam@mdanderson.org
Affiliation:
5Department of Medicine, Houston Methodist Hospital, Houston, TX 77030, USA
ORCID: https://orcid.org/0009-0000-1888-2839
Affiliation:
5Department of Medicine, Houston Methodist Hospital, Houston, TX 77030, USA
ORCID: https://orcid.org/0000-0001-7531-1178
Affiliation:
6Section of Nephrology, Department of Medicine, Baylor College of Medicine, Michael E. DeBakey VA Medical Center, Houston, TX 77030, USA
Affiliation:
5Department of Medicine, Houston Methodist Hospital, Houston, TX 77030, USA
Email: Aesmail@houstonmethodist.org
ORCID: https://orcid.org/0000-0002-2337-8351
Explor Med. 2025;6:1001353 DOI: https://doi.org/10.37349/emed.2025.1001353
Received: June 06, 2025 Accepted: August 20, 2025 Published: September 01, 2025
Academic Editor: Yingyong Zhao, Northwest University, China
Patients who have lupus nephritis are usually asymptomatic. A few lupus nephritis patients may experience edema, hypertension, nocturia, polyuria, and foamy urine. Foamy urine or nocturia are early indicators of proteinuria, indicating tubular or glomerular dysfunction. Membranous-like glomerulopathy with masked IgG kappa deposits (MMMD) represents a form of immune complex deposition marked by concealed IgG kappa-restricted deposits, which are located in the subepithelial and mesangial areas as seen on electron microscopy. We report a rare case of a 26-year-old Hispanic woman with a history of systemic lupus erythematosus (SLE) diagnosed in 2015, who was initially evaluated for proteinuria and underwent a renal biopsy in 2019. The biopsy demonstrated membranous glomerulonephritis consistent with class V lupus nephritis. The patient volunteered to participate in a clinical trial for lupus nephritis in mid-2023. The second renal biopsy done at this visit (4 years after the initial renal biopsy) reported membranous glomerulonephritis, consistent with lupus class V and MMMD. Given the new finding of MMMD, a search for monoclonal gammopathy was initiated by looking for flow cytometry, serum protein electrophoresis (SPEP), and serum-free light chains, all of which were reported as negative. As the workup for monoclonal gammopathy and monoclonal gammopathy of renal significance (MGRS) was negative, MMMD was considered a secondary manifestation of lupus nephritis, a rare renal presentation of the condition.
Membranous-like glomerulopathy with masked IgG kappa deposits (MMMD) is a pattern of immune complex deposition characterized by masked deposits that show IgG kappa restriction and are subepithelial and mesangial by electron microscopy (EM) [1, 2]. These biopsies are typically negative for IgG on immunofluorescence (IF) and only reveal kappa or lambda light chain deposits on paraffin IF [1, 3]. This entity was first reported by Larsen et al. [4] in 2014 and has since been recognized more often as a distinct clinicopathologic diagnosis, most commonly seen in young women who present with proteinuria and variable autoimmune serology findings.
To identify the precise underlying etiologic disease state, substantial serologic tests and careful clinicopathologic correlation are required [2–4]. The diagnosis of membranous glomerulopathy, which is an immune complex-mediated glomerulopathy with primarily subepithelial immune deposits and IgG as the main Ig, based on renal biopsy, is an excellent example [1–4]. This recently described entity (MMMD) is associated with the presence of auto-antibodies but not with clearly defined autoimmune diseases. Here we present a case of a patient with systemic lupus erythematosus (SLE) and biopsy-proven class V lupus nephritis whose repeat biopsy showed Membranous-like glomerulopathy with masked monoclonal deposits MMMD.
A 26-year-old Hispanic female was admitted for the evaluation of persistent proteinuria. She had a history of hypertension, hearing loss, anxiety, anemia, deep vein thrombosis (DVT), migraine headaches, and miscarriage due to pre-eclampsia. She was diagnosed with SLE in 2015 and underwent a kidney biopsy in 2019 for proteinuria > 1 g that returned with positive dsDNA, anticardiolipin antibody (IgG and IgM), and low C4.
The initial biopsy demonstrated membranous glomerulonephritis consistent with class V lupus nephritis. The IgA was glomeruli with focal segmental trace peripheral granular staining, IgG was glomeruli with diffuse and global, peripheral granular staining 3+, IgM was glomeruli with diffuse, segmental to global, mesangial, and peripheral granular staining 2+, C3 was glomeruli with focal and segmental weak peripheral staining, arterioles +, C1q was glomeruli with diffuse and global, peripheral granular staining 1+ to 2+.
She was prescribed prednisone 10 mg and mycophenolate mofetil (MMF) 1,000 mg for her class V lupus nephritis without significant improvement in her proteinuria.
