Affiliation:
1Department of Medicine, Maastricht University Medical Center, 6202 AZ Maastricht, The Netherlands
Affiliation:
2Department of Biochemistry, University of Maastricht, 6200 MD Maastricht, The Netherlands
3Cardiovascular Research Institute Maastricht (CARIM), University of Maastricht, 6200 MD Maastricht, The Netherlands
ORCID: https://orcid.org/0000-0001-7867-6957
Affiliation:
1Department of Medicine, Maastricht University Medical Center, 6202 AZ Maastricht, The Netherlands
Affiliation:
1Department of Medicine, Maastricht University Medical Center, 6202 AZ Maastricht, The Netherlands
3Cardiovascular Research Institute Maastricht (CARIM), University of Maastricht, 6200 MD Maastricht, The Netherlands
ORCID: https://orcid.org/0000-0001-7750-8249
Affiliation:
1Department of Medicine, Maastricht University Medical Center, 6202 AZ Maastricht, The Netherlands
3Cardiovascular Research Institute Maastricht (CARIM), University of Maastricht, 6200 MD Maastricht, The Netherlands
Email: p.deleeuw@maastrichtuniversity.nl
ORCID: https://orcid.org/0000-0002-4949-5812
Explor Med. 2025;6:1001321 DOI: https://doi.org/10.37349/emed.2025.1001321
Received: January 21, 2025 Accepted: April 18, 2025 Published: May 21, 2025
Academic Editor: Akiko Mammoto, Medical College of Wisconsin, USA
Aim: The VitaK-CAC (vitamin K-coronary artery calcification) trial is a double-blind, randomized, placebo-controlled trial in patients with pre-existent CAC who were treated for two years with either placebo or the vitamin K2-analogue menaquinone-7 (MK-7). The purpose of the present analysis of the VitaK-CAC trial was to assess the degree of adherence to supplementation with MK-7 during the implementation and persistence phases.
Methods: We estimated adherence in three different ways: 1) by pill counts, 2) by measuring plasma levels of MK-7, and 3) by measuring plasma levels of dephosphorylated, uncarboxylated matrix Gla-protein (dp-ucMGP), a marker of the functional bioactivity of vitamin K.
Results: Estimated adherence based on pill counts was 90%, but it was lower (80%) when plasma levels of vitamin MK-7 were taken as reference. Changes in dp-ucMGP appeared not to be independent from those of MK-7.
Conclusions: We conclude that none of the three methods that we applied in our study is absolutely reliable to estimate adherence to supplementation with MK-7. Yet, the measurement of MK-7 levels provides the best information (the study has been registered at clinicaltrials.gov as NCT01002157).
Several studies suggest that vitamin K supplementation may reduce vascular calcification. However, meta-analyses of trials have not shown consistent results [1, 2]. This could be explained, at least in part, by heterogeneity in design and patient population among the studies. However, poor adherence to supplementation has been largely overlooked in some trials. Unfortunately, little is known about adherence to dietary vitamin K supplementation. Recently, our group completed the VitaK-CAC (vitamin K-coronary artery calcification) trial. This provided a unique opportunity to assess adherence to long-term supplementation with menaquinone-7 (MK-7), a vitamin K2-analogue. In that study, we evaluated whether supplementation with MK-7 over a period of two years can slow down the progression of CAC [3]. To assess adherence, we measured plasma levels of vitamin K and of dephosphorylated, uncarboxylated matrix Gla-protein (dp-ucMGP), a marker of the functional bioactivity of vitamin K [4], in addition to pill counts. Plasma levels of MK-7 were used to ascertain that the supplementation was taken. Since MK-7 plasma levels steadily increase after oral administration [5], a lack of increase likely indicates non-adherence. Given that dp-ucMGP levels are expected to decrease following MK-7 administration [6–9], we argued that inadequate intake would result in a smaller-than-expected reduction. Here, we present our findings on adherence to long-term MK-7 supplementation and its effects on dp-ucMGP and plasma MK-7 levels.
The VitaK-CAC trial is a double-blind, randomized, placebo-controlled trial in patients with pre-existent CAC. The study included 180 patients of at least 18 years of age with complaints suggestive of coronary artery disease (CAD), and a baseline CAC score between 50 and 400 Agatston units (AU). They were randomly assigned (1:1) to treatment with either placebo or 360 micrograms of MK-7 (MenaQ7, Nattopharma AS, Oslo, Norway—now part of Gnosis by Lesaffre, Lille, France). Patients were seen at six-month intervals during follow-up. At baseline, and again one and two years after starting treatment, patients underwent CT-scans and comprehensive blood tests, including assessment of vitamin K status. The protocol of the study has been described in detail elsewhere [3], and the study has been registered at clinicaltrials.gov as NCT01002157.
The trial was approved by the Ethical Review Committee of the Maastricht University Medical Center (MEC09-2-075, NL27372.068.09), and all patients gave written informed consent.
According to the 2012 taxonomy for describing and defining adherence [10] and the EMERGE guidelines [11, 12], our study focuses on the implementation and persistence phases of adherence. We operationalized adherence assessment using three different approaches. First, we estimated tablet intake by counting the number of pills participants returned to the laboratory at each follow-up visit. When 20% or less of the prescribed pills were returned, this was considered adequate adherence. Because pill counts may misrepresent actual adherence, we also measured vitamin K1 and MK-7 levels from the blood samples, which were taken after an overnight fast at the one-year and two-year visits. These data would allow to conclude whether MK-7 levels had risen sufficiently, even with less-than-optimal intake of tablets. In patients receiving MK-7, an increase in plasma levels above the upper interquartile range (IQR) observed in the placebo group was taken as evidence of supplementation adherence. When this occurred both at 12 months and at 24 months, this was taken as a robust indicator of good persistence [13]. We also measured K1 to rule out the possibility that participants were independently taking over-the-counter vitamin K supplements.
Finally, we measured levels of dp-ucMGP at the same time points that blood was taken for the vitamin assays. Since vitamin K administration is supposed to lead to a fall in dp-ucMGP concentration, changes in the levels of this protein could also serve as a marker of adherence. Taking the assay variability into account, we considered a fall in dp-ucMGP by more than 10% as indicative of a positive effect of MK-7 supplementation and, hence, as a proxy for good adherence. Accordingly, we assessed adherence from three perspectives: intake (pill counts), bioavailability (vitamin K levels), and functional activity (dp-ucMGP levels).
Immediately after sampling, blood was centrifuged at 3,000 g for 10 min to prepare plasma, which was then stored at –80°C until shipped on dry-ice for centralized analysis. We measured vitamin K using liquid chromatography tandem mass spectroscopy (LCMSMS) consisting of an initial sample purification step prior to tandem MS detection (Magtivio BV, Nuth, the Netherlands). Assay variation ranged from 1.0–5.9% for K1 and 3.5–14.6% for MK-7. The assay performance was evaluated through participation in the international KEQAS scheme.
EDTA-plasma was used to measure dp-ucMGP levels using the commercially available IVD CE-marked chemiluminescent InaKtif dp-ucMGP assay on the IDS-iSYS system (IDS, Boldon, UK) as described earlier [14]. The within-run and total variations of this assay were 0.8–6.2% and 3.0–8.2%, respectively. To minimize assay variability, all samples were processed in a single analytical run.
We measured CAC scores using the Agatston method with a dual-source CT-scanner (Somatom Definition Flash, Siemens Medical Solutions, Forchheim, Germany) as previously described [3]. Data acquisition parameters were 2 × 128 × 0.6 mm slice collimation, a gantry rotation time of 280 ms, and a tube voltage of 100 kV or 120 kV depending on patients’ height and weight.
Using generalized estimating equations (GEE) to account for correlated repeated measures, we tested whether plasma levels of vitamin K1, MK-7, and dp-ucMGP changed over time within and between treatment groups. Results were adjusted for age, sex, and body mass index (BMI). Group comparisons at each time point were tested by means of the Mann-Whitney U-test for non-normally distributed data. Where appropriate, we applied the Bonferroni correction for multiple comparisons.
We used linear regression analysis (in case of continuous variables) and logistic regression analysis (in case of categorical variables) to test the association between pill counts and demographic variables and between changes in MK-7 and those in dp-ucMGP.