She came back for a follow-up visit in mid-2021, she was complaining of easy bruising and joint pain, especially around her time of menstrual cycle, a lab assessment was done during this visit and her lab values were abnormal as elevated protein in the urine (+3 g), positive blood in the urine (+2), low iron in the blood (34 μg/dL), positive dsDNA, low platelet and low C4. She was prescribed lisinopril 10 mg daily and hydrochlorothiazide (HCTZ) 25 mg daily to control her proteinuria and the possibility of kidney disease.
The second renal biopsy was done at this visit (4 years after the initial renal biopsy) was reported with light microscopic of a complex pattern of renal pathology, the sample includes up to 17 glomeruli, with three showing global sclerosis but the remaining glomeruli exhibit various abnormalities, such as diffusely thick and rigid capillary loops, large subepithelial deposits, mild mesangial matrix expansion, and hypercellularity, some glomeruli display ischemic changes and segmental sclerosis (Figure 1). In the interstitium, there is mild fibrosis and tubular atrophy affecting approximately 10% of the cortex, accompanied by a mild mononuclear inflammatory infiltrate and focal foam cells, and tubular epithelial cells show swelling with protein reabsorption droplets, but no red blood cell casts are observed. Vascular changes include minimal arteriolar hyalinosis, and a toluidine blue stain highlights an arcuate caliber artery with minimal intimal fibrosis. Special stains, including PAS, Jones silver, trichrome, and SMMT, were used to evaluate different aspects of basement membranes, deposits, and scarring. Controls for these stains are routinely run and verified. Notably, there is no evidence of karyorrhexis, wire loops, fibrinoid necrosis, crescent formation, or vasculitis.
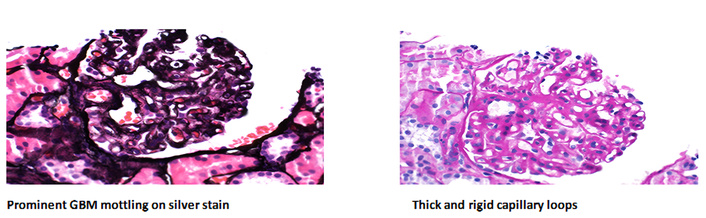
Light microscopic results. Renal biopsy with Jones’ methenamine silver stain showed increased cellularity. Jones’ methenamine silver stain of the renal biopsy demonstrated increased cellularity in the glomeruli, with thickened capillary loops and large subepithelial deposits. GBM: glomerular basement membrane. Magnification: 400×.
The IF analysis by this biopsy revealed features consistent with glomerular pathology, showing up to 12 glomeruli with segmentally prominent capillary loop mottling. Positive staining was observed for IgM, C3, kappa, and lambda light chains within the glomeruli, while IgG, IgA, C1q, albumin, fibrinogen, and other light chains showed no significant glomerular staining. Pronase digestion provided additional insight, indicating IgG, kappa, and lambda light chain involvement. Furthermore, diffuse positive staining for serum amyloid P (SAP) in glomeruli suggested potential amyloid deposition. Importantly, no substantial extraglomerular staining was detected. The glomeruli showed segmental to global mesangial and segmental capillary loop granular staining by IgM (2+), C3 (1+), and kappa (1–2+), and lambda (1–2+) light chains versus by IgG (2–3+) and kappa (3+) light chain. There was a very segmental paramesangial and rare capillary loop stained by a lambda light chain (trace-1+).
EM of this biopsy revealed glomerulus basement membranes with segmental thickening and remodeling associated with segmental subepithelial electron dense deposits to include mesangial hinge regions. Some of the deposits were large and hump-shaped. Several intramembranous electron-dense deposits and a few electron lucencies were also present. No subendothelial electron-dense deposits were seen. There was no capillary loop hypercellularity. There were frequent deep mesangial electron-dense deposits present, accompanied by an increase in mesangial matrix. There was severe epithelial foot process effacement. The tubular basement membranes demonstrated no deposits.
Following the new diagnosis of MMMD, an evaluation for monoclonal gammopathy was undertaken, including flow cytometry, serum protein electrophoresis (SPEP), and serum free light chain testing. All results were negative (see Table 1). Given the negative workup for monoclonal gammopathy/monoclonal gammopathy of renal significance (MGRS), the MMMD was considered secondary to lupus nephritis, a rarely reported renal manifestation of lupus nephritis.
Flow cytometry and SPEP and serum-free light chain data, and other markers.
| Tests | Values |
|---|---|
| T-cell/NK-cell markers | |
| CD2 | Positive; subset |
| CD3 | Positive; subset |
| CD4 | Positive; subset |
| CD5 | Positive; subset |
| CD7 | Positive; subset |
| CD8 | Positive; subset |
| CD56 | Positive; small subset |
| B-cell markers | |
| CD19 | Positive; subset |
| CD20 | Positive; subset |
| CD22 | Positive; subset |
| Kappa/CD19+ | Polyclonal |
| Lambda/CD19+ | Polyclonal |
| Myeloid markers | |
| CD117 | Negative |
| CD14 | Negative |
| Plasma cell markers | |
| CD38+/cKappa | Negative |
| CD38+/cLambda | Negative |
| CD138 | Negative |
| Kappa-lambda quat. flow with ratio | |
| Kappa quat. free light chains | 3.30–19.40 mg/L |
| Lambda quat. free light chains | 5.71–26.30 mg/L |
| Kappa/lambda free light chain ratio | 0.26–1.65 |
| SPEP | |
| T protein | 7.2 g/dL |
| Albumin | 3.8 g/dL |
| Alpha 1 | 0.3 g/dL |
| Alpha 2 | 0.8 g/dL |
| Beta | 1.0 g/dL |
| Gamma | 1.4 g/dL |
SPEP: serum protein electrophoresis.