The study was powered on the basis of its primary outcome variable, i.e., the CAC score. To detect an absolute difference of 15% in CAC progression between treatment groups with 90% power, 59 patients per group were required [3].
Continuous data are expressed as medians with IQR and categorical data as percentages, unless stated otherwise.
A p-value < 0.05 was deemed statistically significant. We used STATA software (version 18 for MacOS, StataCorp, College Station, TX, USA) and Prism software (version 10 for MacOS, GraphPad software, Boston, Mass) for all statistical analyses and graphs.
Of the 180 patients who were initially randomized (90 to each group), 13 dropped out for various reasons before any baseline measurements were obtained. Thus, baseline data are available for 167 patients (Table 1). All of them completed the one-year follow-up evaluation. During the second year, however, 7 of the 82 patients in the placebo group and 10 of the 85 patients in the active group dropped out. Consequently, 150 patients completed the entire follow-up period, 75 on placebo and 75 on MK-7.
Baseline characteristics of the follow-up population
| Variable | Placebo (n = 75) | MK-7 (n = 75) |
|---|---|---|
| Age (years) | 62 (54–66) | 59 (55–65) |
| Sex (M/F, %) | 43/32 (57/43) | 46/29 (61/39) |
| Height (m) | 1.71 (1.64–1.79) | 1.72 (1.65–1.78) |
| Weight (kg) | 82 (73–92) | 81 (72–90) |
| Vitamin K1 (μg/L) | 0.549 (0.410–0.724) | 0.597 (0.392–0.762) |
| Vitamin MK-7 (μg/L) | 0.518 (0.352–0.639) | 0.499 (0.315–0.775) |
| dp-ucMGP (pmol/L) | 349 (285–402) | 329 (273–409) |
| CAC score (AU) | 145 (90–220) | 135 (95–221) |
Data presented as median and interquartile range (IQR) for continuous variables and as percentages for categorical variables. CAC: coronary artery calcification; AU: Agatston units; MK-7: menaquinone-7; dp-ucMGP: dephosphorylated, uncarboxylated matrix Gla-protein
Due to inconsistent bottle returns by many participants, reliable pill counts could be obtained for only half of the patients. Overall, the median percentage of returned pills was 10% (IQR 7–14%) in the placebo group and 10% (IQR 6–13%) in the MK-7 group (difference not significant), suggesting an adherence of approximately 90% in both groups. Ordinary and logistic regression analyses showed that the number of returned pills was not influenced by age, sex, or BMI.
Plasma levels of vitamin K1 and MK-7 could be obtained in all patients who completed the one- and two-year follow-up. Figure 1 shows how these levels evolved over time. In both groups, K1 levels fell significantly during the first year of treatment but remained constant thereafter. No significant difference in K1 levels was observed between the placebo and treatment groups at any time point.
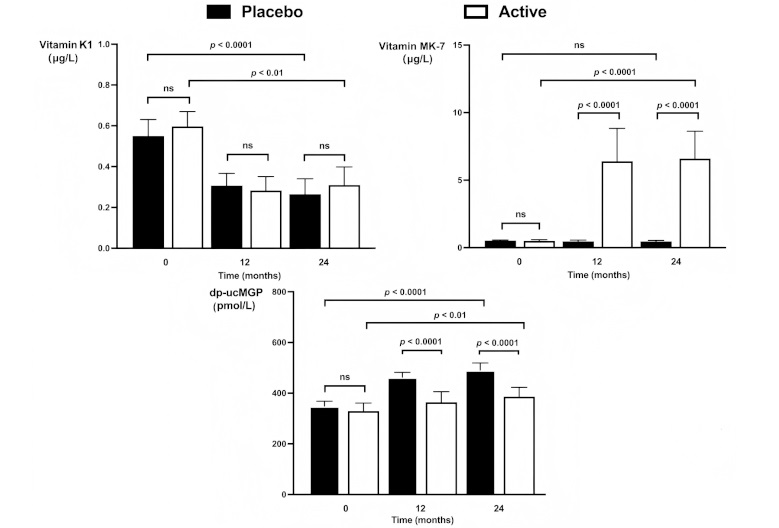
Plasma levels of vitamin K1 (upper left panel), vitamin MK-7 (upper right panel), and dp-ucMGP (lower panel) in the placebo group and actively treated group at baseline as well as after 12 and 24 months of treatment. Generalized estimating equations (GEE) statistics were used to test whether changes over time and between groups were statistically significant. Significance levels as derived from the GEE are given for the whole period. Significance levels for group comparisons at each time point are based on the Mann-Whitney U-test for non-normally distributed data. ns: not significant; MK-7: menaquinone-7; dp-ucMGP: dephosphorylated, uncarboxylated matrix Gla-protein
Baseline MK-7 levels were similar between the two groups. During the first year of the study, MK-7 levels did not change in the placebo group but rose significantly in the actively treated group. No further rise was observed thereafter. Age, sex, and BMI had no modifying effect on the increase in MK-7 levels.
In Figure 2, the individual changes in MK-7 levels are displayed for the actively treated patients. It appears that there is substantial variation in these levels, not only between patients but also between the various time points. In six patients (8%), MK-7 levels remained within the placebo range after the first year, possibly due to poor adherence or absorption. However, they all increased their levels during the second year. Conversely, in a substantial number of patients who had sizeable increments in MK-7 during the first year, levels fell back to the normal range during the second year. In ten patients (13%), MK-7 levels at the end of the study did not differ from those in the placebo group. In total, 60 of the 75 participants who received MK-7 had elevated plasma levels throughout the study, suggesting a persistence rate of 80%.
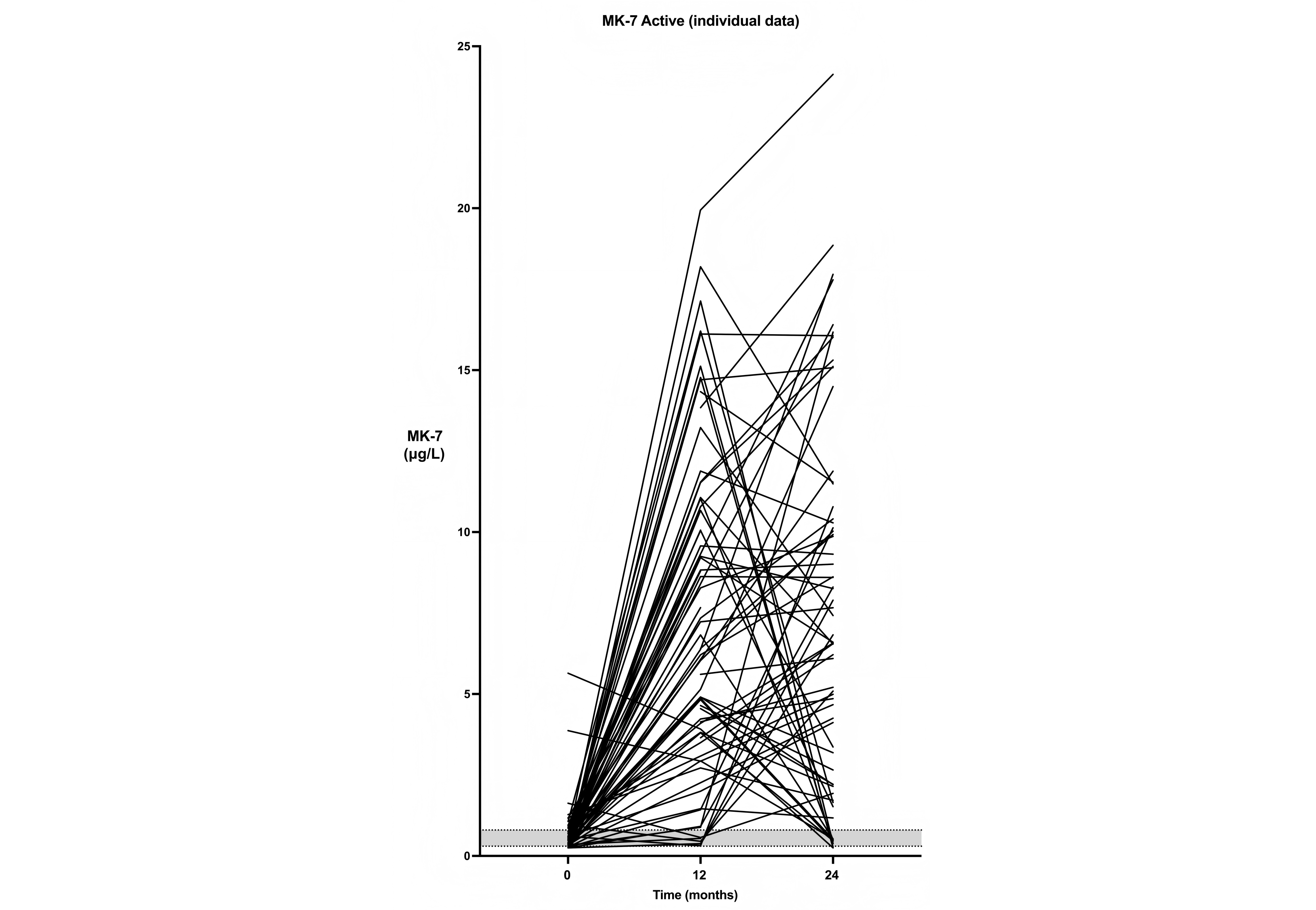
Plasma levels of MK-7 in all individual patients from the actively treated group. The shaded area represents the interquartile range for the placebo group. MK-7: menaquinone-7
As shown in Figure 1, levels of dp-ucMGP rose significantly in both groups, although to a lesser extent in the group with active treatment (p < 0.0001). In the placebo group, dp-ucMGP levels increased by a median of 39% (IQR 21–67%) over two years. In the actively treated group, dp-ucMGP increased by less than 39% in 67% of the patients and less than 21% in 57% of them. If the reduced rise in dp-ucMGP is considered a proxy for adherence, this suggests approximately 60% adherence in the MK-7 group.
GEE analysis showed that, except for the treatment group, BMI (p < 0.001) was a significant independent predictor of dp-ucMGP levels (i.e., higher levels at higher BMI). Age and sex had no modifying effect, and there was no interaction between time and treatment.
At closer examination, we found substantial interindividual variations with both falls and rises in dp-ucMGP levels during treatment in the two groups. However, the proportion of patients with a ≥ 10% decrease in dp-ucMGP, indicating enhanced vitamin K activity, was four times greater in the treatment group compared to placebo (20 versus 5%; p = 0.003). In 21 of the 75 patients (28%), the rise in MK-7 was sufficiently great for levels of dp-ucMGP to decrease.
At the end of the 24-month follow-up, changes in dp-ucMGP levels from baseline were inversely correlated with changes in MK-7 levels (R2 = 0.19; p < 0.04). When we combined these data with the results of the pill counts, this inverse relationship appeared to be similar for all patients, regardless of pill counts. The regression lines for patients with pill count adherence ≥ 80% and < 80% had identical slopes and intercepts, allowing the data to be represented by a single regression line (Figure 3). The proportion of patients with an adherence of 80% or more, as based on pill counts, did not differ between patients in whom dp-ucMGP rose and those in whom dp-ucMGP fell.