The patient is currently on mycophenolate 1,000 mg, hydroxychloroquine 200 mg, lisinopril 20 mg, HCTZ 25 mg, and carvedilol 3.125 mg. Based on recent lab results, there was no significant improvement in her proteinuria. Furthermore, she underwent a subsequent kidney biopsy.
Renal manifestations of SLE represent 30–50% of prevalence that include proteinuria ≥ 500 mg/24 h (30–50%), cellular casts (30–50%), nephrotic syndrome (25%), and end-stage renal disease (5–10%) [5].
A new entity has been described called MMMD, which is identified as a pattern of immune complex depositions that can be examined by using EM. These deposits are subepithelial and mesangial and exhibit IgG kappa limitation [3].
The gold standard for recognizing and characterizing immune deposits is routine direct IF on fresh, unfixed tissue. “Masked deposits” are Igs that can be seen when IF is repeated on tissue that has been formalin-fixed and paraffin-embedded, but is not seen during standard IF staining.
Nasr et al. [6] provided the first description of the masked deposits that this approach revealed. Fourteen instances were described by Larsen et al. [4] as having several big subepithelial deposits that could be seen by EM and C3-predominant staining and negative IgG staining on regular IF.
Repeat IF after the formalin-fixed, paraffin-embedded tissue was digested with a pronase to produce intense, localized IgG staining. Having a mean age of 26 years, young patients with masked membranous nephropathy (MN) have comprised the majority of those documented.
Our patient is a young Hispanic female. When there are no glomeruli in the tissue submitted for standard IF, pronase digestion of the paraffin block for IF is most frequently used as a salvage procedure. The cause of some deposits stained by paraffin-embedded tissue IF rather than by standard IF is mainly unknown.
The relationship between lupus nephritis and MMMD remains incompletely understood. Lupus nephritis is classically associated with a full house pattern of immune complex deposition (IgG, IgA, IgM, C3, and C1q). MMMD, on the other hand, is characterized by a more restricted IgG-kappa light chain pattern that may be missed on routine IF. This brings up the question of whether MMMD represents a unique histopathologic manifestation of lupus nephritis or only a coincidental finding in patients with underlying autoimmunity. Currently, there are no treatment guidelines tailored specifically for MMMD. Management generally follows the conventional immunosuppressive therapies used for lupus nephritis. Further prospective studies are needed to determine whether MMMD in SLE has unique prognostic significance or requires different therapeutic considerations.
In conclusion, our case illustrates that patients with SLE can develop membranous glomerulonephritis with masked monoclonal deposits as a renal manifestation of the SLE in addition to classic class V lupus nephritis. In addition, this case shows the importance of performing a renal biopsy in SLE patients as well as submitting the tissue to EM and pronase digestion of paraffin-embedded tissue.
EM: electron microscopy
HCTZ: hydrochlorothiazide
IF: immunofluorescence
MGRS: monoclonal gammopathy of renal significance
MMMD: membranous-like glomerulopathy with masked IgG kappa deposits
SLE: systemic lupus erythematosus
SPEP: serum protein electrophoresis
The abstract section of this manuscript was presented in the form of an abstract at the Kidney Week 2023 and is included in the conference abstract collection (https://www.asn-online.org/education/kidneyweek/2023/program-abstract.aspx?controlId=3944740).
MA: Writing—original draft, Writing—review & editing, Conceptualization, Methodology, Investigation, Resources. SM: Writing—original draft, Writing—review & editing, Conceptualization, Methodology, Investigation, Resources, Project administration, Supervision. AM: Writing—original draft, Writing—review & editing, Data curation, Formal analysis, Visualization, Investigation, Resources, Validation. DB, EAN, and BK: Writing—original draft, Writing—review & editing, Investigation, Resources. SA: Writing—original draft, Writing—review & editing, Data curation, Investigation, Resources, Validation. AE: Writing—original draft, Writing—review & editing, Investigation, Resources, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The approval number IRB-2023-0457 at the PCCR, Houston, TX.
The authors certify that they have obtained the appropriate patient consent forms.
The patient has given her consent for her clinical information to be reported in the journal.
The data of this case report that supports our results are available on request from the corresponding author, Sreedhar Mandayam.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2095
Download: 10
Times Cited: 0