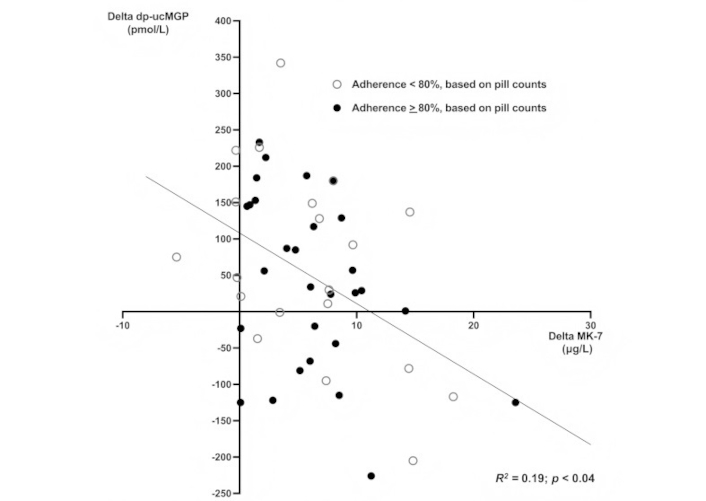
The relationship between changes from baseline in MK-7 and dp-ucMGP after 24 months of follow-up. Open circles: patients with an adherence, based on pill counts, of 80% or more. Closed circles: patients with an adherence less than 80%. Regression line for the pooled data from the two groups [adjusted for body mass index (BMI)]
No difference in the evolution of the CAC score was found between patients with an adherence below or above 80%, as determined by pill counts. However, when patients with persistence throughout the study, as based on the MK-7 levels, were compared to those who were not, the former group showed an attenuated progression of their CAC score. The median CAC score increased by 43 AU (IQR 17–80) in the persistent group compared to 68 AU (IQR 46–109) in the less persistent group (p < 0.05). A similar difference was found between patients with stable or decreased dp-ucMGP and those with increased levels, but this difference disappeared after adjusting for concurrent changes in MK-7 levels.
This study demonstrates that, although adherence to MK-7 supplementation was likely adequate, accurately estimating adherence remains challenging. We took a three-step approach, looking at tablet intake, bioavailability of the vitamin and its bioactivity. Based on the returned tablet counts, approximately 90% of participants appeared to take the study medication as prescribed, suggesting reasonably good adherence. However, while pill count and similar methods are commonly used to estimate medication adherence [15], they have limitations, as our experience also confirmed. Nearly half of the patients failed to bring their pillboxes to one or more follow-up visits. If this group would also be more forgetful in taking the study medication, true adherence would be much lower. Moreover, two patients with the same pill count may still have had a substantially different adherence pattern [16]. Another argument that arises from our study against the usefulness of pill counts is the fact that there was no difference in the response of MK-7 levels and in the primary outcome (CAC progression) between patients with or without sufficient adherence.
Indeed, even with pill counts, it is impossible to be certain that participants took their tablets correctly. Precisely for that reason, hypertension specialists for whom non-adherence is almost a daily problem now recommend chemical adherence testing [17]. In line with this recommendation, we also measured plasma levels of MK-7. Based on these data, we estimated adherence, or more precisely persistence, to be 80%, which is probably a better approximation than adherence based on pill counts. Furthermore, the fact that the progression of coronary calcification in patients with persistence throughout the study was attenuated in comparison to less adherent individuals is a strong argument in favour of estimating adherence based on MK-7 plasma levels.
Nevertheless, the individual plots in Figure 2 reveal substantial fluctuations in plasma levels over time. Whether these fluctuations are due to inconsistent adherence or to some other factor(s) remains elusive. Certainly, we cannot exclude the possibility that variations in absorption or metabolism have contributed to, or may even be entirely responsible for, the observed swings in plasma levels. However, in most patients, MK-7 levels remained well above those observed in the placebo group. In this respect, it is interesting that the levels of vitamin K1 fell during the study. As this occurred both in the placebo and in the actively treated group, this cannot be attributed to an effect of the supplementation itself. One possible explanation is that participants altered their diet and consumed less K1-rich food. A higher consumption of plant-based foods in lieu of animal-based foods, for instance, would be expected to lead to lower bioavailability of vitamin K1 [18]. However, in the absence of dietary intake data, we cannot draw definitive conclusions on this point.
Another chemical estimate that we introduced in this study is the measurement of dp-ucMGP levels, which can be used as proxy for the adequacy of MK-7 supplementation [6–8]. Variations in dp-ucMGP were considerable. On average, levels of this protein increased in both groups, albeit to a lesser extent in the actively treated group. This may reflect the age-related increase in dp-ucMGP levels [19, 20]. Consequently, it seems fair to consider at least 28% of the patients in whom dp-ucMGP levels fell as adherent because it is extremely unlikely that these levels would fall spontaneously. However, since dp-ucMGP normalization is not always possible [9], it is reasonable to consider participants adherent even when their dp-ucMGP changes do not exceed the lower interquartile limit of the placebo group. This was the case in about 60% of the patients who received MK-7.
Nevertheless, caution is warranted. Theoretically, it is still possible that less-adherent participants took their MK-7 pills only shortly before their visit to the clinic, with an attenuated rise of dp-ucMGP during that period alone. In that case, relying on dp-ucMGP concentrations would lead to overestimation of adherence. This is, however, unlikely because MK-7 levels peak 4 hours after intake [5] while dp-ucMGP levels take a much longer time to stabilize. Conversely, dp-ucMGP measurements may underestimate adherence if adequate MK-7 intake is accompanied by variability in absorption or carboxylation efficiency.
Moreover, to conclude that adherence based on dp-ucMGP measurements is around 60% is not justified. First of all, BMI had an impact on dp-ucMGP as well. This finding is not unexpected, as dp-ucMGP levels tend to be higher in obese individuals, particularly those with abdominal adiposity [4, 21]. As the effect of MK-7 on dp-ucMGP per our analysis was less in heavier participants, the implication of this finding may be that changes in dp-ucMGP are less suitable as a marker of adherence to supplementation with MK-7 in the obese. A further argument against the use of dp-ucMGP can be distilled from the data in Figure 3. Although these data confirm earlier findings [6–9], the regression line intersects the x-axis at a level of approximately 10 μg/L. This indicates that, on average, MK-7 levels have to increase by at least that amount before dp-ucMGP can be expected to fall. Whether this reflects intrinsic interindividual differences in vitamin K carboxylation efficiency cannot be determined from our data.
Taken together, our data reveal a striking discrepancy in adherence estimates across the three methods used. However, all of these methods are fallible, which makes it impossible to conclude which one is best. Because the relationship between the changes in dp-ucMGP and MK-7 appeared to be independent of pill counts, our findings suggest that at least pill counts are probably less reliable for estimating adherence than the chemical assays. As discussed, changes in dp-ucMGP appear less reliable for assessing adherence than MK-7 levels, making plasma MK-7 measurement likely the most accurate method for confirming supplementation intake.
One limitation of our study is the absence of an electronic monitoring system. Unfortunately, this was not feasible within the context of our study. However, we did apply a medication event monitoring system (MEMS) in the past but found that this method was not superior to pill counts and even somewhat less accurate [22]. Likewise, smart blister packages with electronic wiring are not ideal either [23]. Despite the attractiveness of such methods, there is still a risk that patients either intentionally or unintentionally do not take their medication as prescribed.
Another limitation is that we did not monitor dietary changes, which could have impacted adherence estimates. However, Lentz and coworkers [8] have shown that a vitamin K-rich diet was not able to induce a significant effect on dp-ucMGP. Thus, any changes in this peptide that were smaller than those observed in the placebo group are likely attributable to the supplementation and not to diet.
Overall, adherence to MK-7 supplementation appears to be approximately 80% based on MK-7 plasma levels, assuming adequate absorption. Although none of the three methods that we applied in our study is absolutely reliable to estimate adherence, the measurement of MK-7 levels may, therefore, provide the best information.
AU: Agatston units
BMI: body mass index
CAC: coronary artery calcification
dp-ucMGP: dephosphorylated, uncarboxylated matrix Gla-protein
GEE: generalized estimating equations
IQR: interquartile range
MK-7: menaquinone-7
The authors wish to thank Dr. Gregory Veldhuizen for his help in the linguistic correction of the manuscript.
LMV, AAK, PWdL: Conceptualization, Methodology, Data curation, Formal analysis, Writing—original draft. LJS: Conceptualization, Methodology, Writing—review & editing. CdH: Data curation, Writing—review & editing. All authors have seen and approved the submitted version.
LJS receives research grants from Gnosis by Lesaffre and Bayer and is the founder and shareholder of Coagulation Profile BV. He has voluntarily refrained from being involved in the analysis of the data. The other authors declare that there are no conflicts of interest.
The trial was approved by the Ethical Review Committee of the Maastricht University Medical Center [MEC09-2-075, NL27372.068.09].
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The data presented in this study are available from the authors at reasonable request.
This study was supported by a grant from the Dutch Heart Foundation [2010B161]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 4848
Download: 14
Times Cited: 0
